Abstract
Background
Patients entering nursing facilities (NFs) are frequently colonized with antibiotic-resistant organisms (AROs). To understand the determinants of ARO colonization on NF admission, we applied whole-genome sequencing to track the spread of 4 ARO species across regional NFs and evaluated patient-level characteristics and transfer acute care hospitals (ACHs) as risk factors for colonization.
Methods
Patients from 6 NFs (n = 584) were surveyed for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis/faecium (VREfc/VREfm), and ciprofloxacin-resistant Escherichia coli (CipREc) colonization. Genomic analysis was performed to quantify ARO spread between NFs and compared to patient-transfer networks. The association between admission colonization and patient-level variables and recent ACH exposures was examined.
Results
The majority of ARO isolates belonged to major healthcare-associated lineages: MRSA (sequence type [ST] 5); VREfc (ST6); CipREc (ST131), and VREfm (clade A). While the genomic similarity of strains between NF pairs was positively associated with overlap in their feeder ACHs (P < .05 for MRSA, VREfc, and CipREc), limited phylogenetic clustering by either ACH or NF supported regional endemicity. Significant predictors for ARO colonization on NF admission included lower functional status and recent exposure to glycopeptides (adjusted odds ratio [aOR], > 2 for MRSA and VREfc/VREfm) or third-/fourth-generation cephalosporins (aOR, > 2 for MRSA and VREfm). Transfer from specific ACHs was an independent risk factor for only 1 ARO/ACH pair (VREfm/ACH19: aOR, 2.48).
Conclusions
In this region, healthcare-associated ARO lineages are endemic among connected NFs and ACHs, making patient characteristics more informative of NF admission colonization risk than exposure to specific ACHs.
Keywords: transmission, genomic epidemiology, antibiotic-resistant organisms, nursing facilities, surveillance
Using a combination of whole-genome sequencing, patient transfer, and clinical data, we discerned the dissemination of 4 high-priority antibiotic-resistant organisms (AROs) in the regional healthcare network, and epidemiolocal drivers underlying the high ARO importation rate into regional nursing facilities.
Healthcare facilities are hotspots for the emergence and spread of antibiotic-resistant organisms (AROs) [1]. Since the 1990s, the use of post–acute care facilities, such as nursing facilities (NFs), has grown dramatically to care for diverse and complex patient populations after discharge from acute care hospitals (ACHs) [2, 3]. NF patients are at particularly high risk of acquiring AROs because they tend to be older, with multiple comorbidities, and are often treated with antibiotics [4]. Recent studies reported that between 30% and 65% of NF patients are colonized with at least 1 ARO, and 4% of NF patients are diagnosed with an ARO infection during their stay [5–7].
Like in ACHs, there is ongoing endemic transmission of AROs within NFs. In addition to within-NF transmission, NF patients are often colonized when admitted. A recent Michigan-based multisite surveillance study found that more than half of NF patients were colonized with at least 1 ARO at the time of admission [8]. This study also provided empirical evidence supporting the importance of regional dissemination of AROs via patient transfer, as nearly all patients were directly admitted from ACHs. However, while a role for patient transfer is apparent, it remains unclear whether the high burden of ARO colonization entering NFs is more greatly influenced by the clinical characteristics of incoming patients or recent exposure to high-risk healthcare facilities whose infection prevention practices, antibiotic stewardship, and/or architectural design increase risk of ARO acquisition [9]. Understanding the relative contribution of these patient- and facility-level factors is essential for the design of optimal interventions to reduce ARO burden both at the level of individual healthcare facilities and across regional healthcare networks.
Here, we sought to improve our understanding of regional ARO dissemination by examining the distribution of ARO lineages across healthcare facilities and characterizing patient factors and prior healthcare exposures that are associated with the risk of colonization at NF admission. We focused our analysis on the 4 most common ARO species observed in our study NFs: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VREfc), VRE faecium (VREfm), and ciprofloxacin-resistant Escherichia coli (CipREc). All 4 pose serious threats to public health according to the Centers for Disease Control and Prevention [10]. We hypothesized that these AROs disseminate regionally with patient transfer, and that both patient clinical characteristics and recent ACH exposure contributed to the risk of colonization at the time of NF admission. By combining whole-genome sequencing (WGS), patient transfer data, and clinical metadata, we investigated the relationship between ARO transmission networks and patient movement patterns, and the relative contributions of patient-level risk factors and prior healthcare exposures to the high rates of importation of a diverse set of AROs into regional NFs.
