Abstract
Background
People with advanced human immunodeficiency virus (HIV) (CD4 < 50) remain at high risk of tuberculosis (TB) or death despite the initiation of antiretroviral therapy (ART). We aimed to identify immunological profiles that were most predictive of incident TB disease and death.
Methods
The REMEMBER randomized clinical trial enrolled 850 participants with HIV (CD4 < 50 cells/µL) at ART initiation to receive either empiric TB treatment or isoniazid preventive therapy (IPT). A case-cohort study (n = 257) stratified by country and treatment arm was performed. Cases were defined as incident TB or all-cause death within 48 weeks after ART initiation. Using multiplexed immunoassay panels and ELISA, 26 biomarkers were assessed in plasma.
Results
In total, 52 (6.1%) of 850 participants developed TB; 47 (5.5%) died (13 of whom had antecedent TB). Biomarkers associated with incident TB overlapped with those associated with death (interleukin [IL]-1β, IL-6). Biomarker levels declined over time in individuals with incident TB while remaining persistently elevated in those who died. Dividing the cohort into development and validation sets, the final model of 6 biomarkers (CXCL10, IL-1β, IL-10, sCD14, tumor necrosis factor [TNF]-α, and TNF-β) achieved a sensitivity of 0.90 (95% confidence interval [CI]: .87–.94) and a specificity of 0.71(95% CI: .68–.75) with an area under the curve (AUC) of 0.81 (95% CI: .78–.83) for incident TB.
Conclusion
Among people with advanced HIV, a parsimonious inflammatory biomarker signature predicted those at highest risk for developing TB despite initiation of ART and TB preventive therapies. The signature may be a promising stratification tool to select patients who may benefit from increased monitoring and novel interventions.
Clinical Trials Registration
Keywords: tuberculosis, biomarker, antiretroviral therapy, early mortality
Our study is the first to identify an inflammatory plasma 6-biomarker signature predictive of incipient TB in advanced HIV that occurs rapidly after the initiation of ART despite tuberculosis screening and preventive therapy.
Tuberculosis (TB) is the leading cause of death due to a single infectious agent worldwide [1]. Human immunodeficiency virus (HIV) infection greatly increases the risk of developing TB, which increases proportionately as CD4+ T-cell counts inexorably decline in persons living with HIV (PLHIV) with an unsuppressed viral load [2–4]. Treatment with antiretroviral therapy (ART) and TB preventive therapy (isoniazid preventive therapy [IPT]) greatly reduce the risk of TB and death [5–7] and are now being widely scaled up. However, PLHIV, especially those with low CD4+ T-cell counts below 200 cells/µL, remain at high risk of mortality and incident TB in high-burden settings, especially during the first 12 months post-ART and IPT initiation [8–10]. A meta-analysis showed that 17% (95% confidence interval [CI] 11–24%) of adults in sub-Saharan Africa die within the first year after ART initiation (defined as early mortality) [11–13]; TB accounts for 5–44% of these deaths. Despite “Treat All” policies adopted by most countries, the proportion of patients who present with advanced HIV remains stubbornly static particularly in low-income settings [10, 14]. TB and mortality risk is not uniform and is thought to be associated with high baseline, host-mediated inflammation, immune dysfunction, and incipient undiagnosed infections [15] despite current TB screening methods [16], ART, and IPT initiation.
Therefore, there is an urgent clinical and public health need to identify the PLHIV who are at high risk of TB and early mortality as they could benefit from increased monitoring and evaluation [1, 15]. Plasma biomarkers that measure host inflammation and immune status are now more readily available and cost effective and have been measured in patients with active TB and healthy controls with and without evidence of TB [17–20]. However, few biomarker studies have determined predictive associations and very few included severely immunosuppressed PLHIV who are at the highest risk of developing active TB or death [21–23]. Identifying such biomarkers may provide insight into the relevant biological pathways involved in the disease process and may suggest potential interventions to prevent incident TB and death. In the present study, we leveraged the multicountry trial AIDS Clinical Trials Group Study 5274, “Reducing Early Mortality and Morbidity by Empiric TB Treatment” (REMEMBER, NCT01380080) [6] to identify inflammatory biomarkers in highly immunosuppressed PLHIV residing in TB/HIV high-burden settings that predict incident TB and death as well as time to TB and death following the initiation of ART.
METHODS
Study Design
We conducted a case-cohort study among participants enrolled in REMEMBER, which was a multisite, international, unblinded randomized treatment strategy trial comparing empiric 4-drug TB treatment with isoniazid preventive therapy (IPT) for reducing death within 48 weeks after ART initiation in adults with HIV with CD4 cell counts <50 cells/µL [6].
Study Population
In brief, the parent REMEMBER trial participants were recruited from 18 outpatient research clinics in 10 countries (Malawi, South Africa, Haiti, Kenya, Zambia, India, Brazil, Zimbabwe, Peru, and Uganda). Individuals were screened for TB before inclusion using a symptom screen, locally available diagnostics, and the Xpert MTB/RIF assay when available. Study candidates with confirmed or suspected TB were excluded. Key inclusion criteria were liver function tests 2.5 times the upper limit of normal or less, a creatinine clearance of at least 30 mL/min, and a Karnofsky score of at least 30.
