Abstract
Background
Strongyloidiasis can cause devastating morbidity and death in immunosuppressed patients. Identification of reliable biomarkers for strongyloidiasis in immunosuppressed patients is critical for the prevention of severe disease.
Methods
In this cross-sectional study of solid organ transplant (SOT) candidates and recipients, we quantified Strongyloides-specific IgG to the recombinant NIE-Strongyloides antigen and/or to a soluble extract of S. stercoralis somatic antigens (“crude antigen”) using enzyme-linked immunosorbent assays (ELISAs). We also measured peripheral eosinophilia, 4 different eosinophil granule proteins, and intestinal fatty acid–binding protein (IFABP).
Results
We evaluated serum biomarkers in 149 individuals; 77 (52%) pre-SOT and 72 (48%) post-SOT. Four percent (6/149) tested positive by NIE ELISA and 9.6% (11/114) by crude antigen ELISA (overall seropositivity of 9.4% [14/149]). Seropositive patients had higher absolute eosinophil counts (AECs) than seronegative patients (P = .004). AEC was positively correlated to the levels of eosinophil granule proteins eosinophil cationic protein (ECP) and eosinophil peroxidase (EPO) (P < .05), while IFABP was positively related to the 2 other eosinophil granule proteins (major basic protein [MBP] and eosinophil-derived neurotoxin [EDN]; Spearman’s r = 0.3090 and 0.3778, respectively; P < .05; multivariate analyses slopes = 0.70 and 2.83, respectively).
Conclusions
This study suggests that, in SOT patients, strongyloidiasis triggers both eosinophilia and eosinophil activation, the latter being associated with intestinal inflammation. These data provide insight into the pathogenesis of S. stercoralis infection in the immunocompromised population at high risk of severe strongyloidiasis syndromes.
Keywords: Strongyloides stercoralis, solid organ transplant, eosinophils, eosinophil granule protein, intestinal fatty acid–binding protein
It is critical to diagnose strongyloidiasis prior to solid-organ transplantation (SOT) to prevent severe strongyloidiasis syndromes. This cross-sectional study evaluated serum biomarkers for eosinophil activation and intestinal inflammation to provide insight into the pathogenesis of strongyloidiasis in SOT patients.
Strongyloides stercoralis is a parasitic nematode endemic to most tropical and subtropical countries where it chronically infects more than 30 million people [1, 2]. Most S. stercoralis infections in the United States are imported through travel to or emigration from endemic areas. Chronic infection can persist with minimal to no symptoms for decades, and often peripheral blood eosinophilia is the only finding present [3].
Strongyloides stercoralis can cause significant disease in the setting of acquired immunosuppression [4, 5], such as solid organ transplantation (SOT) or corticosteroid use. The immunosuppressed state of these patients increases the risk of hyperinfection syndrome and disseminated strongyloidiasis, conditions that are often underdiagnosed because they present with nonspecific signs such as gram-negative bacterial sepsis and/or meningitis. In SOT recipients, these severe manifestations are almost always due to accelerated autoinfection rather than new infection, although cases of donor-derived S. stercoralis hyperinfection have been described [6]. The large population of immigrants from S. stercoralis–endemic countries living in the United States constitutes a significant group at risk for both hyperinfection post-SOT and transmission of infection after organ donation [7, 8]. The American Society of Transplantation recommends that all potential solid organ donors and recipients from endemic areas undergo serologic screening for strongyloidiasis before SOT [9], although adherence to this recommendation is far from universal [10].
Detection of S. stercoralis infection in patients preparing for SOT is crucial to prevent severe disease post-SOT. The current standard for diagnosis of S. stercoralis infection is the direct detection of larvae in stool; however, this method has significant limitations [11]. In practice, serologic screening is the most commonly used method for strongyloidiasis diagnosis. Most commercial clinical laboratories in the United States use an enzyme-linked immunosorbent assay (ELISA) that measures the immunoglobulin G (IgG) response to a crude soluble extract of Strongyloides somatic antigens derived from the filariform larvae [11] (“crude antigen ELISA”). These assays are reasonably sensitive (73–100%) but often lack specificity (29–93%) due to antigenic cross-reactivity with other helminths [11]. Moreover, the crude antigen ELISA is less sensitive in immunocompromised individuals [12]. In contrast, NIE-Strongyloides, a 31-kDa recombinant antigen identified from S. stercoralis filariform larvae cDNA libraries [13], has been used in both ELISA and luciferase immunoprecipitation system assays and performs better than the crude antigen ELISA [14] because of its more than 99% specificity.