METHODS
Overview of Study Design and Study Population
Leveraging surveillance cultures collected in a recent study [8], the current study used genomic and patient metadata to characterize the regional transmission and risk factors associated with the colonization of the 4 different ARO lineages simultaneously. At the time of funding, data from 584 patients (90% of total patients enrolled in the parent study; N = 651) were complete and included in the analysis. In brief, between November 2013 and October 2015, patients were enrolled from 6 NFs in southeast Michigan, shortly after admission (mean, 5.6 days [standard deviation, 3.0 days]; range, 0–14 days). Patient demographic information, clinical data, and recent healthcare exposure were gathered at enrollment. More than 95% of patients were directly discharged from an ACH. Details about antibiotic use prior to NF admission were manually extracted from medical records review. We focused our antibiotic analysis to the top 3 classes administered in this study population—namely, first/second-generation cephalosporins (n = 61), third/fourth-generation cephalosporins (n = 80), and glycopeptides (n = 73). Functional status was measured using the Physical Self-Maintenance Scale, ranging from full independence (score of 6) to full dependence (score of 30) in 6 categories of self-maintenance activities. Multiple body sites (hands, nares, oropharynx, feeding tube insertion site, suprapubic catheter site, groin, perianal area, and wounds) were cultured at enrollment, days 14 and 30, and monthly thereafter for up to 6 months. MRSA, VRE, and CipREc were isolated as described previously [8].
Genomic and Population Structure Analyses
For each ARO of interest, the earliest isolate from each patient was used for WGS and analyzed using a customized pipeline as described in detail in the Supplementary Materials. Sequence data are available under BioProject PRJNA435617.
We focused our analysis on dominant sequence types (STs) or genetically similar isolates that comprised the majority of the collection for each ARO species. In patients who were culture-negative at admission and became culture-positive during stay, their isolates were removed from subsequent analyses if they were closely related to another patient’s isolate from the same NF. WGS data allowed us to identify single-nucleotide variants between isolates within the same species, which were then used to estimate gene flow between NFs using an adaptation of Wright F statistic (Fsp) [11]. For Fsp analysis, only facilities with > 5 isolates were included. A low Fsp suggests that 2 NFs share a genetically homogenous population, and vice versa. Permutation tests were used to evaluate whether isolates were more likely to cluster by healthcare facility (ACH or NF) more than expected by chance alone. See the Supplementary Materials for detailed methods.
Patient Transfer Network Analysis
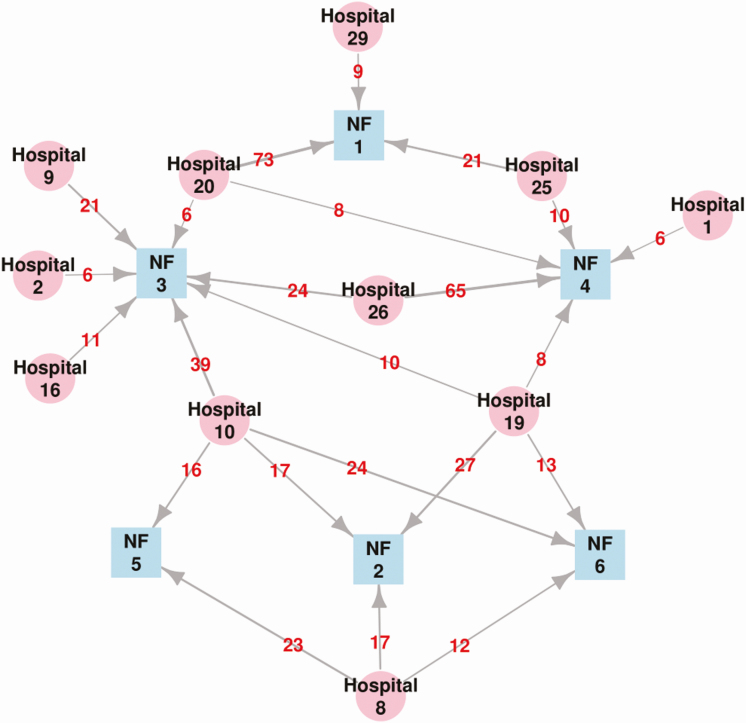
The identity of the discharge hospital of each enrolled patient was collected at the time of enrollment. The connectedness between NFs was calculated by quantifying the similarity in the distribution of discharge ACHs from where they received their patients using the Kullback-Leibler divergence method. In brief, the proportion of patients in a specific NF admitted from a set of ACHs was compared to that of patients in another NF. If 2 NFs had patients admitted from the same set of ACHs in identical proportions, the difference in patient transfer pattern would be zero for that NF pair (no divergence/dissimilarity), and vice versa. Only ACHs discharging at least 10 patients were included in the analysis. See the Supplementary Materials for R packages used for calculation.
