Dear Editor
Toxic epidermal necrolysis (TEN) and Stevens‐Johnson syndrome (SJS) present with extensive epidermal detachment of the skin and mucous membranes as well as systemic involvement. 1 Drug hypersensitivity is the major culprit for TEN, while mycoplasma pneumonia infection, vaccinations, acute graft‐vs‐host disease, and systemic lupus erythematosus (SLE) are among other causes. 2 Prior studies have reported a rare primary presentation of LE as SJS/TEN, which is defined as a new clinical entity (SJS/TEN‐like lupus erythematosus). 3
Herein, we report a challenging case of SJS/TEN‐like LE that highlights the importance of considering LE as a rare cause of SJS/TEN like syndrome.
A 26‐year‐old female presented with a 2‐week history of generalized targetoid papule and purpuras and erosive lesions of mucosa. Her past medical history and drug history were insignificant.
Lesions involved almost 70% of her body surface including palms and soles with positive Nikolsky sign (Figure 1).
FIGURE 1.
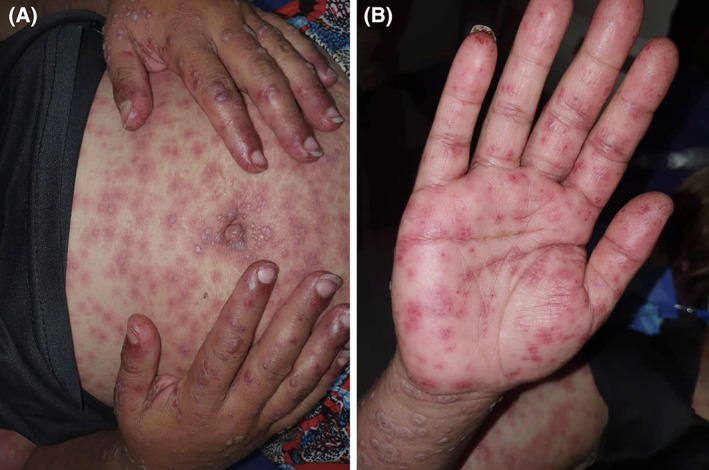
A, Generalized targetoid and purpuric macules of variable size with central bullae formation in some lesions and B, Multiple targetoid and purpuric macules on the palms
Laboratory data showed 2‐fold rise in liver enzymes level, 4 g proteinuria in 24 h‐urine analysis and elevated serum creatinine. Anti‐nuclear antibody and anti‐ds‐DNA, anti‐LA and anti‐RO antibody were strongly positive. Investigations for SARS‐CoV‐2 infection came back negative.
Of note, mild ascites was detected in her abdominal cavity ultrasonography. After primary rheumatology consultation, the patient received methylprednisolone 500 mg as pulse therapy for three consecutive days.
Skin biopsy was obtained from one of the target lesions which showed near total epidermal necrosis and subepidermal blister formation containing fibrinous material and necrotic cells admixed with some lymphocytes, neutrophils and a few eosinophils. Moreover, there was a mild perivascular infiltration of lymphocytes and a few eosinophils and neutrophils in the superficial dermis, which produced lichenoid interface reaction with Civatte body formation and melanin incontinence (Figure 2). The direct immunofluorescence (DIF) study showed focal 2+ granular anti‐IgM deposits along dermoepidermal junction. Considering the earlier corticosteroid pulse therapy, DIF was in favor of a partially treated lupus erythematosus (Figure 3).
FIGURE 2.
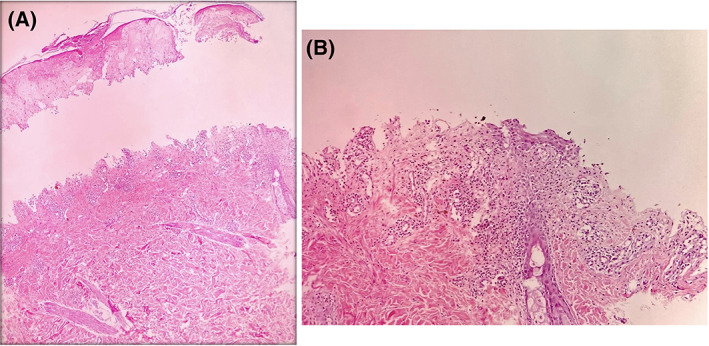
A, Histopathologic examination showed a subepidermal blister with full thickness epidermal necrosis and mild perivascular dermatitis (H&E, ×40); B, Higher magnification shows mild superficial perivascular dermatitis and focal lichenoid tissue reaction (H&E, ×100)
FIGURE 3.
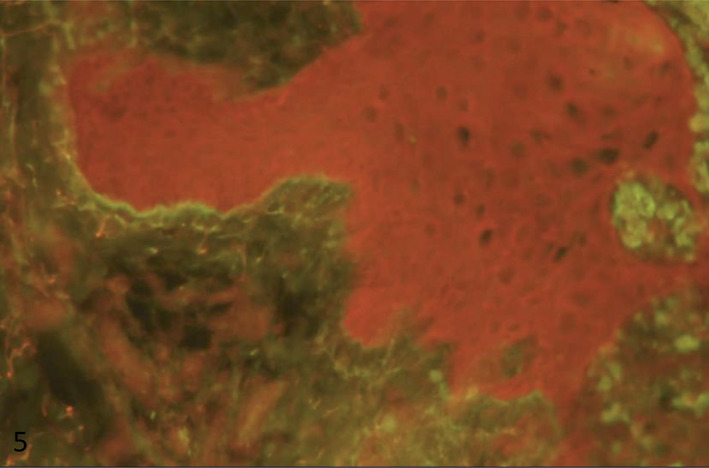
Immunofluorescence study: focal immunoreactivity for anti‐IgM in dermoepidermal junction
Corticosteroid pulse therapy was then changed to 30 mg/day oral prednisolone plus 1 g/kg/day intravenous immunoglobulin infusion (IVIg) for 3 days resulting marked improvement of skin lesions over a week. Unfortunately, she presented with SARS‐CoV‐2 infection 4 days after her discharge, although repeated screenings for COVID‐19 were negative during her admission period. Despite urgent ICU admission, she died of acute respiratory distress syndrome (ARDS) 3 days later.