Peripheral eosinophilia is a common finding in immunocompetent hosts with strongyloidiasis, and the decline in absolute eosinophil count (AEC) posttreatment has been used as a marker of treatment success [3]. Eosinophil granule proteins (major basic protein [MBP], eosinophil cationic protein [ECP], eosinophil-derived neurotoxin [EDN], and eosinophil peroxidase [EPO]) are highly proinflammatory and have been used as markers of eosinophil activation in both parasitic [15] and noninfectious inflammatory [16] conditions. Similarly, intestinal fatty acid–binding protein (IFABP), a serum marker of intestinal epithelial cell injury [17], has been studied in immunocompetent populations with chronic intestinal parasitic infections [18] but not, to our knowledge, in immunocompromised individuals.
The main objective of this study was to assess the utility of the NIE and crude antigen ELISAs as screening tools for chronic S. stercoralis infection in SOT patients who have immigrated to the United States from endemic regions. We have also identified a potential mechanistic pathway for strongyloidiasis-related intestinal injury in these immunocompromised patients.
METHODS
Ethics Statement
This protocol (H-37115) was approved by the institutional review boards of Baylor College of Medicine and Baylor St Luke’s Medical Center (BSLMC). All participants provided written informed consent. No individuals younger than 18 years old were included. Patients consented in their primary language if not conversant in English.
Study Population
Patients were recruited from the inpatient service at BSLMC and the outpatient Infectious Diseases clinic at Baylor Clinic in Houston, Texas, from April 2017 to May 2018. To be eligible, patients must have been born in a S. stercoralis–endemic region outside of the continental United States. All patients were either listed for or had received a kidney, liver, heart, or lung transplant (6 patients were listed for or received dual-organ transplants). Patients were excluded if they reported a history of treatment for S. stercoralis at any point in the past. Patients were also excluded if they had received albendazole or ivermectin for any reason in the previous year or were unable to consent due to alteration in mental status.
Data regarding patient demographics, country of origin, and immunosuppressant regimen at the time of consent were obtained. Patients seen in the hospital were assessed for signs or symptoms of S. stercoralis hyperinfection syndrome or disseminated disease.
Sample Size Calculation
In prior studies, eosinophilia was present in 5–35% of patients who tested positive for Strongyloides [7, 19, 20]. Assuming a lower bound of Strongyloides seropositivity of 6% [20] and standard 1-proportion confidence intervals (CIs) at 80% power and 95% accuracy, we required a minimum of 130 subjects for this study.
Sample Collection
We enrolled 149 patients by convenience sampling as described above. Whole blood was collected from these patients in standard EDTA tubes (for complete blood count with differential) as part of routine clinical care. Blood samples were taken to the Laboratory of Clinical Parasitology and Diagnostics, National School of Tropical Medicine, Baylor College of Medicine (NSTM), for the investigational assays described below (except for the Strongyloides crude antigen ELISA, which was performed by commercial laboratories as described). Blood samples were centrifuged to obtain plasma and stored at −80°C in the NSTM until use.
Strongyloides Crude Antigen IgG ELISA
Strongyloides crude antigen ELISA was performed by a commercial laboratory (Quest Diagnostics, Secaucus, NJ) if sent from BSLMC or ARUP Laboratories (Salt Lake City, UT) if sent from Baylor Clinic. Qualitative results were reported through the institutional electronic medical record. Only 114 of 149 enrolled patients underwent crude antigen ELISA testing due to physician ordering preference. To avoid selection bias error, intention to test was implemented, and no patients were excluded from calculations.
Strongyloides NIE IgG ELISA
Stored patient plasma was used to perform NIE ELISA on all 149 samples, as previously described [13]. Cutoffs for positives and negatives were based on receiver operator curve analysis using sera from stool-positive Strongyloides-infected patients and normal healthy controls. For the NIE ELISA, a standard curve was used. The cutoff for a positive NIE ELISA result was 11.88 units/mL. The threshold was determined by setting the sensitivity to 100% and specificity to 76.47%. This threshold was chosen to optimize the number of positives in this high-risk immunocompromised population. Because the crude antigen ELISA is the current serologic standard of practice, patients who were seropositive by either crude antigen or NIE ELISA were considered to have strongyloidiasis.
Intestinal Fatty Acid–Binding Protein
Intestinal fatty acid–binding protein was measured at the NSTM using a patient serum as previously described [21, 22].
