Abstract
Background
Direct oral anticoagulants (DOACs) have become first-line treatment for venous thrombotic events. DOAC prescribing trends among people living with human immunodeficiency virus (PWH) are not well described. The coadministration of DOACs with the antiretroviral (ARV) pharmacokinetic boosters ritonavir (RTV) or cobicistat (COBI) may be complicated by pharmacokinetic interactions.
Methods
A longitudinal cohort study was conducted using the D.C. Cohort Database in Washington, D.C., from January 2011 to March 2017, to describe oral anticoagulant prescribing among PWH ≥ 18 years old and the prevalence of DOAC use with RTV or COBI. Data collection included demographic and clinical characteristics, ARV and anticoagulant prescriptions, and International Classification of Diseases Ninth and Tenth Edition diagnosis codes.
Results
Among 8315 PWH, there were 236 anticoagulant prescriptions (96 DOAC, 140 warfarin) for 206 persons. PWH prescribed anticoagulants were predominantly Black (82%) and male (82%), with a mean age at anticoagulant initiation of 56 years. DOAC use increased from 3% of total anticoagulant prescribing in 2011 to 43% in 2016, accounting for 64% of all newly recorded anticoagulant prescriptions by 2016. There were 19 bleeding events recorded among 16 individuals. Despite the Food and Drug Administration label recommendation to avoid rivaroxaban with boosted ARVs, 41% remained on boosted ARVs after rivaroxaban initiation.
Conclusions
DOAC use increased substantially in PWH by 2016. Although rivaroxaban is not recommended with RTV or COBI, concomitant use was recorded in 41% of rivaroxaban recipients in this cohort. As DOAC usage increases, clinicians need to be aware of potential DOAC/ARV interactions in order to select the most appropriate oral anticoagulant and monitoring plan for PWH.
Keywords: antiretrovirals, anticoagulation, HIV, cobicistat, ritonavir
Direct oral anticoagulant (DOAC) prescribing has increased substantially in people living with human immunodeficiency virus (PWH). Clinicians need to be aware of potential DOAC and antiretroviral interactions to select the most appropriate oral anticoagulant and monitoring plan for PWH.
Warfarin was the only commercially available oral anticoagulant until 2010. However, warfarin therapy is complicated by drug interactions, dietary limitations, a narrow margin of safety and efficacy, and the need for frequent monitoring of the international normalized ratio and dosage adjustments. Since 2010, the United States Food and Drug Administration (FDA) has approved 5 direct-acting oral anticoagulants (DOACs): dabigatran, rivaroxaban, apixaban, edoxaban, and betrixaban. The 2016 American College of Chest Physicians guidelines recommend DOACs as first-line agents over warfarin for the treatment of deep venous thrombosis and pulmonary embolism [1]. For atrial fibrillation, excluding patients with prosthetic heart valves, both warfarin and DOACs have Class I recommendations by the 2014 American Heart Association guidelines [2]. By the end of 2016, DOAC usage accounted for 42.9% of the oral anticoagulant US market share, based on filled prescriptions [3].
The benefits of anticoagulation therapy must be weighed against the risk of serious adverse events and the optimal management of drug-drug interactions. Risk stratification tools, such as CHA2DS2-VASc (congestive heart failure, hypertension, age [ > 65 = 1 point, > 75 = 2 points], diabetes, previous stroke/transient ischemic attack (2 points)- vascular disease) and HAS-BLED (hypertension, abnornal renal and liver function, stroke, bleeding tendency/disposition, elderly, drugs or alcohol) scores, may not be reliable in people living with human immunodeficiency virus (HIV; PWH) [4]. According to the Institute for Safe Medication Practices, data gathered from the FDA Adverse Event Reporting System in 2016 reported that the largest percentage of serious adverse events in the United States, primarily bleeding events, were attributed to the use of oral anticoagulants [3]. Oral anticoagulants accounted for 18 978 serious injuries and 3018 deaths. The drugs implicated in these events were rivaroxaban (n = 15 043; 68.4%), apixaban (n = 3148; 14.3%), dabigatran (n = 1944; 8.8%), warfarin (n = 1753; 8%), and edoxaban (n = 108; less than 1%).
Several studies demonstrate that PWH are more likely to develop venous thromboembolism (VTE) at rates of up to 10 times those of individuals living without HIV [5–9]. Treatment of thromboembolism in PWH can be challenging due to significant drug-drug interaction potential with oral anticoagulants and antiretrovirals (ARVs). Whereas warfarin can be readily monitored for efficacy and toxicity based on well-defined international normalized ratio ranges, there are several challenges to the use of DOACs among PWH on antiretroviral therapy (ART). ARVs that are strong cytochrome P450 (CYP3A4) and p-glycoprotein (p-gp) inhibitors, namely protease inhibitors and pharmacokinetic boosters (ritonavir and cobicistat), are either not recommended for coadministration (rivaroxaban) or may require DOAC dosage reduction [10–15]. Additional practice challenges with DOACs include the lack of well-established surrogate markers for monitoring efficacy and toxicity, as well as complex dosing regimens that vary based on clinical indications and patient characteristics, such as age or renal function.
This analysis aimed to assess trends in oral anticoagulant prescribing within an observational cohort of PWH receiving care in Washington, D.C., between 2011 and 2017. In this report, we describe the type, frequency, and indication of their usage and assess the prevalence of potential drug interactions between oral anticoagulants and antiretrovirals.
METHODS
This analysis used data collected from an ongoing clinic-based, cohort study of PWH in Washington, D.C. (D.C. Cohort), that began enrolling participants in January 2011. The overall objectives of the cohort are to characterize HIV outcomes in the District of Columbia and to improve the quality of care for PWH. This analysis includes data from 11 participating outpatient sites: 7 are hospital-based clinics and 4 are community-based clinics. During the time frame for the current analysis, 3 additional clinics contributed data to the D.C. Cohort; however, no participants at those sites had a record of being prescribed oral anticoagulants and, thus, were excluded from this analysis. All participants provided written informed consent for inclusion in the cohort, which is approved by the Institutional Review Boards of George Washington University and each site’s own Institutional Review Board (when applicable). Details of the D.C. Cohort Study design have been previously described [16].