The current case describes a patient with acute cutaneous lupus erythematosus (ACLE) with SJS/TEN‐like primary presentation. There are few points that may help to differentiate between the classic SJS/TEN and SJS/TEN‐like LE. For instance, the absence of exposure to etiologic factors for SJS/TEN and photo distributed patterns of skin lesions are in favor of SJS/TEN‐like LE. Further, the interval between the first rash to epidermal detachment in SJS/TEN‐like LE seems to be longer (weeks to months) than the classic SJS/TEN (hours to days). 4 SJS/TEN‐like LE skin lesions may appear on palms and soles more frequently than SJS/TEN 5 .
Moreover, lupus specific lesions, which is characterized by interface dermatitis response, could turn the diagnosis toward SJS/TEN‐like LE. 6 Presence of continuous IgM, IgG, and C3 deposition at basement membrane zone may be observed in DIF and is of great value in distinguishing these two entities as well. 5
Previous reports indicated that the high level of lupus antibody profile (anti‐nuclear antibody, anti‐ds‐DNA, anti‐RO, and anti‐LA) are detected in SJS/TEN‐like LE. 7
Involvement of internal organs in the setting of SLE that often need immunosuppressive treatment increases susceptibility to severe forms of COVID‐19; this should be taken into account before initiation of treatment in these patients. 8
Although there is no meticulous guidelines for treating SJS/TEN‐like LE, IVIg has been reported as an efficient treatment in the literature. The possible explanation for such efficiency could be explained by the fact that IVIg blocks Fas‐Fas ligands which has a crucial role in apoptosis in both LE and SJS/TEN. 3 On the other hand, IVIg may be considered as a safe option for those who are suffering from comorbidities and have higher risk of developing severe infection in the current pandemic. 9 , 10 It is noteworthy that many unusual skin presentations can occur in the setting of COVID‐19 11 ; however, we ruled out this condition by conducting repeated screening test for COVID‐19 during her admission period.
CONFLICT OF INTEREST
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
AUTHOR CONTRIBUTIONS
Mohammad Shahidi‐Dadras: Editing draft, Investigation, Conceptualization. Farnaz Araghi: Writing draft. Arman Ahmadzadeh: Data acquisition. Azadeh Rakhshan: Data acquisition. Mohammadreza Tabary: Editing draft, Visualization. Sahar Dadkhahfar: Editing draft, Supervision.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Caproni M, Torchia D, Schincaglia E, et al. Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens–Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2006;155(4):722‐728. [DOI] [PubMed] [Google Scholar]
- 2. Singthong S, Kwangsukstid O, Sudtikoonaseth P. Toxic epidermal necrolysis‐like lupus erythematosus: a case report. Thai J Dermatol. 2019;35(1):25‐33. [Google Scholar]
- 3. Mandelcorn R, Shear NH. Lupus‐associated toxic epidermal necrolysis: a novel manifestation of lupus? J Am Acad Dermatol. 2003;48(4):525‐529. [DOI] [PubMed] [Google Scholar]
- 4. Lee H, Tey H, Pang S, Thirumoorthy T. Systemic lupus erythematosus presenting as Stevens–Johnson syndrome and toxic epidermal necrolysis: a report of three cases. Lupus. 2011;20(6):647‐652. [DOI] [PubMed] [Google Scholar]
- 5. Tankunakorn J, Sawatwarakul S, Vachiramon V, Chanprapaph K. Stevens‐Johnson syndrome and toxic epidermal necrolysis‐like lupus erythematosus. J Clin Rheumatol. 2019;25(5):224‐231. [DOI] [PubMed] [Google Scholar]
- 6. Perić J, Lekić B, Bosić M, Škiljević D. Toxic epidermal necrolysis‐like subacute cutaneous lupus erythematosus: a case report. Serbian J Dermatol Venereol. 2019;11(4):129‐132. [Google Scholar]
- 7. Merklen‐Djafri C, Bessis D, Frances C, et al. Blisters and loss of epidermis in patients with lupus erythematosus: a clinicopathological study of 22 patients. Medicine. 2015;94(46):e2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID‐19) in a series of 17 patients with systemic lupus erythematosus under long‐term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79(6):837‐839. [DOI] [PubMed] [Google Scholar]
- 9. Ferro F, Elefante E, Puxeddu I, et al. COVID‐19: the new challenge for rheumatologists. First update. Clin Exp Rheumatol. 2020;38(3):373‐382. [PubMed] [Google Scholar]
- 10. Nobari NN, Goodarzi A. Patients with specific skin disorders who are affected by COVID‐19: what do experiences say about management strategies? A systematic review. Dermatol Ther. 2020;e13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