Our case-cohort comprised of (1) a randomly selected subcohort of 193 participants all of whom had available archived plasma specimens for determination of biomarker levels prior to (for baseline analyses) and at least 1 time-point after ART initiation but prior to development of our two outcomes of interest: TB or death (for longitudinal analyses) and (2) all additional TB and death cases (n = 64) in REMEMBER outside of the randomly selected subcohort in accordance to case-cohort design principles (Supplemental Figure 1) [24, 25]. In the parent trial in the 48 weeks postentry, there were a total of 57 TB events and 52 competing death events before TB could occur. We studied 52 (91%) of these 57 TB cases and 47 (90%) of the 52 death events. The cohort sampling was balanced by country and treatment arm.
Definitions
We defined incident TB cases as persons developing TB within 48 weeks of randomization (as adjudicated in REMEMBER) [6]. We defined death cases as persons who died within 48 weeks after randomization. All available clinical data collected on trial participants was included in the case-cohort study.
Laboratory Procedures
Plasma samples were thawed from storage at −80°C. Thawed samples were filtered using 0.65 µm Ultrafree-MC Centrifugal Filters (Millipore- UFC30DV00) followed by 0.22 µm Ultrafree-MC Centrifugal Filters (Millipore- UFC30GVNB), and filtered samples were aliquoted and frozen again for storage at −80°C until ready for use to minimize subsequent freeze-thaw cycles during analysis. The following analytes were quantified using MESO SCALE DISCOVERY (MSD) multiplexed immunoassay kits as per manufacturer’s recommendations (www.mesoscale.com): V-PLEX Proinflammatory Panel 1 Human Kit (K15049D; interferon γ [IFN-γ], interleukin [IL]-1β, IL-2, IL-6, IL-8, IL-10, IL-13, tumor necrosis factor α [TNF-α]), V-PLEX Cytokine Panel 1 Human Kit (K15050D; GM-CSF, IL-1α, IL-12/IL-23p40, IL-15, IL-16, IL-17A, TNF-β, VEGF-A), and V-PLEX Chemokine Panel 1 Human Kit (K15047D; Eotaxin (CCL-11), macrophage inflammatory protein [MIP]-1β, TARC (CCL-17), CXCL-10, MIP-1α, IL-8, monocyte chemoattractant protein [MCP]-1, myeloid dendritic cell [MDC], MCP-4). Plates precoated with capture antibodies as supplied by the manufacturer were washed, incubated with sample or calibrator at the kit-specified dilution factors for 2 hours at room temperature with shaking, washed again, incubated with detection antibody for 2 hours at room temperature with shaking, washed again, and incubated with kit-supplied Read Buffer prior to acquisition on the MESO QUICKPLEX SQ 120 machine. MSD Discovery Workbench 4.0 was used to acquire and compute concentration values. For soluble CD14 (sCD14) and IL-1 R1 analysis, R&D Systems Human CD14 DuoSet enzyme-linked immunosorbent assay (ELISA) (DY383) and Human IL-1 RI DuoSet ELISA (DY269) were used, respectively, as per manufacturer’s recommendations (www.rndsystems.com). SoftMax Pro 5.3 was used to acquire and compute concentration values.
Statistical Analysis
Descriptive statistics were performed to characterize the study populations. Several complementary approaches were used to identify which biomarkers were associated with TB and death outcomes: Cox regression, hierarchical clustering, discriminant canonical correlation analysis, and cross-validation modeling [26]. Analysis of the case-cohort data was modeled using Cox proportional hazards using the linear biomarker values and then separately using the proportion greater than the 3rd quartile. To account for the case-cohort design, we used a weighted Cox regression with Barlow method of weighting. Mean values of log10-transformed plasma concentrations of each biomarker for the population were z-score normalized and illustrated in a heatmap which grouped biomarkers using hierarchical clustering (Ward’s method with 100× bootstrap). Profiles were stratified by incident TB or death.
A multivariate discriminant analysis model using sparse canonical correlations was employed to test and quantify the accuracy of combined biomarkers to predict incident TB or death. For each marker, Spearman correlations corrected for multiple measurements (Holm-Bonferroni’s method) were calculated for each marker with either time to TB diagnosis or death.
Lastly, cross-validation was used to develop and validate a parsimonious combination of biomarkers that best predict incident TB. Excluding specimens from participants who had died, 235 samples were randomly stratified split into a 80% training set, and a 20% testing set that each has the same positive class ratio as the whole data set (0.22). The training set was log transformed, centered, and scaled for feature selection. In the feature/signature selection step, for each model in lasso logistic regression, xgboost and random forest, feature weights were calculated by applying a 5-fold cross validation in modeling training set with the target variable being TB or not (1/0) and then normalized between 0 and 1. Average weights from the three models were saved. Then a ridge logistic regression model using top features from the previous run was performed; the top proteins ranked were selected by leave-one-out cross-validation. We performed this procedure 100 times. One hundred sets of identified signatures were saved. From the 100 sets of signatures, the top N proteins (N = average signature length) were selected based on the appearing times. To build an incident TB prediction model, we did hyperparameter tuning using a random forest model with the training set containing only the 6-protein biomarkers we identified. The final prediction model was selected based on a 5-repeats, 5-fold cross validation with hyperparameter tuning. To evaluate the unbiased model performance and generalization ability, we then normalized the testing set, performed 50 rounds bootstrap sampling from the testing set, and predicted all 50 bootstrapped testing set using the final prediction model.