Statistical Analysis
We used Spearman rank correlation to test the association between geographical distance, NF connectedness, and ARO genomic similarity. Logistic regression models were used to determine the odds ratios (ORs) and associated 95% confidence intervals (95% CIs) of risk factors. We constructed a separate model for each ARO and included colonized patients and patients not colonized with any ARO at enrollment as controls. We used 1-way analysis of variance to identify patient characteristics that differed significantly between ACHs. The final multivariate model for admission colonization was adjusted for risk factors with a P value of < .1 in univariate analyses. All statistical analyses were performed in R.
RESULTS
The Burden of High-priority ARO Species Was Due to Regional Dissemination of Epidemic Lineages
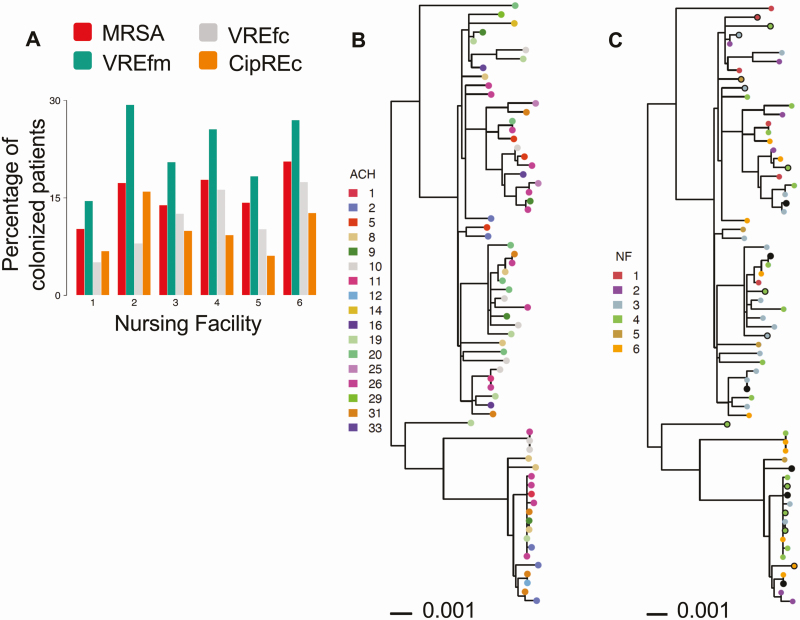
To characterize the strains circulating among regional healthcare facilities, we performed WGS on the first isolate of the 4 high-priority AROs cultured from each colonized patient (MRSA, n = 117; VREfm, n = 129; VREfc, n = 75; CipREc, n = 64). The majority of isolates for all 4 ARO species were collected upon admission (Supplementary Figure 1). Extraction of multilocus sequencing types (MLSTs) from WGS data revealed that the vast majority of isolates from all 6 NFs belonged to STs commonly associated with healthcare facilities (Supplementary Figure 2). In particular, 76.0% of MRSA isolates belonged to ST5 and closely related isolates (n = 89/117), 90.6% of CipREc isolates belonged to a subclade of ST131, ST43, or were closely related to ST43 (n = 58/64) [12], and 90.1% of VREfc isolates analyzed were ST6 (n = 68/75). While multiple STs were observed for VREfm, ST412, the most dominant ST in our VREfm population, belongs to the hospital-associated clade A [13]. The close genetic relatedness of isolates in our collection suggests that the VREfm strains circulating in regional facilities were also likely members of clade A [14] (Supplementary Figure 3). The presence of common ARO lineages in all 6 NFs over the 3-year study period suggests these ARO strains are endemic to the regional healthcare network (Figure 1A). This notion was further supported by the genetic intermixing of isolates from the current study and a previous surveillance conducted during May 2010–April 2013, involving 12 southeast Michigan NFs not included in the current study [15] (Supplementary Figure 4).