Eosinophil Granule Proteins
The concentrations of 4 eosinophil granule proteins (MBP, ECP, EDN, and EPO) were measured in patient serum using a multiplex suspension array system at the National Institutes of Health, as previously described [23].
Patient Follow-up
All patients with either a positive qualitative crude antigen ELISA or a positive NIE ELISA result were notified, as was their transplant provider. Patients were offered treatment with 2 daily doses of 200 µg/kg of ivermectin.
Statistical Analysis
Clinical continuous variables were compared using Mann-Whitney tests for nonparametric variables. Spearman correlation tests were used to determine relationships between continuous variables. Analysis of covariance was utilized for comparison of linear correlations. P < .05 was considered significant. All of these tests were performed using GraphPad Prism 8.2.1 (GraphPad Software, San Diego, California, USA), while univariate regression, multivariate regression, and multivariate regression using interaction covariates were performed using the lm function included in the R version 3.6.1. Covariates for the regression analyses included age, sex, number of immunosuppressive medications (0–3 medications), Strongyloides seropositivity, and AEC.
RESULTS
Testing and Demographic Information
Demographic data for patients enrolled in the study are described in Table 1. Age and sex distributions were similar for S. stercoralis–seropositive participants compared with those who were seronegative. Participants were from 5 different continents, although the majority were from North America, specifically Mexico. Our study cohort had similar numbers of participants awaiting SOT (n = 77, 52%) as post-SOT (n = 72, 48%). The most common transplanted organ was kidney (56% of post-SOT patients), followed by liver (32%), heart (6%), and lung (6%). Two patients underwent dual liver/kidney transplants, and 1 patient underwent a dual heart/lung transplant.
Table 1.
Demographic Data and Results of Strongyloides Serological Testing
| All (N = 149) | Strongyloides Positive (n = 14) | Strongyloides Negative (n = 135) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P | |
| Age in years (median) | 149 | 58 years | 14 | 9.4 | 135 | 90.6 | |
| Sex | .95 | ||||||
| Male | 92 | 63 | 9 | 9.8 | 83 | 90.2 | |
| Female | 53 | 37 | 5 | 9.4 | 48 | 90.6 | |
| Nationality | .90 | ||||||
| African | 5 | 3 | 0 | 0 | 5 | 100 | |
| Asian | 22 | 15 | 3 | 14 | 19 | 86 | |
| Latin | 116 | 78 | 11 | 9 | 105 | 91 | |
| Middle Eastern | 5 | 3 | 0 | 0 | 5 | 100 | |
| European | 1 | 1 | 0 | 0 | 1 | 100 | |
| Continent of origin/exposure | .048 | ||||||
| South America | 4 | 3 | 0 | 0 | 4 | 100 | |
| Central America | 21 | 14 | 6 | 28.6 | 15 | 71.4 | |
| North America | 89 | 60 | 4 | 4.4 | 82 | 95.5 | |
| Africa | 5 | 3 | 0 | 0 | 5 | 100 | |
| Asia | 23 | 15 | 1 | 4.3 | 22 | 95.6 | |
| Middle East | 5 | 4 | 0 | 0 | 5 | 100 | |
| Europe | 1 | 1 | 0 | 0 | 1 | 100 | |
| Transplant status | .78 | ||||||
| Pretransplant | 77 | 52 | 9 | 11.7 | 68 | 88.3 | |
| Posttransplant | 72 | 48 | 5 | 6.9 | 67 | 93.1 | |
| Absolute eosinophil count, cells/mL | … | … | 212 (0–1620) | 82 (0–700) | .004 | ||
| MBP, ng/mL | … | … | 233.2 (45.2–504.5) | 217.5 (0–1273.0) | .57 | ||
| ECP, ng/mL | … | … | 616.2 (190.8–3257.8) | 718.2 (171.8–8464.1) | .30 | ||
| EPO, ng/mL | … | … | 0.0 (0–39.5) | 0.0 (0–39.5) | .62 | ||
| EDN, ng/mL | … | … | 46.4 (0–319.4) | 364.6) | .39 | ||
| IFABP, pg/mL | … | … | 1710 (719.1–2968) | 1731 (46.6–3969) | .70 |
Data are presented as number and percentage unless otherwise indicated.
Abbreviations: ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPO, eosinophil peroxidase; IFABP, intestinal fatty acid–binding protein; MBP, major basic protein.