Participants’ clinical data, including body mass index (BMI), laboratory results (renal function, CD4 cell count, HIV-1 RNA), and International Classification of Diseases Ninth and Tenth Edition (ICD-9 and ICD-10) diagnoses, are manually entered or electronically exported from patient medical records and entered into a web-based data entry system, Discovere (Cerner Corporation, Kansas City, MO). Prescription information is exported directly from electronic prescribing orders. Additional data available include demographic information and pertinent medical history, including comorbidities, HIV history, potential indication for anticoagulation, concomitant medications, and bleeding events. Bleeds were categorized as major or minor. Major bleeds were defined as those requiring hospitalization and/or a medical intervention (reversal agent, blood transfusion). All other bleeds were considered minor. Comorbidities, anticoagulant indications, and bleeding events were defined using ICD-9 and ICD-10 standards or the Healthcare Cost and Utilization Project Clinical Classifications Software (CCS; Supplementary Tables 1–3). CCS codes were used to provide a method for classifying ICD-9/10 diagnoses into clinically meaningful categories [17].
Study Population and Design
All participants who were prescribed oral anticoagulants (warfarin or DOACs) and enrolled in the D.C. Cohort from 1 January 2011 to 31 March 2017 were included. Yearly prescribing trends are reported through 2016. Each individual course of a medication was considered a unique event. Participant variables, including sex, race, age, and social and HIV histories, were recorded in the database. Bleeding events were defined as a new CCS or ICD-9/10 code following the initiation of warfarin or a DOAC (Supplementary Table 4). Reported events were further assessed via manual chart reviews to confirm participant characteristics and anticoagulant-ARV details. The severity of bleeding events, concomitant medications, and anticoagulant dosing were also evaluated.
Statistical Analysis
Descriptive statistics were used to describe the baseline characteristics of the participants by anticoagulant agent. Baseline participant characteristics at the time of initial anticoagulant prescription were reported as frequencies (%) for categorical data and means (standard deviations) for continuous data, and compared between those on DOACs versus warfarin using Fisher Chi-square and Student’s t tests, respectively. Additional comparisons were made for anticoagulant prescriptions (DOAC versus warfarin) for comorbidities occurring following an anticoagulant prescription and were reported as frequencies (%) for the presence of the diagnosis. The absence of a diagnosis was recorded as no diagnosis present. Prescription trends were described as overall trends and newly recorded trends. Overall trends were defined as the number of warfarin or DOAC prescriptions over the total number of prescriptions (warfarin and DOACs) recorded in the D.C. Cohort in a given year. Trends in newly recorded prescriptions were defined as the number of warfarin or DOAC prescriptions recorded for the first time over the total number of newly recorded prescriptions (warfarin and DOACs) in the D.C. Cohort in a given year. Differences in the proportions of warfarin and total DOAC prescriptions by year were analyzed using a Fisher Chi-square test. A Poisson regression analysis was stratified by anticoagulant to assess whether there was a significant increase in the total count of either DOAC or warfarin over time. All analyses were performed using the Statistical Analysis System (SAS) statistical software package, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Demographics and Baseline Characteristics: Overall Cohort and Participants on Anticoagulation Therapy
A total of 8315 PWH were enrolled in the D.C. Cohort through the study period. There were 236 oral anticoagulant prescriptions identified in 206 persons; 116 (56.3%) persons were prescribed warfarin and 90 (43.6%) were prescribed DOACs. Demographics and baseline characteristics for participants who were prescribed anticoagulants are summarized in Table 1. The only marginally significant difference in a characteristic between participants receiving DOACs versus warfarin was body mass index at anticoagulant start date (DOACs, 26.9 ± 6.6; warfarin, 29.4 ± 9.6; P = .03). There were no additional statistically significant differences in demographic characteristics (P > .05). Participants who received anticoagulation were predominantly male (81.5%) and Black (82.5%). The average age of participants at the time of anticoagulant initiation was 56 ± 10.9 years. Approximately 30% were below the age of 50. Histories of smoking and recreational drug use were documented in 65.5% and 43.7% of participants, respectively.
Table 1.
Demographics and Baseline Characteristics of Study Population
| Dabigatran, n = 11 | Rivaroxaban, n = 63 | Apixaban, n = 16 | Total DOAC, n = 90 | Warfarin, n = 116 | Overall, n = 206 | P Value | |
|---|---|---|---|---|---|---|---|
| Male (%) | 10 (90.9) | 52 (82.5) | 14 (87.5) | 76 (84.4) | 92 (79.3) | 168 (81.5) | .3460 |
| Age at anticoagulant initiation, years, mean ± SD | 55.4 ± 8.7 | 58.7 ± 11.1 | 55.6 ± 9.6 | 57.7 ± 10.6 | 55 ± 11.1 | 56 ± 10.9 | .0989 |
| Age range at cohort enrollment | … | … | … | … | … | … | .2357 |
| <40 years (%) | 0 (0) | 6 (9.5) | 1 (6.3) | 7 (7.8) | 15 (12.9) | 22 (10.7) | |
| 40–49 years (%) | 5 (45.5) | 9 (14.3) | 5 (31.3) | 19 (21.1) | 20 (17.2) | 39 (18.9) | |
| 50–59 years (%) | 3 (27.3) | 21 (33.3) | 6 (37.5) | 30 (33.3) | 50 (43.1) | 80 (38.8) | |
| 60–69 years (%) | 3 (27.3) | 22 (34.9) | 4 (25.0) | 29 (32.2) | 24 (20.7) | 53 (25.7) | |
| ≥70 years (%) | 0 (0) | 5 (7.9) | 0 (0) | 5 (5.6) | 7 (6.0) | 12 (5.8) | |
| Race/ethnicity | … | … | … | … | … | … | .6761 |
| NH Black, n (%) | 7 (63.6) | 51 (81.0) | 13 (81.3) | 71 (78.9) | 99 (85.3) | 170 (82.5) | |
| NH White, n (%) | 3 (27.3) | 9 (14.3) | 0 (0) | 12 (13.3) | 11 (9.5) | 23 (11.2) | |
| Hispanic, n (%) | 0 (0) | 0 (0) | 2 (12.5) | 2 (2.2) | 2 (1.7) | 4 (1.9) | |
| Other/Unknown, n (%) | 1 (9.09) | 3 (4.8) | 1 (6.3) | 5 (5.6) | 4 (3.4) | 9 (4.4) | |
| Weight, kg, mean ± SD; n | 90.6 ± 17.8; 11 | 81.9 ± 24.5; 63 | 87.1 ± 19.4; 16 | 83.9 ± 23.0 | 89.8 ± 29.6; 113 | 87.2 ± 27.0 | .1110 |
| BMI, kg/m2, mean ± SD; n | 27.6 ± 5.2; 11 | 26.3 ± 7.2; 63 | 28.7 ± 4.7; 16 | 26.9 ± 6.6 | 29.4 ± 9.6; 112 | 28.2 ± 8.5 | .0304 |
| Substance use history at enrollment,a n (%) | |||||||
| Alcohol use | 1 (9.1) | 23 (36.5) | 5 (31.3) | 29 (32.2) | 39 (33.6) | 68 (33.0) | .1385 |
| Smoking history | 6 (54.6) | 42 (66.7) | 10 (62.5) | 58 (64.4) | 77 (66.4) | 135 (65.5) | .5663 |
| Recreational and other drug useb | 5 (45.5) | 26 (41.3) | 8 (50.0) | 39 (43.3) | 51 (44.0) | 90 (43.7) | .1550 |
| History of intravenous drug use | 2 (18.2) | 10 (15.9) | 3 (18.8) | 15 (16.7) | 15 (12.9) | 30 (14.6) | .3071 |
| Any history of bleeding event prior to anticoagulant initiation | 0 (0) | 3 (4.8) | 3 (18.8) | 6 (6.3) | 7 (6.1) | 13 (6.4) | .8622 |
Abbreviations: BMI, body mass index; DOAC, direct oral anticoagulant; IQR, interquartile range; NH, non-Hispanic; SD, standard deviation.