Ethics Statement and Role of the Funding Source
This study was approved by ethics committees and institutional review boards at Johns Hopkins University and participating site institutions. The sponsors of the study had no role in study design, data collection, analysis or interpretation, or in the writing of the paper.
RESULTS
Study Population
Of 850 participants enrolled in REMEMBER from October 31, 2011, to June 9, 2014, 257 were included in the case-cohort (n = 193 in randomly selected subcohort and n = 64 additional cases) with 52 TB (Supplemental Figure 1) and 47 death cases in total occurring within 48 weeks post-ART initiation. Of 47 participants who died [27], 13 also had antecedent incident TB. Twelve of 52 TB cases were microbiologically confirmed (10 pulmonary TB and 2 extrapulmonary TB) and the remaining met the independent endpoint committee review of clinical TB [6]. The majority (33/52; 63%) of TB cases occurred within 12 weeks post-ART initiation (Supplemental Figure 2). The case-cohort characteristics are shown in Table 1. There was no difference in the distribution of cases across countries by sub-cohort and additional cases (P-value = .20).
Table 1.
Baseline Characteristics of the Case Cohort
| Characteristics | Random Subcohorta n = 193 | TB | Death | ||||
|---|---|---|---|---|---|---|---|
| Subcohort Control n = 180 | TB Case n = 52 | P-value | Sub-cohort Control n = 180 | Death Case n = 47 | P-value | ||
| Age, median (IQR) | 36 (31–43) | 36 (31–43) | 36 (31–43) | .95 | 36 (31–43) | 41 (33–48) | .01 |
| Female, no. (%) | 96 (50%) | 92 (51%) | 24 (46%) | .64 | 92 (51%) | 21 (45%) | .51 |
| BMI < 18.5 kg/m2, no. (%) | 55 (29%) | 49 (27%) | 18 (35%) | .58 | 51 (28%) | 15 (32%) | .15 |
| Karnofsky score | |||||||
| 30–59 | 2 (1%) | 2 (1%) | 0 | .70 | 1 (1%) | 4 (9%) | .001 |
| 60–89 | 67 (35%) | 62 (35%) | 21 (41%) | 61 (34%) | 23 (49%) | ||
| ≥ 90 | 122 (64%) | 114 (64%) | 30 (59%) | 116 (65%) | 20 (43%) | ||
| WHO stage 3 or 4, no. (%) | 67 (34%) | 62 (34%) | 18 (35%) | > .95 | 61 (34%) | 21 (45%) | .18 |
| Absence of poor prognostic factor, no. (%)a | 130 (67%) | 123 (68%) | 33 (63%) | .52 | 123 (68%) | 27 (57%) | .17 |
| Absolute CD4+ T Cells, median (IQR) | 22 (10–37) | 22 (10–37) | 24 (15–36) | .48 | 22 (10–38) | 15 (7–24) | .01 |
| log10 Human Immunodeficiency Virus -1 RNA, median (IQR) | 5.36 (4.98–5.70) | 5.36 (4.96–5.69) | 5.44 (5.09–5.87) | .22 | 5.35 (4.945–5.69) | 5.43 (4.86–5.85) | .41 |
| Hemoglobin < 8 µg/dL, no. (%) | 6 (3%) | 6 (3%) | 3 (6%) | .42 | 5 (3%) | 7 (15%) | .004 |
| Albumin, median (IQR) | 4.8 (3.6–38) | 4.7 (3.6–38.3) | 27 (3.1–37) | .29 | 4.8 (3.6–38.3) | 4.1 (3.2–33.9) | .02 |
| Absolute neutrophil count, median (IQR) | 1.67 (1.08–2.30) | 1.61 (1.07–2.28) | 2.15 (1.43–3.64) | < .001 | 1.60 (1.07–2.27) | 2.12 (1.46–3.94) | < .001 |
| Creatinine, median (IQR) | 0.70 (0.60–0.86) | 0.7 (0.60–0.84) | 0.70 (0.60–0.80) | .54 | 0.71 (0.60–0.87) | 0.70 (0.58–1.0) | .67 |
| WBC (109 cells/L), median (IQR) | 3.07 (2.40–3.97) | 3.05 (2.35–3.90) | 3.50 (2.70–4.70) | .01 | 3.0 (2.4–3.9) | 3.6 (2.9–5.2) | .002 |
| Monocyte:lymphocyte ratio, median (IQR) | 0.43 (0.29–0.62) | 0.43 (0.29–0.62) | 0.49 (0.32–0.74) | .14 | 0.43 (0.29–0.62) | 0.42 (0.28–0.67) | > .95 |
| CD4 to CD8 ratio × 100, median (IQR) | 4.44 (2.15–8.11) | 4.32 (2.1–8.35) | 5.01 (2.86–6.94) | .31 | 4.42 (2.1–8.1) | 3.52 (1.83–6.41) | .15 |
Abbreviations: BMI, body mass index; IQR, interquartile range; N, number; TB, tuberculosis; WBC, white blood cell; WHO, World Health Organization.
aAbsence of poor prognostic factor defined as not having any of the following: hospitalization within past 30 days, BMI <18.5 or anemia <8 g/dl). Fischer exact p values were used to compare proportions and Wilcoxon rank sum tests to compare medians.