Strongyloides Serology Results: Crude Antigen ELISA and NIE ELISA
Of 149 participants tested using the NIE ELISA, 6 (4.0%) were positive. Of 114 participants tested using the crude antigen ELISA, 11 (9.6%) were positive. Of these 11 participants who were seropositive by the crude antigen ELISA, only 3 were also positive by the NIE ELISA. One-hundred and three participants tested negative via the crude antigen ELISA and, of these, 3 (2.9%) tested positive using the NIE ELISA. To reduce selection bias from the incomplete commercial testing, we considered positive serology results from either test showing an overall S. stercoralis seroprevalence of 9.4% (14/149 participants).
Eosinophilia Is Associated With Strongyloides stercoralis Seropositivity
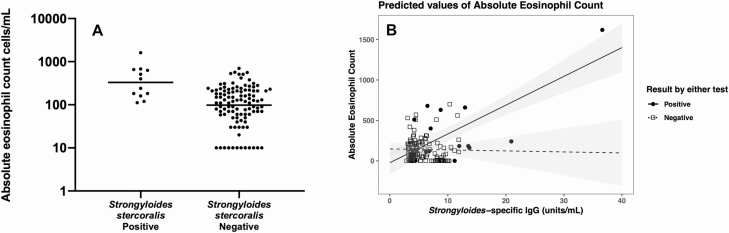
Strongyloides stercoralis–seropositive participants had significantly higher geometric mean (GM) AECs compared with those who were seronegative (212 vs 82 cells/mL, P = .004) (Figure 1A). This finding was driven by the pre-SOT population (AEC = 240 cells/mL for seropositive participants vs 84 cells/mL for seronegative; P = .0014), with a positive trend in post-SOT patients (GM, 183 vs 30 cells/mL; P = .34). Multivariate analysis showed a positive relationship between Strongyloides-specific IgG and AEC (Figure 1B). This model revealed that AEC increased by 36.9 cells/mL for every 1-unit/mL increase in Strongyloides-specific IgG in positive Strongyloides serology samples (95% CI, 20.84–52.87; P < .001).
Figure 1.
A, Absolute eosinophil counts were elevated for Strongyloides-seropositive versus -seronegative patients (320 vs 122 cells/mL, P = .004). B, Peripheral eosinophilia is associated with S. stercoralis seropositivity. AEC increased by an estimated 36.86 cells/mL for every 1 unit/mL S. stercoralis IgG (95% CI, 20.99–52.73; P < .001). Abbreviations: AEC, absolute eosinophil count; CI, confidence interval; IgG, immunoglobulin G.
Eosinophil Activation Is Associated With Strongyloides-induced Eosinophilia
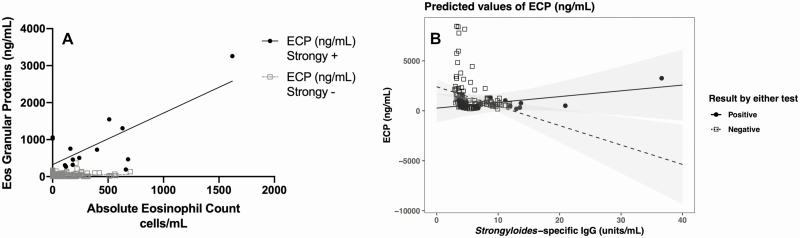
Using linear regression, the eosinophil granule protein ECP showed a positive relationship to AEC in S. stercoralis–seropositive compared with –seronegative patients (R2 = 0.55 vs 0.003, respectively; P < .001) (Figure 2A). Multivariate analysis showed a positive relationship between S. stercoralis–specific IgG and 2 eosinophil granule proteins (ECP and EPO) (Figure 2B). These models revealed that in Strongyloides–positive serology samples, every 1-unit/mL increase in S. stercoralis–specific IgG related to a 251.9-ng/mL increase in ECP (Figure 2B) and a 1.2-ng/mL increase in EPO. An inverse relationship was noted for MBP (see Supplementary Figure 2).
Figure 2.
Strongyloides seropositivity is associated with increased eosinophil granule proteins. A, Strongyloides stercoralis–seropositive patients had eosinophilia associated with increased ECP and EPO compared with seronegative patients. B, For every 1-unit/mL increase in S. stercoralis IgG, ECP increased by 251.9 ng/mL, and EPO increased by 1.2 ng/mL. Multivariate analysis results for all eosinophil granule proteins were compared to S. stercoralis IgG concentration (see Supplementary Figure 2). Abbreviations: ECP, eosinophil cationic protein; Eos, eosinophil; EPO, eosinophil peroxidase; IgG, immunoglobulin G; Strongy, Strongyloides stercoralis.