aDefined as a history of use (previous or current) by research assistants at enrollment.
bVariable is a summary measure of several drug use questions captured by research assistants at baseline, including any cocaine, heroin, marijuana, crystal meth, LSD, intravenous drugs, and other recreational drug use.
In 53% of participants, there was a history of an AIDS-defining condition (Table 2). The average number of years from an HIV diagnosis to anticoagulant initiation was 15 ± 8 years. At the time of anticoagulant initiation, 98.1% of participants were receiving ART, 78.2% had CD4 counts >200 cells/mm3, and 78.6% had an HIV viral load <200 copies/mL. Raltegravir was the most commonly prescribed integrase strand transfer inhibitor (65%) and boosted darunavir was the most commonly prescribed protease inhibitor (52%). Ritonavir and cobicistat boosted regimens were prescribed in 47% and 11% of participants, respectively.
Table 2.
Human Immunodeficiency Virus History of Study Population
| Dabigatran, n = 11 | Rivaroxaban, n = 63 | Apixaban, n = 16 | Total DOAC, n = 90 | Warfarin, n = 116 | Overall, n = 206 | P Value | |
|---|---|---|---|---|---|---|---|
| Prescribed ART at time of anticoagulant initiation, n (%) | 11 (100) | 62 (98.4) | 16 (100) | 89 (98.9) | 113 (97.4) | 202 (98.1) | .1609 |
| Known years from HIV diagnosis to anticoagulant initiation, mean +/− SD | 18.0 ± 10.3 | 15.5 ± 8.9 | 18.5 ± 6.2 | 16 ± 9 | 15.6 ± 8.1 | 15 ± 8 | .5168 |
| Most recent measured CD4 prior to anticoagulant initiation, cells/mm3, n (%) | .4616 | ||||||
| CD4 <200 cells/mm3 | 1 (9.1) | 8 (12.7) | 3 (18.8) | 12 (13.3) | 13 (11.2) | 25 (12.1) | |
| CD4 200–500 cells/mm3 | 3 (27.3) | 23 (36.5) | 3 (18.8) | 29 (32.2) | 50 (43.1) | 79 (38.4) | |
| CD4 >500 cells/mm3 | 5 (45.5) | 28 (44.4) | 6 (37.5) | 39 (43.3) | 43 (37.1) | 82 (39.8) | |
| Unknown | 2 (18.2) | 4 (6.4) | 4 (25.0) | 10 (11.1) | 10 (8.6) | 20 (9.7) | |
| Most recent VL prior to anticoagulant initiation, copies/ml, n (%) | .4163 | ||||||
| <200 copies/mL | 8 (72.7) | 47 (74.6) | 13 (81.3) | 68 (75.6) | 94 (81.0) | 162 (78.6) | |
| ≥200 copies/mL | 1 (9.1) | 9 (14.3) | 1 (6.3) | 11 (12.2) | 14 (12.1) | 25 (12.1) | |
| Unknown | 2 (18.2) | 7 (11.1) | 2 (12.5) | 11 (12.2) | 8 (6.9) | 19 (9.2) | |
| AIDS defining diagnoses at first anticoagulant,a n (%) | 11 (5.3) | 29 (46.0) | 10 (62.5) | 43 (47.8) | 68 (58.6) | 111 (53.9) | .1215 |
Abbreviations: ART, antiretroviral therapy; DOAC, direct oral anticoagulant; HIV, human immunodeficiency virus; SD, standard deviation; VL, viral load.
aParticipants with an AIDs defining diagnosis at anticoagulant start date, but only shows the first reported AIDS defining diagnosis.
The most commonly documented comorbidities were hypertension (49.0%), chronic kidney disease (37.0%), and diabetes (25.0%), and 23.0% of participants had a documented history of malignancy. The most commonly documented indications for anticoagulation included nonpulmonary VTE (25.8%), pulmonary embolism (19.9%), and atrial fibrillation (11.0%; Table 3).
Table 3.