Baseline Plasma Biomarker Levels Distinguish PLHIV Who Develop TB After ART from Those Who Do Not
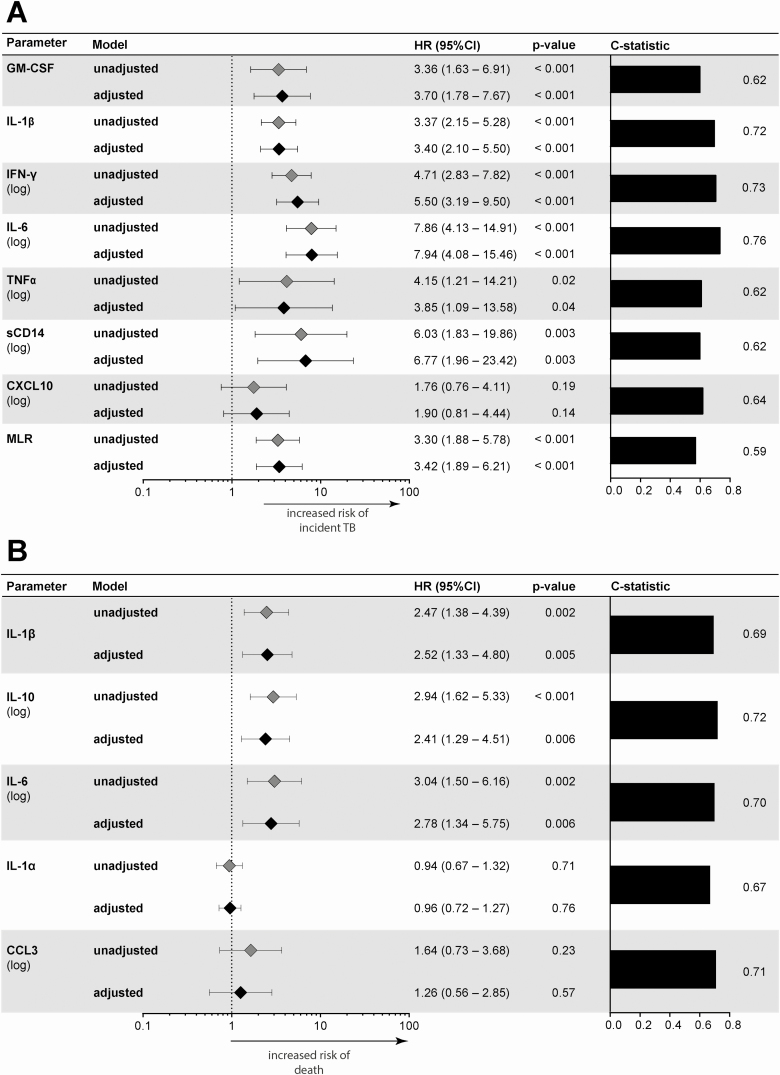
Unadjusted baseline plasma biomarker levels in TB cases, deaths, and controls are shown in Figure 1. Absolute values are shown in Supplemental Table 1. Adjusting for age, sex, and body mass index, biomarkers that were associated with incident TB (Supplemental Table 2) had partial overlap with those that predicted death (Supplemental Table 3). Notably adjusted hazard ratios of GM-CSF, IL-1β, IFN-γ, IL-6, TNF-α, sCD14, and the monocyte lymphocyte ratio (MLR) [28] were significantly increased in participants that developed TB, whereas increased IL-1β, IL-10, and IL-6 were significantly associated with death.
Figure 1.
Cox regression model for biomarkers. Association with (A) incident TB, adjusted model includes age, sex, BMI; (B) death, adjusted model includes age, sex, and BMI presented as a Forest plot. Panels on the right display the c-statistics values. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MLR, monocyte to lymphocyte ratio; TB, tuberculosis.
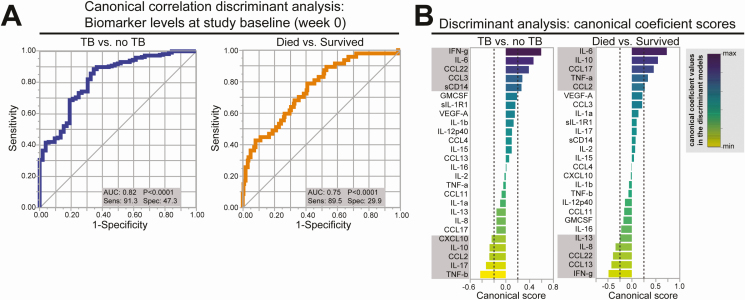
Using canonical discriminant models to further characterize the association of all 26 biomarkers combined with incident TB or death, the area under the ROC curve (AUC) was 0.82 (P < .0001) and AUC 0.75 (P < .0001), respectively (Figure 2A). By further plotting the canonical scores of each marker used in the discriminant model, we identified biomarkers (IFN-γ, IL-6,CCL-22,CCL3, sCD14, CXCL-10, IL-10, CCL2, IL-17, TNF-β) that were most influential in the ROC curve to predict subsequent TB overall (Figure 2B). For death, canonical scores identified IL-6, IL-10, CCL-17, TNF-α, CCL2, IL-13, IL-6, CCL22, CCL13, and IFN-γ as being the most influential in the model. When analyses were stratified by treatment arm, the biomarkers most influential in predicting incident TB were overlapping, although slightly different from the overall analysis (Supplemental Figure 3); both arms, however, had high AUCs (0.87 P < .0001). The AUCs for death when stratified by arm however were less robust.