Intestinal Inflammation Associated With Eosinophil Activation
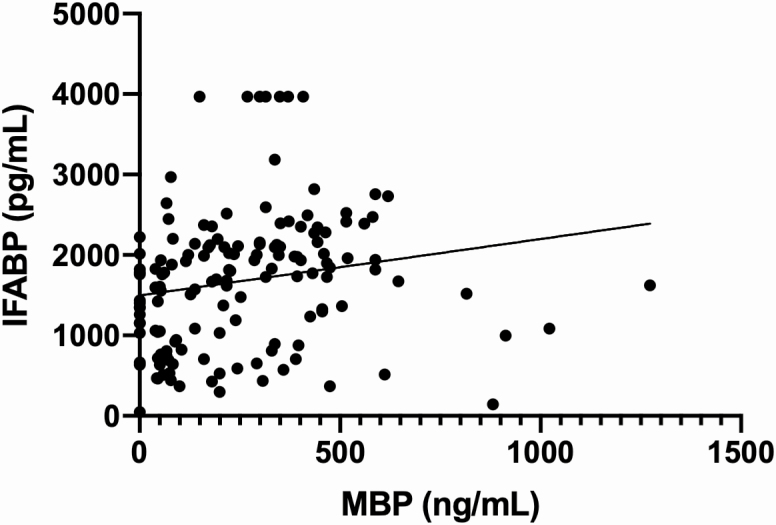
Two eosinophil granule proteins, MBP and EDN, were associated with increasing IFABP (Figure 3). This correlation was confirmed via both univariate (Spearman’s r: 0.03090 and 0.3778, respectively; P < .05) and multivariate testing (slope of 0.70 and 2.83, respectively; P < .05).
Figure 3.
Increased eosinophil granule proteins MBP and EDN (data not shown) correlated with increasing IFABP, a marker of intestinal inflammation. This correlation was validated with both univariate and multivariate testing (see Supplementary Figure 3). Abbreviations: EDN, eosinophil-derived neurotoxin; IFABP, intestinal fatty acid–binding protein; MBP, major basic protein.
DISCUSSION
Strongyloidiasis is one of few helminth infections that can last lifelong in untreated patients [24]. Infection is usually subclinical in immunocompetent hosts; in fact, strongyloidiasis is often diagnosed incidentally when the clinician notes unexplained peripheral eosinophilia. In contrast, in immunosuppressed hosts, S. stercoralis can cause significant morbidity and mortality in the absence of early clinical indicators such as eosinophilia. Dangerous syndromes caused by this parasite, including hyperinfection and dissemination, occur in individuals with acquired immune system alterations, including infection with human T-lymphotropic virus (HTLV)-1, treatment with corticosteroids or other immunosuppressive medications, or hematopoietic stem cell or SOT [4].
One objective of this study was to assess the utility of 2 different serologic tests (the crude antigen ELISA and the NIE ELISA) as screening tools for chronic S. stercoralis infection in SOT patients who have emigrated to the United States from endemic regions. Four percent of our study subjects were seropositive by the specific NIE-based ELISA, whereas 9.6% were positive by the crude antigen ELISA (overall prevalence was 9.4%). One explanation for the discordance noted between the crude antigen ELISA and NIE ELISA results is the possibility of crude antigen cross-reactivity with homologous helminth proteins found among various nematodes. Our overall seroprevalence rate is higher than that noted in a previous study of Latin American immigrants awaiting SOT (Fitzpatrick et al [20] measured a 6.7% seroprevalence using a crude antigen ELISA). The relatively high S. stercoralis seroprevalence in SOT candidates and recipients from endemic parts of the world highlights the need to test all at-risk patients prior to immunosuppression and, where diagnostic tools are not available, to empirically treat all at-risk patients with ivermectin.
Besides determining the seroprevalence of strongyloidiasis in peri-SOT patients from endemic regions, an additional aim to was to investigate strongyloidiasis-associated eosinophilia (including degranulation cascades involving the eosinophil granule proteins) and its relationship to intestinal inflammation. The existing literature strongly supports a role for the eosinophil in the pathogenesis of a handful of disease categories, including tissue-invasive parasitic diseases [25]. Eosinophils have been shown to have both pro- and anti-inflammatory properties and have been thought to play an essential role in tissue remodeling. Thus, we used peripheral eosinophilia as a surrogate marker of innate immune activation related to possible parasitic infection. As anticipated, AEC was higher in patients who were S. stercoralis seropositive (Figure 1).