Reported Comorbidities and Indications for Anticoagulant Use
| Dabigatran, n = 14 | Rivaroxaban, n = 64 | Apixaban, n = 18 | Total DOAC, n = 96 | Warfarin, n = 140 | Overall, n = 236 | P Value | |
|---|---|---|---|---|---|---|---|
| Comorbidities, n (%) | |||||||
| Hepatic | |||||||
| Hepatitis B | 2 (14.3) | 5 (7.8) | 2 (11.1) | 9 (9.4) | 6 (4.3) | 15 (6.4) | .1727 |
| Hepatitis C | 5 (35.7) | 10 (15.6) | 6 (33.3) | 21 (21.9) | 25 (17.8) | 46 (19.5) | .5044 |
| Hepatitis, other or unspecified | 3 (21.4) | 13 (20.3) | 5 (27.8) | 21 (21.9) | 30 (21.4) | 51 (21.6) | 1.0000 |
| Hepatic impairment or liver disease | 2 (14.3) | 8 (12.5) | 3 (16.7) | 13 (13.5) | 19 (13.5) | 32 (13.6) | 1.0000 |
| Cardiovascular | |||||||
| Hypertension | 8 (57.1) | 29 (45.3) | 13 (72.2) | 50 (52.1) | 67 (46.9) | 117 (49.0) | .5963 |
| Cardiac dysrhythmias | 5 (35.7) | 14 (21.9) | 2 (11.1) | 21 (21.9) | 23 (16.4) | 44 (18.6) | .3109 |
| Coronary atherosclerosis/ other heart disease | 1 (7.1) | 5 (7.8) | 2 (11.1) | 8 (8.3) | 13 (9.3) | 21 (8.9) | 1.0000 |
| Heart valve disorders | 0 (0) | 5 (7.8) | 1 (5.6) | 6 (6.3) | 12 (8.6) | 18 (7.6) | .6213 |
| Congestive heart failure, non-hypertensive | 0 (0) | 9 (14.1) | 0 (0) | 9 (9.4) | 20 (14.3) | 29 (12.3) | .3153 |
| Pulmonary | |||||||
| COPD/Bronchiectasis | 0 (0) | 19 (29.7) | 1 (5.6) | 20 (20.8) | 19 (13.5) | 39 (16.5) | .1559 |
| Renal | |||||||
| Chronic kidney disease | 4 (28.6) | 23 (35.9) | 6 (33.3) | 33 (34.4) | 54 (38.5) | 87 (37.0) | .5832 |
| Acute/unspecified renal failure | 2 (14.3) | 6 (9.4) | 1 (5.6) | 9 (9.4) | 24 (17.0) | 33 (13.9) | .1256 |
| Endocrine | |||||||
| Diabetes mellitus | 4 (28.6) | 15 (23.4) | 5 (27.8) | 24 (25.0) | 35 (25.0) | 59 (25.0) | 1.0000 |
| Other | |||||||
| Malignancy | 3 (21.4) | 14 (21.9) | 5 (27.8) | 22 (22.9) | 32 (23.0) | 54 (23.0) | 1.0000 |
| Anemia | 3 (21.4) | 12 (18.8) | 0 (0) | 15 (15.6) | 30 (21.4) | 51 (21.6) | .3130 |
| Indications, n (%) | |||||||
| Phlebitis; thrombophlebitis, and thromboem bolism | 5 (37.5) | 15 (23.4) | 4 (22.2) | 24 (25) | 37 (26.4) | 61 (25.8) | .8801 |
| Acute or chronic embolism and thrombosis of various veins | 4 (28.6) | 14 (21.9) | 2 (11.1) | 20 (20.8) | 41 (29.3) | 61 (25.8) | .1737 |
| Pulmonary embolism | 2 (14.3) | 18 (28.1) | 2 (11.1) | 22 (22.9) | 24 (17.1) | 47 (19.9) | .3164 |
| Atrial fibrillation | 3 (21.4) | 5 (7.8) | 1 (5.6) | 9 (9.4) | 17 (12.1) | 26 (11.0) | .5347 |
| Acute cerebrovascular disease | 1 (7.1) | 7 (10.9) | 1 (5.6) | 9 (9.4) | 6 (4.2) | 15 (6.4) | .1727 |
| Aortic and peripheral arterial embolism or thrombosis | 0 (0) | 3 (4.7) | 0 (0) | 3 (3.1) | 3 (2.1) | 6 (2.5) | .6894 |
| Arterial embolism at various sites | 0 (0) | 3 (4.7) | 0 (0) | 3 (3.1) | 3 (2.1) | 6 (2.5) | .6894 |
| Chronic pulmonary embolism | 1 (7.1) | 2 (3.1) | 0 (0) | 3 (3.1) | 1 (.7) | 4 (1.7) | .3068 |
| Antiphospholipid syndrome | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.4) | 2 (.85) | .5153 |
| Acute myocardial infarction | 0 (0) | 1 (1.6) | 0 (0) | 1 (1) | 3 (2.1) | 4 (1.7) | .6478 |
Participants may have ≥1 diagnosis.
Abbreviations: COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; n, participants with a particular comorbidity or indication by anticoagulant prescription.
Anticoagulant Usage Patterns
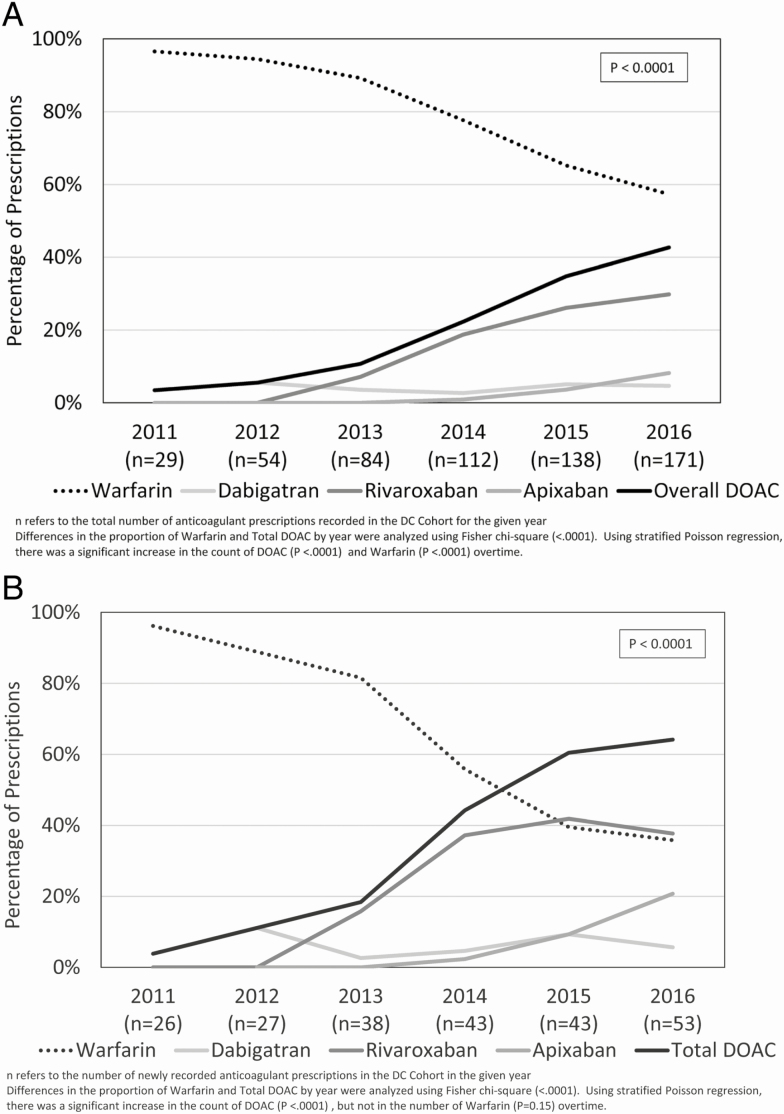
Across the entire period, there were 236 oral anticoagulant prescriptions, with 140 and 96 prescriptions for warfarin and DOACs, respectively. There was a decrease in the overall proportion of warfarin between 2011 and 2016, from 97% to 57% of all anticoagulant prescriptions. Meanwhile, the overall proportion of DOAC use increased from 3% to 43% (P < .0001) of all anticoagulant prescriptions (see Figure 1A).