Figure 2.
Canonical discriminant analysis of baseline biomarkers most influential for incident TB and death. A, ROC curve analysis of plasma levels of all biomarkers measured combined at study baseline (week 0) to distinguish TB vs. no TB or patients that died from those who survived. B, Canonical discriminant analyses of the biomarkers were performed independently for TB and death. Those above 0.2 and below −0.2 were considered most influential in the ROC curve analyses and are shown in the grey boxes. Abbreviations: AUC, area under the curve; min, minimum; max, maximum; ROC, receiver operator characteristics; sens, sensitivity; spec, specificity; TB, tuberculosis.
Using Cross-validation to Develop and Validate a Parsimonious Biomarker Signature to Predict Incident TB
Lastly, to determine whether a parsimonious biomarker signature could predict TB, we used a cross-validation approach. From the 100 sets of identified signatures, the top N proteins (N = average signature length) selected based on the appearing times were CXCL-10, IL-1β, IL-10, sCD14, TNF-α, and TNF-β, many of which were also identified in Cox regression, hierarchical clustering analyses described above. The final prediction model using this 6-protein biomarker signature measured at baseline with a predicted probability cut-off of 0.226, predicted incident TB with a high sensitivity of 0.90 (95% CI: .87–.94), a specificity of 0.71 (95% CI: .68–.75), and an AUC of 0.81 (95% CI: .78–.83).
Death Is Associated With a Persistent Inflammatory Signature
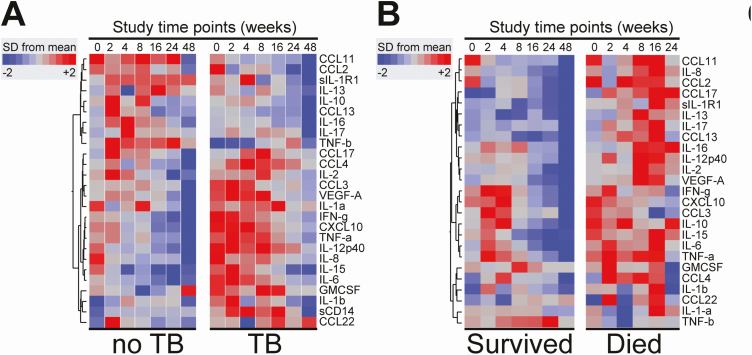
Using hierarchical clustering of z-score normalized, prospectively assessed plasma inflammatory biomarker values, the heatmap in Figure 3A shows overall trends in levels over time of follow-up; many inflammatory biomarkers are highly expressed at baseline in participants that went on to develop TB. Importantly, there is a more diffuse and persistent pattern of inflammatory biomarker expression in those that subsequently died compared to controls without TB and compared to those that developed TB (Figure 3B).
Figure 3.
Plasma biomarker levels measured serially in patients stratified by clinical endpoints. A–B, Mean values of log10-transformed concentration of each plasma marker per time point were calculated for the entire population and also per clinical outcomes. Biomarker values were z-score normalized and illustrated in a heatmap in which biomarkers were grouped using hierarchical clustering (Ward’s method with 100× bootstrap). Dendrograms represent Euclidean distance. Abbreviation: SD, standard deviation; TB, tuberculosis.
Highest Inflammatory Biomarker Levels With Shortest Time to Active TB \After ART
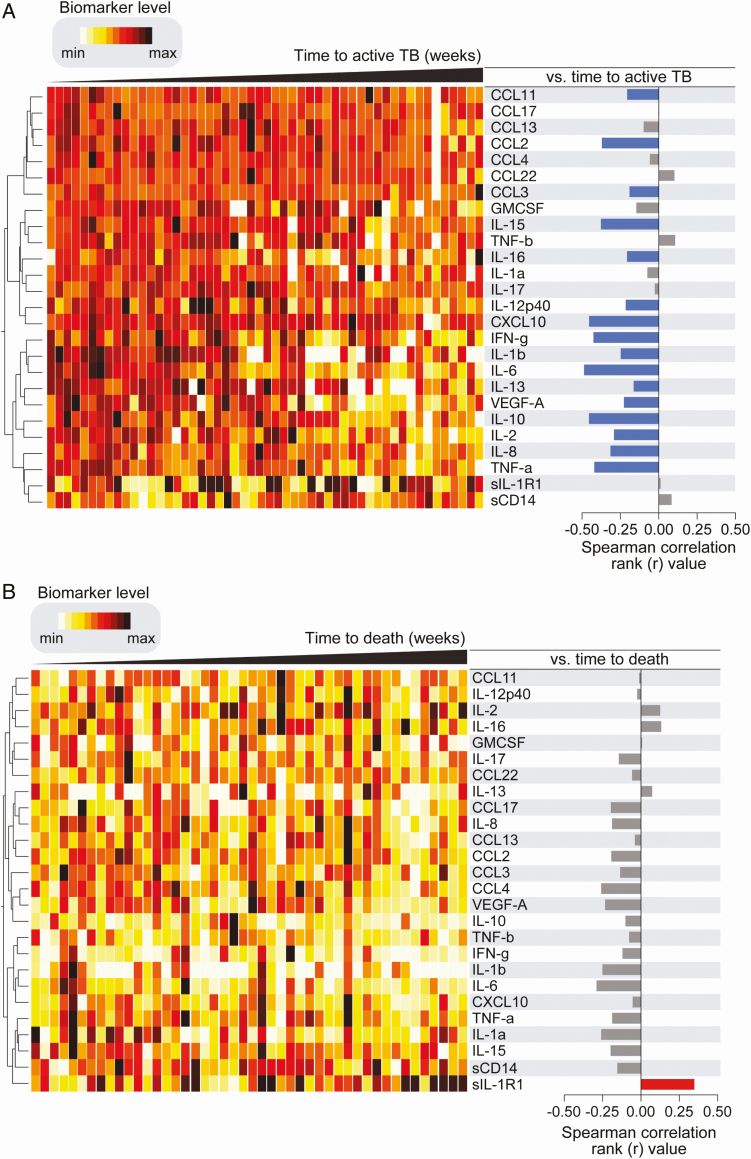
We further assessed correlations between plasma inflammatory biomarker levels and time to developing TB or death. The heatmap shows the highest inflammatory biomarker levels with shortest time to incident TB after ART (Figure 4A). Spearman correlation matrices revealed that increased levels of several plasma inflammatory biomarkers were significantly associated with rapid progression to TB suggesting that incipient TB has a more exaggerated inflammatory signature (Figure 4A, right-hand panel). Conversely, individuals that displayed lower baseline levels of such markers developed TB at later time points. A similar analysis failed to find a distinct biomarker profile associated with time to death (Figure 4B). Only the anti-inflammatory marker IL-1R1 correlated with time to death. Individuals that had higher baseline levels of IL-1R1 died at later time points compared to those with lower concentrations.
Figure 4.