Figure 1.
High prevalence of antibiotic-resistant organisms (AROs) in regional nursing facilities due to endemic spread of epidemic lineages. A, Percentage of patients colonized with prevalent AROs within major healthcare lineages (methicillin-resistant Staphylococcus aureus [MRSA]: sequence type [ST] 5 and ST5-like; vancomycin-resistant Enterococcus faecalis [VREfc]: ST6; ciprofloxacin-resistant Escherichia coli [CipREc]: ST43/131 and ST43/131-like, and clade A vancomycin-resistant Enterococcus faecium [VREfm] isolates) at the time of enrollment or during nursing facility stay, by nursing facility. Each color corresponds to the colonization prevalence of a specific ARO. B and C, Phylogenetic tree of VREfc isolates labeled by patient’s most recent acute care hospital (ACH) exposure (B) and patient’s nursing facility (NF) residence (C) at the time of isolate detection. Isolates collected at the time of admission are shown as solid circles, and those collected during follow-up visits are shown as filled circles. Follow-up isolates pruned from analysis due to close genetic distance with an admission or earlier isolate within the same NF are shown as solid black circles. Sequence type of each isolate is shown on the right. The tree was inferred from maximum likelihood (RAxML) analysis with midpoint rooting. Scale bar represents substitutions per nucleotide site.
High Interconnectivity Among Study NFs Leads to a Lack of Genetic Clustering of ARO Strains by Facility
We next set out to determine if the strain-level resolution provided by WGS would allow tracking the spread of these endemic healthcare-associated lineages across regional facilities. To maximize our sampling of strain diversity within each NF, we included both enrollment and follow-up isolates that were phylogenetically distinct from other isolates within the same NF, as they may represent isolates acquired from patients not enrolled in the study, or were present at a level below limit of detection at enrollment. Regardless of the underlying basis, we included all phylogenetically nonredundant isolates in our subsequent analyses of regional transmission (Supplementary Figure 5; see Supplementary Methods for removal of redundant follow-up isolates).
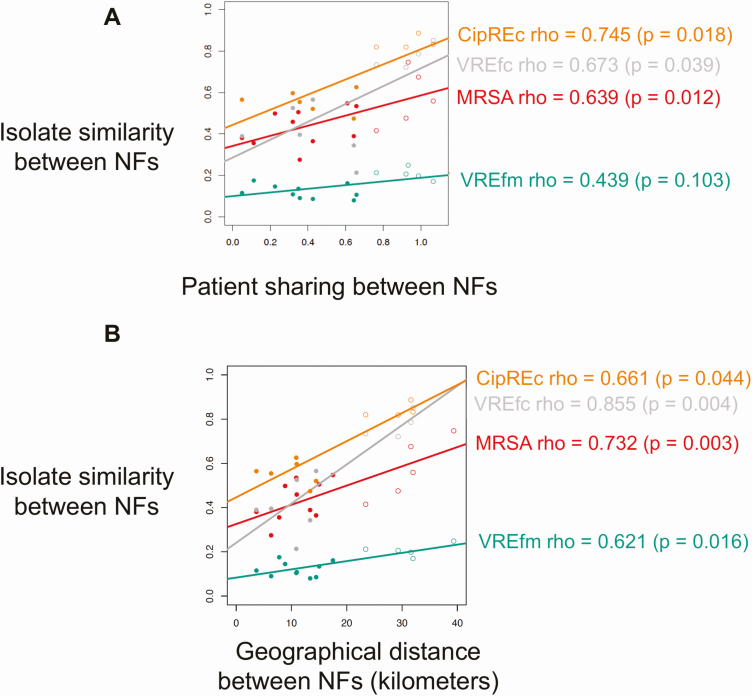
Based on the premise that patient transfer mediates regional spread, we hypothesized that NFs receiving patients from the same feeder ACHs would harbor more genetically similar strains. However, inspection of the core-genome phylogeny did not support this hypothesis, as strains appeared highly intermixed when labeled by feeder ACHs. The lack of clustering by facility was corroborated by phylogenetic permutation tests, which found no statistical support for clustering by most ACHs or NFs on any of the ARO core-genome