Figure 1.
A, Trends in overall oral anticoagulant prescriptions in the D.C. Cohort, by drug, 2011–2016, where n is the total number of prescriptions in a given year. Differences in the proportion of warfarin and total DOAC by year were analyzed using a Fisher Chi-square test (<.0001). Using a stratified Poisson regression, there were significant increases in the counts of DOAC (P < .0001) and warfarin (P < .0001) over time. B, Newly recorded oral anticoagulant prescriptions in the D.C. Cohort, by drug and year, 2011–16, where n is the number of newly recorded prescriptions in a given year. Differences in the proportion of warfarin and total DOAC by year were analyzed using a Fisher Chi-square test (<.0001). Using a stratified Poisson regression, there was a significant increase in the count of DOAC (P < .0001), but not in warfarin (P = .15) over time. Abbreviation: D.C., District of Columbia; DOAC, direct oral anticoagulants.
DOACs accounted for 3.8% of all new oral anticoagulant prescriptions in 2011; this increased to 64% by 2016 (see Figure 1B). While there was not a significant decrease in the number of warfarin prescriptions (P = .15) over time, there was a significant, average increase in the number of DOACs prescribed over time (P < .0001).
Rivaroxaban was the most commonly prescribed DOAC, accounting for 64 of the 96 (66.7%) total DOAC prescriptions and for 70% of DOAC use as of 2016, followed by apixaban (19%) and dabigatran (11%). Over the study time frame, 25 participants had their anticoagulants switched from warfarin to a DOAC, whereas only 1 participant was switched from a DOAC to warfarin.
Warfarin and Direct Oral Anticoagulant Use with Antiretroviral Boosting Agents
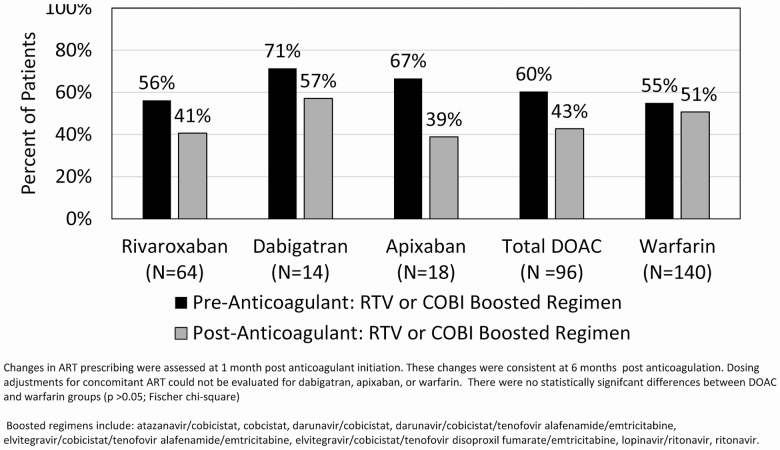
While on oral anticoagulants, 47% of participants were recorded to be receiving boosted ART. Of the participants initiated on warfarin, 55% were receiving cobicistat- or ritonavir-boosted ART prior to anticoagulation and, 1 month after initiation, 51% remained on boosted therapy (Figure 2). Of the participants initiated on a DOAC, 60% were receiving boosted ART prior to anticoagulation, and this decreased to 43% at 1 month after initiation. For participants started on rivaroxaban, 56% were reported as receiving a cobicistat- or ritonavir-based ARV regimen prior to anticoagulation. At 1 month after rivaroxaban initiation, 41% of participants were still reported as receiving a boosted ARV regimen (Figure 2). The reported percentages of coadministration were similar at both 3 and 6 months after anticoagulant initiation (data not shown).
Figure 2.
Boosted ART prescribing patterns before and 1 month after anticoagulant initiation. Changes in ART prescribing were assessed at 1 month postanticoagulant initiation. These changes were consistent at 6 months postanticoagulation. Dosing adjustments for concomitant ART could not be evaluated for dabigatran, apixaban, or warfarin. There were no statistically significant differences between the DOAC and warfarin groups (P > .05; Fisher Chi-square). Boosted regimens include atazanavir/cobicistat, cobicistat, darunavir/cobicistat, darunavir/cobicistat/tenofovir alafenamide/emtricitabine, elvitegravir/cobicistat/tenofovir alafenamide/emtricitabine, elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitabine, lopinavir/ritonavir, and ritonavir. Abbreviations: ART, antiretroviral therapy; COBI, cobicistat; DOAC, direct oral anticoagulants; RTV, ritonavir.
DOACs are largely renally eliminated; hence, an assessment of renal function is an important consideration in dose selection and adjustment. Within 6 months prior to the initiation of a DOAC, 69.8% of participants were reported to have a calculated creatinine clearance (CrCl) ≥ 60 ml/min. A CrCl was not available for 12.5% of participants (data not shown). Among the 17 participants with severe renal dysfunction (<30 ml/min), 12 (70.6%) were prescribed warfarin, compared to 5 (29.4%) prescribed DOACs. Of the DOACs, apixaban was the only DOAC prescribed in participants with CrCls <30 ml/min. No participants were documented to be on dialysis.
Reported Bleeding Events
Between 2011 and 2016, a total of 19 bleeding events were recorded based on ICD-9/10 codes in 16 participants on ART and receiving oral anticoagulants. The median age of these 16 individuals was 63.5 years (range, 51–74), and 7 had documented chronic kidney disease. There were 15 gastrointestinal bleeds and 4 cases of epistaxis documented.
More overall bleeding (major and minor) events were reported with warfarin (n = 12) than with DOACs (n = 7). Between boosted and unboosted ART groups, 8 and 11 bleeding events were documented, respectively.
Of the 12 bleeding events in the warfarin group, 6 had boosted antiretrovirals documented at the time of the bleed. Of the 7 bleeding events in the DOAC group, rivaroxaban was the documented DOAC in 6 of the events, and 2 events had boosted antiretrovirals documented at the time of the bleed.
A total of 5 bleeds required hospitalization. Of these, 3 major bleeds occurred in the warfarin group, and 2 participants were on boosted ART (ritonavir boosted darunavir and atazanavir). The other 2 major bleeds were in the DOAC group (rivaroxaban only), and both participants were on unboosted ART (dolutegravir based).