A. Associations between baseline plasma inflammatory biomarkers and time to TB diagnosis. Left: Data were log-transformed and ranked and colored in a heatmap from minimum to maximum values detected for each marker. Patients were ordered based on time to TB diagnosis (in weeks) and plasma biomarkers were clustered (Ward’s method with 100× bootstrap) according to the distribution profile in the study population. Dendrograms represent Euclidean distance. Right: Spearman correlations for each marker and time to TB diagnosis. Blue bars indicate statistically significant correlations after corrections for multiple measurements (Holm-Bonferroni’s method). Abbreviation: TB, tuberculosis. B. Associations between baseline plasma inflammatory biomarkers and time to death. Left: Data were log-transformed and ranked and colored in a heatmap from minimum to maximum values detected for each marker. Patients were ordered based on time to death (in weeks) and plasma biomarkers were clustered (Ward’s method with 100× bootstrap) according to the distribution profile in the study population. Dendrograms represent Euclidean distance. Right: Spearman correlations or each marker and time to death. Only soluble IL1-R1 remained statistically significant after corrections for multiple measurements (Holm-Bonferroni’s method). Only participants with valid values for all markers were included (n = 46). Abbreviation: IL1-R1, interleukin 1 receptor, type 1 min, minimum; max, maximum.
DISCUSSION
In a large, geographically diverse cohort of severely immunocompromised PLHIV starting ART, we found that several baseline inflammatory biomarkers were independently associated with incident TB after ART initiation using Cox models. To test the robustness of the key inflammatory biomarkers, we used a canonical discriminant analysis and confirmed that INF-γ, IL-6, sCD14, and IL-1β were most influential to predict TB in both models. Finally, a plasma inflammatory 6-biomarker signature was developed and validated and could be developed into a non-sputum-based stratification tool with a sensitivity of 0.90 (AUC of 0.81) at the modeled cutoff.
The REMEMBER trial had 2 arms; in one arm, participants received empiric 4 drug anti-TB treatment, and in the other arm, participants received 6 months of IPT. It is particularly striking that the host biomarkers upregulated at baseline in the hierarchical cluster analysis in participants that were treated with empiric TB treatment and developed TB is very similar to the cytokines that predict a shorter time to active TB. This cytokine signature may represent co-prevalent TB or could be termed “incipient” TB that could not be microbiologically confirmed at baseline given the severe immunosuppression of the participants in this cohort. Previously published expression profiling of non-HIV patients [29] as well as PLHIV [30] who develop TB corroborate a similar inflammatory profile in patients as they approach clinically recognizable TB.
Our data contribute and expand that of our previous studies showing the importance of IFNγ and CXCL10 (IP-10) in robustly classifying active TB in patients with advanced HIV starting ART [22, 31]. In a cohort of HIV-positive, less immunosuppressed patients that were starting isoniazid prophylactic therapy, Lesosky et al compared plasma biomarkers from those with incident TB to those with either prevalent TB or no TB. They found several blood TB biomarkers that overlap with our study, including CXCL10 and IFNγ [21]. Two other studies in PLHIV also identified CXCL10 as predictive of incident TB [31, 32]. Baseline IL-6 predicted both TB and death in our analysis and corroborates findings in other studies [33–38]. IL-1β also independently predicted death, whereas TNF-α was important in the canonical discriminant analysis. Taken together, these data suggest that these plasma inflammatory biomarkers are part of a continuum where some biomarkers (INF-γ, sCD14, CXCL-10, GMCSF) may classify those with incipient TB at high risk for unmasking with ART despite specific therapy, whereas other biomarkers (IL-6, IL-1β, and TNF-α) represent a pathway of immune activation and dysregulation that are shared between those who develop TB and/or death or uniquely associated with death (IL-10), as well as persistent inflammation over follow-up time in those who died. These inflammatory biomarkers reflect the substantial innate immune activation and increase in Th1 responses that is associated with the immune response to Mycobacterium tuberculosis [39]. Interestingly, the inflammatory biomarkers overlap with that in TB-IRIS shown in transcriptomic profiling, which showed overexpression of innate immune mediators and activators of the inflammasome [40].