phylogenies (Figure 1B and 1C showing VREfc phylogeny as a representative example; Supplementary Figures 6–8). To further evaluate the relationship between the genomic similarity of strains in different NFs and the overlap in their feeder ACHs, we computed the patient-sharing similarity between each pair of NFs and compared this to the genomic similarity of strains for each NF pair (Figure 2). While we observed a positive correlation between patient sharing and the genomic similarity of NF strains for each ARO lineage investigated (Spearman ρ = 0.44–0.75; P < .05 for MRSA, VREfc and CipREc, P = .10 for VREfm), we found that the correlation was heavily driven by NF 1, which was located geographically further from the other 5 NFs, and had less overlap in feeder ACHs (Figure 3; Supplementary Figures 9–11). This suggests that while patient transfer and geographical distance influence the genomic similarity of bacterial populations between healthcare facilities, for highly interconnected facilities that are geographically proximate, the frequent intermixing of prevalent ARO lineages can preclude a clear delineation of such relationships.
Figure 2.
Patient-sharing network between regional acute care hospitals (ACHs) and study nursing facilities (NFs). Visualization of patient sharing network involving 6 NFs (blue nodes) and 11 ACHs (pink nodes) in southeast Michigan, 2013–2016. Directed arrows represent patient flow from an ACH to an NF, with the number of patients transferred shown in red. Only ACH/NF pairs with ≥5 patients transferred are shown.
Figure 3.
Genomic relatedness among antibiotic-resistant organisms (AROs) isolates from different nursing facilities (NFs) associated with patient sharing and geographic proximity. Relationship between the genomic similarity of isolates between each pair of nursing facilities (NFs) and overlap in feeder acute care hospitals (ACHs) between the NF pair (A), and geographical distance between NF pair (B). Patient sharing between NFs (x-axis, A) indicates the extent of divergence in the proportion of patients from feeder ACHs between 2 NFs. Lower values indicate higher similarity. Isolate similarity (y-axis, A and B) indicates the divergence of the population structure of each ARO. Lower values indicate more genomic homogeneity between 2 NFs. Spearman rank correlation coefficients are shown on the right. The colors of the data points and regression lines correspond to different AROs. Closed circles denote NF pairs excluding facility 1; open circles denote NF pairs including facility 1. Abbreviations: CipREc, ciprofloxacin-resistant Escherichia coli; MRSA, methicillin-resistant Staphylococcus aureus; NF, nursing facility; VREfc, vancomycin-resistant Enterococcus faecalis; VREfm, vancomycin-resistant Enterococcus faecium.