DISCUSSION
To our knowledge, this observational study represents the largest data set characterizing the usage of both warfarin and DOACs in an urban cohort of PWH to date. Between January 2011 and March 2017, 206 (2.5%) PWH on ART in the D.C. Cohort received oral anticoagulation with warfarin or a DOAC. The average age of participants receiving oral anticoagulation was representative of an aging cohort of PWH, which is consistent with Centers for Disease Control and Prevention national estimates of advancing age (over the age of 50) among PWH [18].
Prescribing trends indicate a significant increase in the proportion of DOAC use between 2011 and 2016. By 2016, DOACs accounted for 64% of all new oral anticoagulant prescriptions, with rivaroxaban being the most frequently prescribed. The increased use of DOACs within this cohort not only calls for attention to the frequency of oral anticoagulant use, but also highlights the shift in oral anticoagulant prescribing patterns.
This change in prescribing practice raises questions as to whether DOACs are being appropriately utilized in this population. Like warfarin, DOACs pose significant potential for drug interactions and increased risks of serious bleeding events [10–15]. As more PWH are being transitioned from warfarin or initiated on DOACs, it remains important to assess for drug interaction potential, appropriate dosage adjustments, and alternative anticoagulant or ARV options, and to weigh risks versus benefits to help inform whether a DOAC would be a safe alternative to warfarin in a given patient scenario. At the very least, prescribers need to be aware of the potential risks of increased oral anticoagulant concentrations when given concomitantly with boosted ART, in order to implement an appropriate monitoring plan.
The current FDA labels for dabigatran and apixaban advise using caution or dose adjusting during concomitant administration of strong dual CYP3A4/p-gp inhibitors [11, 13]. However, dabigatran and apixaban prescriptions could not be assessed for dosage adjustments in our cohort. The FDA label for rivaroxaban recommends against the concomitant use of medications that are strong dual inhibitors of p-gp and CYP3A4 (ie, a ritonavir or cobicistat regimen) due to the possible increased risk of bleeding [12]; yet in our cohort, 41% of rivaroxaban recipients were documented to have been prescribed these combinations. There are published case reports describing the increased risk of bleeding due to rivaroxaban-ritonavir drug interactions [19, 20]. In this study cohort, an overall small number of bleeding events were documented, with more episodes reported in the warfarin (n = 12) versus DOAC (n = 8) group, as well as in the unboosted (n = 12) versus boosted (n = 8) ART group. Given the small number of recorded bleeding events, the clinical interpretation of this data and associated risk factors is limited.
Notably, drug interaction queries from www.hiv-druginteractions.org (University of Liverpool) for warfarin, rivaroxaban, and apixaban were among the top 50 most searched comedications in 2018 [21]. To date, however, there is a paucity of drug interaction data with ARVs and DOACs. DOACs are primarily metabolized by CYP3A4 and/or transported by p-gp. Recommendations on managing drug interactions with strong CYP3A4 and p-gp inhibitors vary based on the specific DOAC. Recommendations may also vary based on the drug information resource utilized, including between the FDA and European Union product labels, tertiary online drug interaction checkers, and the Department of Health and Human Services ARV guidelines (see Table 4), introducing yet another challenge for providers when prescribing these concomitant medications [10–15, 22–31]. Available data on ARV-DOAC drug interactions were primarily from studies conducted with ritonavir in healthy volunteers. Notably, these studies were not conducted within the population of PWH, did not utilize the clinically relevant dose of ritonavir, and did not study ritonavir in combination with a complete antiretroviral regimen [11–15].
Table 4.
Direct Oral Anticoagulant Dosing Recommendations with Boosted Antiretrovirals Across Various Tertiary Resources
| FDA Labelsa [11–13, 27–31] | DHHS [22] | EU Labelsa [23] | Liverpool and EACS [24] | Lexicomp [25] | Micromedexb [26] | |
|---|---|---|---|---|---|---|
| Dabigatran | ||||||
| Elvitegravir/cobicistat | Dose adjustment may be necessaryc | Dose adjustment may be necessaryc | Contraindicated | Do not coadminister | Monitor therapy | Major interaction |
| Atazanavir/ ritonavir | Dose adjustment may be necessaryc | Dose adjustment may be necessaryc | Coadministration not recommended | Potential interaction | Consider therapy modification | Major interaction |
| Atazanavir/cobicistat | Dose adjustment may be necessaryc | Dose adjustment may be necessaryc | Clinical monitoring is recommended | Do not coadminister | Monitor therapy | Major interaction |
| Darunavir/ ritonavir | No dosage adjustment | No data available for dose recommendation | Contraindicated | Potential interaction | Consider therapy modification | Major interaction |
| Darunavir/ cobicistat | No dosage adjustment | No data available for dose recommendation | Contraindicated | Do not coadminister | Monitor therapy | Major interaction |
| Rivaroxaban | ||||||
| Elvitegravir/cobicistat | Coadministration not recommended | Do not coadminister | Coadministration not recommended | Do not coadminister | Avoid combination | Major interaction |
| Atazanavir/ ritonavir | Coadministration not recommended | Do not coadminister | Coadministration not recommended | Do not coadminister | Avoid combination | Major interaction |
| Atazanavir/cobicistat | Coadministration not recommended | Do not coadminister | Coadministration not recommended | Do not coadminister | Avoid combination | Major interaction |
| Darunavir/ ritonavir | Coadministration not recommended | Do not coadminister | Coadministration not recommended | Do not coadminister | Avoid combination | Major interaction |
| Darunavir/ cobicistat | Coadministration not recommended | Do not coadminister | Co-administration not recommended | Do not coadminister | Avoid combination | Major interaction |
| Apixaban | ||||||
| Elvitegravir/cobicistat | Dose-dependent recommendationsd | Dose-dependent recommendationsc | Coadministration not recommended | Do not coadminister | Monitor therapy | Major interaction |
| Atazanavir/ ritonavir | Dose-dependent recommendationsd | Dose-dependent recommendationsc | Coadministration not recommended | Do not coadminister | Consider therapy modification | Major interaction |
| Atazanavir/cobicistat | Coadministration not recommended | Dose-dependent recommendationsc | Coadministration not recommended | Do not coadminister | Monitor therapy | Major interaction |
| Darunavir/ ritonavir | Dose-dependent recommendationsd | Dose-dependent recommendationsc | Coadministration not recommended | Do not coadminister | Consider therapy modification | Major interaction |
| Darunavir/ cobicistat | Dose-dependent recommendationsd | Dose-dependent recommendationsc | Coadministration not recommended | Do not coadminister | Monitor therapy | Major interaction |
All resources had consistent recommendations for international normalized ratio monitoring when listed ARVs are used with warfarin.