Despite expanding access to ART to all PLHIV regardless of CD4 T-cell count, late presentation with advanced HIV persists in settings where TB coinfection is high [1, 10]. The REMEMBER trial showed that despite rigorous baseline TB screening, ART initiation, and preventive/preemptive therapy (either IPT or empiric 4 drug TB therapy), participants remained at risk for incident TB and for death in the ensuing 48 weeks.
Our study had several limitations. We assayed only 26 biomarkers. In addition, the trial into which our study was nested had 2 treatment arms, isoniazid, which, although recommended, has variable coverage across sub-Saharan Africa [1] and 4 drug anti-TB therapy. We analyzed the data according to arm and found that the results were robust across arms and showed that there may be a group of patients in whom even provision of empiric therapy will not avert TB disease, as has been shown in other studies of empiric TB treatment [41, 42]. Further work examining this inflammatory biomarker signature prospectively across a range of CD4 values would also be important as we studied a population with very advanced HIV (CD4 < 50), who, although at very high risk of TB, now account for <25% of ART initiators [8]. Examining cell surface markers of immune activation on PBMC and CD4 T cells specifically in addition to measuring type I interferon responses [43] and circulating immune complexes (CIC) will be an important next step in placing these results in context; previous data have shown increased type I interferon signaling and complement pathway gene activation, which bind CIC in patients with subclinical and early active TB disease [43]. Although the biomarkers of immune activation seem overlapping with those described in HIV/AIDS, particularly considering the profound immunosuppression in this cohort, our analysis shows in this selected group that there are differences in the profiles between those with incipient or unmasked incident TB (IFN-γ and CXCL-10 induced by IFN-γ) compared to those who die (IL-6 and TNF-α). If the sample were larger, it would have been interesting to compare those who die early (in the first 3 months) compared to those who died later.
In summary, our data indicate that a baseline inflammatory biomarker signature identifies PLHIV that fail to control the infection. These data also provide a “window” into immunologic pathways along the spectrum of clinical active TB disease to clinically recognizable active disease despite anti-TB treatment. Our predictive 6-biomarker signature could be used to identify those at highest risk of TB after ART initiation and who may benefit from additional monitoring, intensified or immune modulatory treatment [5, 7]. Further work to validate our inflammatory biomarker signature in stratifying PLHIV initiating ART is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Ritesh Ramchandani and Sachiko Miyahara at the Harvard Center for Biostatistics in AIDS Research for their help in assembling the database from the main study. They acknowledge Maria Arriaga Gutierrez and Dr. Kiyoshi F. Fukutani (Fundação Oswaldo Cruz) for help with statistical analysis and data visualization, and Tiffany Long, Rebecca LeBlanc, Evelyn Hogg who helped organize contributing sites and sample shipping. The AIDS Clinical Trials Group, the Tuberculosis Transformative Science Group for helpful suggestions at project inception, and Gavin Churchyard for thoughtful review of the manuscript. They also thank the participants and the ACTG trial sites and the co-investigators that participated in A5274 REMEMBER.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Research reported in this publication was supported by the AIDS Clinical Trials Group, which is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. The work was also supported by The Johns Hopkins Baltimore-Washington-India Clinical Trials Unit (BWI CTU) (NIH/NIAID UM1AI069465 to A. G., N. G., and Y. C. M.). Additional salary support provided for Y. C. M. from the Fogarty International Center at the NIH (D43TW009771). The work of B. B. A. was supported by the intramural research program from FIOCRUZ and from the NIH (U01AI115940).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: The 49th Union World Conference on Lung Health, The Hague, The Netherlands from 24 to 27 October 2018. Abstract number OA09-263-25.