Eosinophils function, in part, by selectively secreting preformed cationic proteins from their secondary granules. We expected to see a direct relationship between peripheral eosinophilia and serum levels of eosinophil granule proteins. To this end, we measured 4 eosinophil granule proteins (MBP, ECP, EDN, and EPO) as markers for tissue-based eosinophil activation/degranulation. Our results indicate that patients with elevated AEC indeed have increased levels of ECP and EPO (Figure 2). ECP is known to be part of the allergic response and has been implicated in the pathogenesis of inflammatory bowel disease (IBD) [26]. EPO is a potent antinematode protein [27] and also has been implicated in IBD [28].
Intestinal fatty acid–binding protein, an intracellular protein released from epithelial cells of the gastrointestinal tract after mucosal damage, also has been studied as a potential biomarker for intestinal inflammation due to parasitic infection. However, its utility in the SOT population is not well defined. Intestinal fatty acid–binding protein has been associated with intestinal parasite infection in immunocompetent individuals [15] and a subset of immunocompromised patients (patients with human immunodeficiency virus coinfected with visceral leishmaniasis) [29]. In our study, we sought to evaluate the utility of IFABP as a marker of strongyloidiasis-related intestinal inflammation. We found that IFABP is associated with the levels of these eosinophil granule proteins (Figure 3). Although we found no difference in IFABP when comparing seropositive with seronegative participants, we did find that IFABP is associated with the levels of 2 eosinophil granule proteins, MBP and EDN. In murine studies, MBP is important in immunologic control of helminth burdens [30]. MBP may also contribute to loss of intestinal mucosal barrier function, which could explain its relationship with IFABP in our study [31]. EDN has an indirect impact on helminths by having a role in activating the toll-like receptor-myeloid dfferentiation primary response 88 (TLR2-MyD88) signal pathway and, eventually, helping to drive a T-helper 2 (Th2) response [32]. Like the other eosinophil granule proteins, EDN has also been implicated in intestinal inflammation seen in IBD [28]. Together, these observations suggest that eosinophil granule protein activation in the setting of strongyloidiasis may drive innate immune responses in the intestinal mucosa that trigger intestinal inflammation.
Strongyloidiasis is challenging to study in immunocompromised patients due to the chronic nature of the infection and imperfect diagnostic methods. In addition, the relatively small numbers of individuals from S. stercoralis–endemic regions who are candidates for transplant in the United States make it difficult to enroll an adequate number of patients to study this disease, even in a diverse immigrant community such as ours. Nevertheless, in our study we were able to detect and treat 14 S. stercoralis–seropositive SOT patients. While prior studies have extensively evaluated both the sensitivity and specificity of the NIE ELISA and the crude antigen ELISA [13, 14], lack of stool samples for microscopy limited our ability to evaluate the performance of these serologic assays in our study participants. Thus, the S. stercoralis prevalence in our study could be underestimated in the immunosuppressed participants [33]. Finally, due to the low sample size we did not include transplant status as a covariate in the multivariate analysis, although the amount of immunosuppressive medications was considered in determining eosinophilia and eosinophil degranulation. More extensive, multicenter studies should be performed to more accurately interpret S. stercoralis prevalence in the US population at large and those undergoing SOT. Studies of this nature would also allow for a thorough evaluation of eosinophil activation and intestinal inflammation in the setting of strongyloidiasis.