Abbreviations: ARV, antiretroviral; DHHS, Department of Health and Human Services; DOAC, direct oral anticoagulant; EACS, European AIDS Clinical Society; EU, European Union; FDA, Food and Drug Administration.
aIf DOAC and ARV product labeling had discordant recommendations, the table reflects the more stringent recommendation.
bA major interaction was defined as an interaction that may be life-threatening and/or require medical intervention to minimize or prevent serious adverse effects.
cBased on indication and renal function.
dDo not coadminister or reduce dose, depending on dose.
Apixaban may be considered a safer alternative to other DOACs in patients with varying degrees of renal dysfunction. A lower rate of major bleeding events is associated with apixaban when compared to warfarin in patients with atrial fibrillation or VTE and chronic kidney disease (not on hemodialysis) [32, 33]. In patients on hemodialysis, rivaroxaban and dabigatran are shown to be associated with an increased risk of major bleeds, while the risk with apixaban is reported to be no different compared to warfarin [32]. In our study, participants had a higher mean baseline serum creatinine in the apixaban group, suggesting that prescribers favored it in participants with renal impairment. Overall, more participants with severe renal dysfunction (CrCl < 30ml/min) received warfarin (70.1%) than apixaban (29.4%).
The variability in recommendations, the general lack of pharmacokinetic data, and the frequency of concomitant DOAC and boosted ART prescribing highlight the benefits of taking a multi-disciplinary approach in treatment decisions. Ideally, communication between the ARV and anticoagulant prescribers should occur at the initiation of an anticoagulant to address potential interactions, select the most appropriate anticoagulant for an individual patient, and establish a mutually agreed upon course of action and monitoring. This is especially important for DOACs, given their relatively recent introduction and the potential unfamiliarity of prescribers with DOAC-ART interactions.
Clinical pharmacy specialists in the practice areas of anticoagulation and HIV can play an important role in the safe provision of these medications by assisting with literature evaluations and the interpretation of pharmacokinetic drug interaction data. Strategies to implement safe prescribing practices and to assist providers and pharmacists in recognizing when to modify a dose and/or treatment agent (oral anticoagulant or ART) are also necessary. These strategies could include intelligent decision support tools, such as electronic medical record prescriber alerts and health-care clinical dashboards. Additionally, patients should be encouraged to utilize the same pharmacy for all prescriptions, which would increase the likelihood of detecting these interactions at the point of dispensing.
An interpretation of these study results should take into account several limitations, including the possible underestimation of the true rate of ART and oral anticoagulant coprescribing and reliance on the accurate entry of ICD9/10 codes. The D.C. Cohort collects data from HIV clinics, not from the anticoagulation clinics where the anticoagulants are prescribed. Additionally, bleeding events managed at hospitals or other clinics, such as primary care and anticoagulation clinics, may not have been captured. Although dabigatran, apixaban, and warfarin may require dosing modifications according to the drug interaction potential and various patient variables, the appropriateness of the prescribed doses could not be determined due to a lack of consistent documentation of drug dosages. Finally, the reliance on the accurate entry of bleeding events using ICD-9/10 codes is a possible limitation when reporting the absolute number of bleeds.
In conclusion, it is evident that the number of PWH requiring oral anticoagulation is substantial and likely will increase as this population ages. Although warfarin was still frequently prescribed as of 2016, the upward trend in DOAC prescriptions is consistent with current recommendations for the management of patients with thromboembolic disease. The identification of coprescriptions of rivaroxaban and boosted ART in 41% of rivaroxaban recipients after anticoagulant initiation underscores the need for continued efforts to promote communication between multiple health-care practitioners, to provide education, and to increase pharmacologic vigilance. Lastly, these data, in addition to the variable DOAC dosing recommendations between tertiary drug information resources, highlight the need for large prospective surveillance studies to characterize the safe and effective use of oral anticoagulants in PWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. M. G. and S. S. K. contributed equally to this manuscript. Data in this manuscript were collected by the District of Columbia (D.C.) Cohort Study Group with investigators and research staff located at the Cerner Corporation (Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Qingjiang Hou, Jeff Naughton, and David Parfitt); Children’s National Medical Center Adolescent (Lawrence D’Angelo) and Pediatric (Natella Rahkmanina) Clinics; the D.C. Department of Health HAHSTA (Michael Kharfen, Senior Deputy Director); Family and Medical Counseling Service (Michael Serlin); Georgetown University (Princy Kumar); The George Washington University Medical Faculty Associates (David Parenti); The George Washington University Department of Epidemiology and Biostatistics (Amanda Castel, Alan Greenberg, Anne Monroe, Lindsey Powers Happ, Maria Jaurretche, Brittany Wilbourn, James Peterson, Matthew Levy, Morgan Byrne, and Yan Ma); Howard University Adult Infectious Disease (Ronald Wilcox) and Pediatric (Sohail Rana) Clinics; Kaiser Permanente Mid-Atlantic (Michael Horberg); La Clinica Del Pueblo (Ricardo Fernandez); Leidos Biomedical Research, Inc (Safia Kuriakose); MetroHealth (Annick Hebou); the National Institutes of Health (Carl Dieffenbach, Jomy George, Colleen Hadigan, Henry Masur, Alice Pau); Providence Hospital (Jose Bordon); Unity Health Care (Gebeyehu Teferi); Veterans Affairs Medical Center (Debra Benator); Washington Hospital Center (Maria Elena Ruiz); and Whitman-Walker Health (Deborah Goldstein).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the District of Columbia (D.C.) Cohort, which is funded by the National Institute of Allergy and Infectious Diseases (grant number 5UM1AI069503); the National Cancer Institute, National Institutes of Health (contract number HHSN261200800001E); the National Institute of Allergy and Infectious Diseases Intramural Research Program; and by a 2015 award from the D.C. Center for AIDS Research, a National Institutes of Health–funded program (award number AI117970) that is supported by the following National Institutes of Health Co-Funding and Participating Institutes and Centers: the National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Heart Lung Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, National Institute on Aging, Fogarty International Center, National Institute of General Medicines, National Institute of Diabetes and Digestive and Kidney Diseases, and Office of AIDS Research. The Frederick National Laboratory for Cancer Research is sponsored by the National Cancer Institute.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
District of Columbia (D.C.) Cohort Executive Committee:
Jeffery Binkley, Rob Taylor, Nabil Rayeed, Cheryl Akridge, Stacey Purinton, Qingjiang Hou, Jeff Naughton, David Parfitt, Lawrence D’Angelo, Natella Rahkmanina, Michael Kharfen, Michael Serlin, Princy Kumar, David Parenti, Amanda Castel, Alan Greenberg, Anne Monroe, Lindsey Powers Happ, Maria Jaurretche, Brittany Wilbourn, James Peterson, Matthew Levy, Morgan Byrne, Yan Ma, Ronald Wilcox, Sohail Rana, Michael Horberg, Ricardo Fernandez, Safia Kuriakose, Annick Hebou, Carl Dieffenbach, Jomy George, Colleen Hadigan, Henry Masur, Alice Pau, Jose Bordon, Gebeyehu Teferi, Debra Benator, Maria Elena Ruiz, and Deborah Goldstein
References
- 1. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–52. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol 2014; 64(21): 2246–2280, [DOI] [PubMed]
- 3.Institute for Safe Medication Practices. Quarter watch – 2016 quarter four. Available at: https://www.ismp.org/quarterwatch/annual-report-2016. Accessed on 29 July 2017.