References
- 1. WHO. Global Tuberculosis report 2018. World Health Organization, 2018. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 8 December 2019. [Google Scholar]
- 2. Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis 2009; 49:965–72. [DOI] [PubMed] [Google Scholar]
- 3. Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS 2007; 21:713–9. [DOI] [PubMed] [Google Scholar]
- 4. Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badje A, Moh R, Gabillard D, et al. ; Temprano ANRS 12136 Study Group Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5:e1080–9. [DOI] [PubMed] [Google Scholar]
- 6. Hosseinipour MC, Bisson GP, Miyahara S, et al. ; Adult AIDS Clinical Trials Group A5274 (REMEMBER) Study Team Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet 2016; 387:1198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007; 21:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4+ cell count at presentation to HIV care, 1992–2011. Clin Infect Dis 2013; 57:1027–37. [DOI] [PubMed] [Google Scholar]
- 10. Zaniewski E, Ostinelli CHD, Maxwell N, et al. Trends in CD4 and viral load testing in Southern Africa: analysis of 6 countries. Seattle, WA: CROI, 2019. [Google Scholar]
- 11. Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008; 22:1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 2011; 6:e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braitstein P, Brinkhof MW, Dabis F, et al. ; Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration; ART Cohort Collaboration (ART-CC) groups Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 2006; 367:817–24. [DOI] [PubMed] [Google Scholar]
- 14. IeDea, Collaborations ARTC, Avila D, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2014; 65:e8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis 2009; 199:437–44. [DOI] [PubMed] [Google Scholar]
- 16. Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis 2011; 204(Suppl 4):S1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobsen M, Repsilber D, Gutschmidt A, et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med (Berl) 2007; 85:613–21. [DOI] [PubMed] [Google Scholar]
- 18. Pokkali S, Das SD, R L. Expression of CXC and CC type of chemokines and its receptors in tuberculous and non-tuberculous effusions. Cytokine 2008; 41:307–14. [DOI] [PubMed] [Google Scholar]
- 19. Dheda K, Van-Zyl Smit RN, Sechi LA, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One 2009; 4:e4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chegou NN, Sutherland JS, Malherbe S, et al. ; AE-TBC consortium Diagnostic performance of a seven-marker serum protein biosignature for the diagnosis of active TB disease in African primary healthcare clinic attendees with signs and symptoms suggestive of TB. Thorax 2016; 71:785–94. [DOI] [PubMed] [Google Scholar]
- 21. Lesosky M, Rangaka MX, Pienaar C, et al. Plasma biomarkers to detect prevalent, or predict progressive, HIV-1-associated tuberculosis. Clin Infect Dis 2019; 69:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verma S, Du P, Nakanjako D, et al. “Tuberculosis in advanced HIV infection is associated with increased expression of IFNγ and its downstream targets”. BMC Infect Dis 2018; 18:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis 2015; 15:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999; 52:1165–72. [DOI] [PubMed] [Google Scholar]
- 25. Onland-Moret NC, van der A DL, van der Schouw YT, et al. Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol 2007; 60:350–5. [DOI] [PubMed] [Google Scholar]
- 26. Schutz C, Barr D, Andrade BB, et al. Clinical, microbiologic, and immunologic determinants of mortality in hospitalized patients with HIV-associated tuberculosis: a prospective cohort study. PLoS Med 2019; 16:e1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bisson GP, Ramchandani R, Miyahara S, et al. ; Adult AIDS Clinical Trials Group A5274 (REMEMBER) Study Team Risk factors for early mortality on antiretroviral therapy in advanced HIV-infected adults. AIDS 2017; 31:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naranbhai V, Hill AV, Abdool Karim SS, et al. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J Infect Dis 2014; 209:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scriba TJ, Penn-Nicholson A, Shankar S, et al. ; other members of the ACS cohort study team Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog 2017; 13:e1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darboe F, Mbandi SK, Naidoo K, et al. ; SATVI Clinical Immunology Team Detection of tuberculosis recurrence, diagnosis and treatment response by a blood transcriptomic risk signature in HIV-infected persons on antiretroviral therapy. Front Microbiol 2019; 10:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shivakoti R, Gupte N, Tripathy S, et al. ; NWCS 319 and PEARLS Study Team Inflammation and micronutrient biomarkers predict clinical HIV treatment failure and incident active TB in HIV-infected adults: a case-control study. BMC Med 2018; 16:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tenforde MW, Gupte N, Dowdy DW, et al. ; ACTG PEARLS and NWCS 319 Study Group C-reactive protein (CRP), interferon gamma-inducible protein 10 (IP-10), and lipopolysaccharide (LPS) are associated with risk of tuberculosis after initiation of antiretroviral therapy in resource-limited settings. PLoS One 2015; 10:e0117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boulware DR, Hullsiek KH, Puronen CE, et al. ; INSIGHT Study Group Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodger AJ, Fox Z, Lundgren JD, et al. ; INSIGHT Strategies for Management of Antiretroviral Therapy (SMART) Study Group Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis 2009; 200:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ledwaba L, Tavel JA, Khabo P, et al. ; Project Phidisa Biomarkers Team Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One 2012; 7:e24243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Janssen S, Schutz C, Ward A, et al. Mortality in severe human immunodeficiency virus-tuberculosis associates with innate immune activation and dysfunction of monocytes. Clin Infect Dis 2017; 65:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siedner MJ, Bwana MB, Asiimwe S, et al. ; META Study Investigators Inflammatory biomarkers prior to antiretroviral therapy as prognostic markers of 12-month mortality in South Africa and Uganda. AIDS 2019; 33:2043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roy Chowdhury R, Vallania F, Yang Q, et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature 2018; 560:644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai RPJ, Meintjes G, Wilkinson KA, et al. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun 2015; 6:8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manabe YC, Worodria W, van Leth F, et al. Prevention of early mortality by presumptive tuberculosis therapy study: an open label, randomized controlled trial. Am J Trop Med Hyg 2016; 95:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blanc FX, Badje AD, Bonnet M, et al. Systematic vs test-guided tuberculosis treatment: data of the STATIS randomized trial. In: Conference on Retroviruses and Opportunistic Infections Boston. Massachusetts, USA, 2018.
- 43. Esmail H, Riou C, Bruyn ED, et al. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annu Rev Immunol 2018; 36:603–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.