CONCLUSIONS
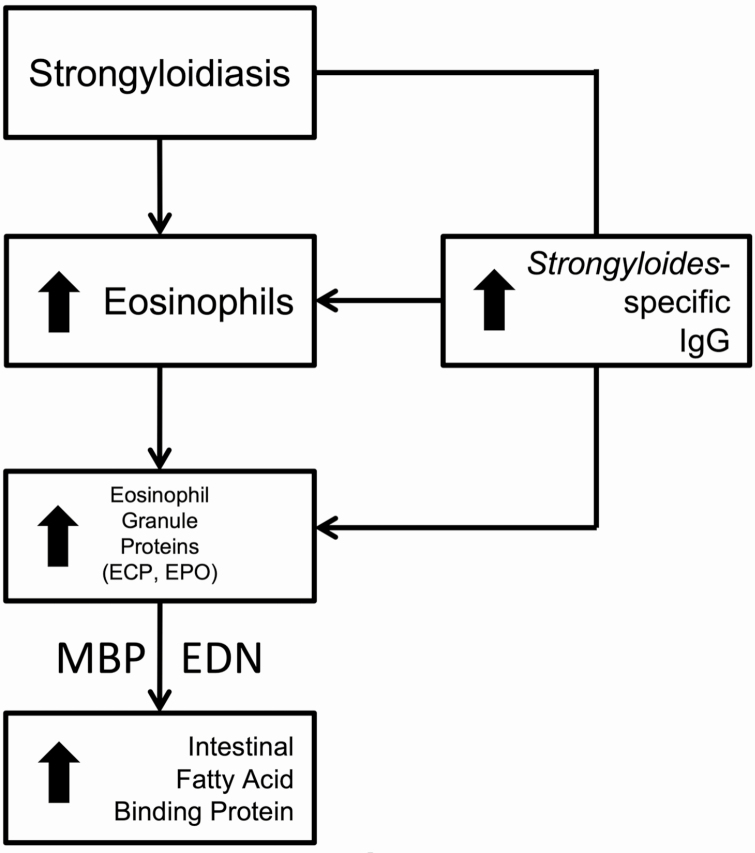
It is critical to diagnose and completely treat strongyloidiasis before immunosuppression in SOT patients to prevent the possibility of hyperinfection and lethal dissemination syndromes. As traditional diagnostic methods can be challenging in this vulnerable population due to their immunosuppressed state, it is essential to understand the mechanism of S. stercoralis intestinal pathophysiology in order to identify surrogate biomarkers of infection that may facilitate diagnosis. This study is the first to evaluate S. stercoralis seroprevalence, eosinophil activation, and intestinal inflammation in an SOT population. As a consequence of our findings, we propose a mechanistic approach to S. stercoralis pathophysiology in SOT patients (Figure 4) that will facilitate strongyloidiasis diagnosis. Those infected will have activated eosinophils, even in the absence of peripheral eosinophilia. These activated eosinophils release specific eosinophil granule proteins, which contribute to intestinal epithelial cell damage. Further study of these pathways will lead to improved strongyloidiasis diagnosis and management strategies in patients undergoing SOT evaluation, as well as a better understanding of Strongyloides-related intestinal inflammation, which likely complicates the SOT patient’s ability to absorb nutrients and medication and places their transplanted organ(s) at risk.
Figure 4.
Solid organ transplant patients with strongyloidiasis had elevated eosinophils and increased ECP and EPO, while MBP and EDN correlated with increasing IFABP. Increasing Strongyloides-specific IgG was associated with increasing eosinophils and ECP, EPO in Strongyloides-seropositive patients (P < .05). Abbreviations: ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPO, eosinophil peroxidase; IFABP, intestinal fatty acid–binding protein; IgG, immunoglobulin G; MBP, major basic protein.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ contributions. R. M. and A. Restrepo conceived of the study. R. M., A. Restrepo, and V. H. obtained funding for the study. A. Restrepo and V. H. recruited and consented patients and obtained clinical samples. E. C., H. P., and R. M. performed NIE Strongyloides ELISA testing. K. B. and R. M. performed Intestinal Fatty Acid Binding Protein. A. Ricciardi and T. B. N. performed eosinophil granule protein studies. E. C., V. H., H. P., A. D., and R. M. performed statistical analysis. E. C., H. P., V. H., and R. M. wrote the first draft of the manuscript. All co-authors contributed to manuscript revision and approved the final draft of the manuscript.
Acknowledgments. The authors thank Tamara Vaughn and the employees of the Baylor St Luke’s Medical Center laboratory for providing blood samples from eligible patients.