- 4.Feinstein M, Hsue P, Benjamin L, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV. A scientific statement from the American Heart Association. Circulation 2019; 139:e1-e27. [DOI] [PMC free article] [PubMed]
- 5.Crum-Cianflone NF, Weekes J, Bavaro M. Review: thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS Patient Care STDs 2008; 22:771–778. [DOI] [PMC free article] [PubMed]
- 6. Matta F, Yaekoub AY, Stein PD. Human immunodeficiency virus infection and risk of venous thromboembolism. Am J Med Sci 2008; 336:402–6. [DOI] [PubMed] [Google Scholar]
- 7. Saber AA, Aboolian A, LaRaja RD, Baron H, Hanna K. HIV/AIDS and the risk of deep vein thrombosis: a study of 45 patients with lower extremity involvement. Am Surg 2001; 67:645–7. [PubMed] [Google Scholar]
- 8. Malek J, Rogers R, Kufera J, Hirshon JM. Venous thromboembolic disease in the HIV-infected patient. Am J Emerg Med 2011; 29:278–82. [DOI] [PubMed] [Google Scholar]
- 9. Rasmussen LD, Dybdal M, Gerstoft J, et al. HIV and risk of venous thromboembolism: a Danish nationwide population-based cohort study. HIV Med 2011; 12:202–10. [DOI] [PubMed] [Google Scholar]
- 10.Bristol-Myers Squibb. Coumadin (warfarin sodium) [prescribing information]. Princeton, New Jersey; Bristol-Myers Squibb, 2017.
- 11.Boehringer Ingelheim Pharmaceuticals, Inc. Pradaxa (dabigatran etexilate mesylate) [prescribing information]. Ridgefield, Connecticut: Boehringer Ingelheim Pharmaceuticals Inc., 2018.
- 12.Janssen Pharmaceutical Companies. Xarelto (rivaroxaban) [prescribing information]. Titusville, New Jersey; Janssen Pharmaceutical Companies, 2019.
- 13.Bristol-Myers Squibb. Eliquis (apixaban) [prescribing information]. Princeton, New Jersey: Bristol-Myers Squibb, 2018.
- 14.Daiichi Sankyo Inc. Savaysa (edoxaban) [prescribing information]. Parsippany, New Jersey; Daiichi Sankyo Inc., 2015.
- 15.Portola Pharmaceuticals. Bevyxxa (betrixaban) [prescribing information]. San Francisco, California: Portola Pharmaceuticals, 2017.
- 16. Greenberg AE, Hays H, Castel AD, et al. ; District of Columbia (D.C.) Cohort Executive Committee Development of a large urban longitudinal HIV clinical cohort using a web-based platform to merge electronically and manually abstracted data from disparate medical record systems: technical challenges and innovative solutions. J Am Med Inform Assoc 2016; 23:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality. Healthcare cost and utilization project (HCUP) tools and software. Available at: https://www.hcup-us.ahrq.gov/tools_software.jsp. Accessed 5 May 2019. [PubMed]
- 18.Centers for Disease Control and Prevention. HIV among people aged 50 and older. Available at: https://www.cdc.gov/hiv/group/age/olderamericans/. Accessed 18 April 2019.
- 19. Corallo CE, Grannell L, Tran H. Postoperative bleeding after administration of a single dose of rivaroxaban to a patient receiving antiretroviral therapy. Drug Saf Case Rep 2015; 2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latakos B, Stoekle M, Elzi L, et al. Gastrointestinal bleeding associated with rivaroxaban administration in a treated patient infected with human immunodeficiency virus. Swiss Med Wkly 2014; 144:1–4. [DOI] [PubMed] [Google Scholar]
- 21.Back D. The challenges of HIV treatment in an era of polypharmacy. In: Program and abstracts of the Conference of Retroviruses and Opportunistic Infections (CROI), Seattle, WA, 2019. Plenary Session. Available at: http://www.croiwebcasts.org/st/plenary?link=nav&linkc=sesstype. Accessed 6 March 2019.
- 22.Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at http://www.aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL.pdf. Accessed 18 February 2020.
- 23.European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines. Accessed 18 April 2019.
- 24.University of Liverpool. HIV drug interaction checker. Available at: https://www.hiv-druginteractions.org. Accessed 18 April 2019.
- 25.Wolters Kluwer Health Inc. Lexicomp: drug interactions. Available at: https://www.online.lexi.com. Accessed 18 April 2019.
- 26.Truven Health Analytics. Micromedex: drug interactions (Columbia Basin College Library ed.) Greenwood Village, Colorado: Truven Health Analytics. Available at: http://www.micromedexsolutions.com. Accessed 18 April 2019.
- 27.Gilead Sciences. Genvoya [prescribing information]. Foster City, California: Gilead Sciences, 2019.
- 28.Bristol-Myers Squibb Company. Reyataz [prescribing information]. Princeton, New Jersey: Bristol-Myers Squibb Company, 2018.
- 29.Bristol-Myers Squibb Company. Evotaz [prescribing information]. Princeton, New Jersey: Bristol-Myers Squibb Company, 2018.
- 30.Janssen Therapeutics. Prezista [prescribing information]. Titusville, New Jersey: Janssen Therapeutics, 2019.
- 31.Janssen Therapeutics. Symtuza [prescribing information]. Titusville, New Jersey: Janssen Therapeutics, 2019.
- 32. Raccah BH, Perlman A, Danenberg HD, Pollak A, Muszkat M, Matok I. Major bleeding and hemorrhagic stroke with direct oral anticoagulants in patients with renal failure: systematic review and meta-analysis of randomized trials. Chest 2016; 149:1516–24. [DOI] [PubMed] [Google Scholar]
- 33. Feldberg J, Patel P, Farrell A, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant 2019; 34:265–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.