Financial support. This work was supported by The Roderick Duncan MacDonald Research Fund (grant number 17RDM006); the US Department of Health and Human Services, Health Resources and Services Administration for Baylor College of Medicine Center of Excellence in Health Equity, Training, and Research (grant number D34HP31024); the Texas Children’s Hospital Center for Vaccine Development; and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. R. M. reports funding from Romark Laboratories, LC. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 2009; 103:967–72. [DOI] [PubMed] [Google Scholar]
- 2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 2013; 7:e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 2001; 33:1040–7. [DOI] [PubMed] [Google Scholar]
- 4. Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep 2011; 13:35–46. [DOI] [PubMed] [Google Scholar]
- 5. Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 2013; 13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abanyie FA, Gray EB, Delli Carpini KW, et al. Donor-derived Strongyloides stercoralis infection in solid organ transplant recipients in the United States, 2009–2013. Am J Transpl 2015; 15:1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ostera G, Blum J, Cornejo C, et al. Strongyloidiasis in Latin American immigrants: a pilot study. J Helminthol 2017; 91:262–6. [DOI] [PubMed] [Google Scholar]
- 8. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 2013; 7:e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clemente WT, Pierrotti LC, Abdala E, et al. ; Working Group on Endemic Disease and Travel Medicine in Solid-Organ Transplantation Recommendations for management of endemic diseases and travel medicine in solid-organ transplant recipients and donors: Latin America. Transplantation 2018; 102:193–208. [DOI] [PubMed] [Google Scholar]
- 10. Abanyie FA, Valice E, Delli Carpini KW, et al. Organ donor screening practices for Strongyloides stercoralis infection among US organ procurement organizations. Transpl Infect Dis 2018; 20:e12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 2013; 7:e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luvira V, Trakulhun K, Mungthin M, et al. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg 2016; 95:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol 2002; 125:73–81. [DOI] [PubMed] [Google Scholar]
- 14. Bisoffi Z, Buonfrate D, Sequi M, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 2014; 8:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajamanickam A, Munisankar S, Bhootra Y, Dolla CK, Nutman TB, Babu S. Elevated systemic levels of eosinophil, neutrophil, and mast cell granular proteins in Strongyloides stercoralis infection that diminish following treatment. Front Immunol 2018; 9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McBrien CN, Menzies-Gow A. The biology of eosinophils and their role in asthma. Front Med (Lausanne) 2017; 4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 2003; 36:529–35. [DOI] [PubMed] [Google Scholar]
- 18. George PJ, Anuradha R, Kumar NP, Kumaraswami V, Nutman TB, Babu S. Evidence of microbial translocation associated with perturbations in T cell and antigen-presenting cell homeostasis in hookworm infections. PLoS Negl Trop Dis 2012; 6:e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nabha L, Krishnan S, Ramanathan R, et al. Prevalence of strongyloides stercoralis in an urban US AIDS cohort. Pathog Glob Health 2012; 106:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzpatrick MA, Caicedo JC, Stosor V, Ison MG. Expanded infectious diseases screening program for Hispanic transplant candidates. Transpl Infect Dis 2010; 12:336–41. [DOI] [PubMed] [Google Scholar]
- 21. Funaoka H, Kanda T, Kajiura S, Ohkaru Y, Fujii H. Development of a high-specificity sandwich ELISA system for the quantification of human intestinal fatty acid-binding protein (I-FABP) concentrations. Immunol Invest 2011; 40:223–42. [DOI] [PubMed] [Google Scholar]
- 22. Rajamanickam A, Munisankar S, Bhootra Y, Dolla C, Nutman TB, Babu S. Microbial translocation associated with an acute-phase response and elevations in MMP-1, HO-1, and proinflammatory cytokines in strongyloides stercoralis infection. Infect Immun 2017; 85. pii: e00772-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makiya MA, Herrick JA, Khoury P, Prussin CP, Nutman TB, Klion AD. Development of a suspension array assay in multiplex for the simultaneous measurement of serum levels of four eosinophil granule proteins. J Immunol Methods 2014; 411:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hauber HP, Galle J, Chiodini PL, et al. Fatal outcome of a hyperinfection syndrome despite successful eradication of Strongyloides with subcutaneous ivermectin. Infection 2005; 33:383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol 2016; 50:214–27. [DOI] [PubMed] [Google Scholar]
- 26. Buonfrate D, Sequi M, Mejia R, et al. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 2015; 9:e0003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol 1990; 144:3166–73. [PubMed] [Google Scholar]
- 28. Levy AM, Gleich GJ, Sandborn WJ, Tremaine WJ, Steiner BL, Phillips SF. Increased eosinophil granule proteins in gut lavage fluid from patients with inflammatory bowel disease. Mayo Clin Proc 1997; 72:117–23. [DOI] [PubMed] [Google Scholar]
- 29. Santos-Oliveira JR, Regis EG, Giacoia-Gripp CB, et al. Microbial translocation induces an intense proinflammatory response in patients with visceral leishmaniasis and HIV type 1 coinfection. J Infect Dis 2013; 208:57–66. [DOI] [PubMed] [Google Scholar]
- 30. Specht S, Saeftel M, Arndt M, et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun 2006; 74:5236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furuta GT, Nieuwenhuis EE, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol 2005; 289:G890–7. [DOI] [PubMed] [Google Scholar]
- 32. Yang D, Chen Q, Su SB, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 2008; 205:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mascarello M, Gobbi F, Angheben A, et al. Prevalence of Strongyloides stercoralis infection among HIV-positive immigrants attending two Italian hospitals, from 2000 to 2009. Ann Trop Med Parasitol 2011; 105:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.