Characteristics of Patients and Transfer Hospital Influence Risk for ARO Colonization on Admission to NFs
The above results suggest that the burden of ARO colonization on NF admission for all 4 tested ARO species is driven by transmission of epidemic lineages at connected healthcare facilities. We next sought to understand the factors that determine which specific patients are at increased risk for ARO colonization on NF admission. In particular, we hypothesized that patient risk will be influenced by a combination of the colonization pressure at connected ACHs (eg, high-risk feeder facilities) and patients harboring clinical features associated with risk of colonization (eg, high-risk patients). To delineate the contribution of patient- and facility-level factors to colonization risk, we constructed an individual multivariate regression model for each ARO species, including patient characteristics with a P < .1 in univariate analyses and recent ACH exposure. We focused on ACHs that discharged at least 50 patients to our 6 study NFs collectively. The remaining ACHs were collapsed into an “Other” group and served as the reference in analyses. Multivariate models for each individual ARO indicated a dominant role of patient factors, with lower functional status (adjusted odds ratio [aOR], > 1 for all 4 AROs), exposure to glycopeptides (aOR, > 2 for VREfm, VREfc, and MRSA) and exposure to third/fourth-generation cephalosporins (aOR, > 2 for MRSA and VREfm) being significant risk factors for colonization at admission (Table 1). After controlling for patient-level factors, transfer facility was a significant risk factor for only 1 ARO/ACH pair (VREfm/ACH19: OR, 2.48 [95% CI, 1.06–5.83]); the only other ARO/ACH pair with an OR > 2 was for CipREc/ACH10 (OR, 2.27 [95% CI, .82–6.46]). However, we note that while we treated antibiotic exposure as a patient-level factor, antibiotic use was significantly different among patients transferred from different ACHs (Table 2), which could be indicative of antibiotic prescribing patterns at connected ACHs influencing NF admission prevalence.
Table 1.
Associations Between Patient Characteristics, Facility Exposure, and Colonization With an Antibiotic-resistant Organism (ARO) Upon Admission to a Nursing Facility, by ARO
| Characteristic | Methicillin-resistant Staphylococcus aureus (n = 67) | Vancomycin-resistant Enterococcus faecium (n = 101) | Vancomycin-resistant Enterococcus faecalis (n = 49) | Ciprofloxacin-resistant Escherichia coli (n = 34) |
|---|---|---|---|---|
| Urinary catheter use in past 30 d | 1.37 (.7–2.6) | 1.69 (.94–3) | – | – |
| Lower functional status | 1.15 (1.08–1.23) | 1.08 (1.02–1.15) | 1.07 (1–1.14) | 1.11 (1.02–1.2) |
| Length of hospital stay | – | 1.06 (1.02–1.11) | – | – |
| Charlson comorbidity score | – | 1.05 (.91–1.19) | 1.17 (1.01–1.35) | – |
| Exposure to 3rd/4th-gen cephalosporins | 2.48 (1.03–5.81) | 3.96 (1.97–8.09) | – | – |
| Exposure to glycopeptides | 2.95 (1.23–6.93) | 2.77 (1.29–5.94) | 2.62 (1.02–6.41) | – |
| Hospital 8 | 0.63 (.19–1.8) | 1.18 (.45–2.94) | 0.43 (.09–1.43) | 0.7 (.1–3.03) |
| Hospital 10 | 0.77 (.32–1.8) | 0.83 (.35–1.9) | 0.59 (.22–1.47) | 2.27 (.82–6.46) |
| Hospital 19 | 1.3 (.44–3.54) | 2.48 (1.06–5.83) | 0.7 (.18–2.18) | 1.64 (.33–6.42) |
| Hospital 20 | 0.51 (.17–1.35) | 0.63 (.22–1.61) | 0.51 (.16–1.42) | 1.53 (.47–4.76) |
| Hospital 26 | 0.93 (.37–2.25) | 1.6 (.7–3.61) | 1.2 (.47–2.92) | 1.53 (.42–5.06) |
Data are presented as odds ratios (95% confidence intervals). An individual multivariate regression model was built separately for each antibiotic-resistant organism (ARO). “–” indicates that the covariate was not included in the final model because the P value was ≥ .1 in the univariate analysis. Risk factors significantly associated with ARO colonization at admission are bolded. Functional status was measured by physical self-maintenance score. Hospitals with < 50 discharges were collapsed and used as the referent group.
Table 2.
Clinical Characteristics of All Patients Discharged From Each Major Acute Care Hospital (≥50 Discharged Patients) and the Prevalence of Antibiotic-resistant Organism Colonization at Nursing Facility Admission, by Discharge Hospital
| Characteristic | Hospital 8 (n = 55) | Hospital 10 (n = 101) | Hospital 19 (n = 61) | Hospital 20 (n = 87) | Hospital 26 (n = 90) | Other Hospitals (n = 190) | P Value |
|---|---|---|---|---|---|---|---|
| Male sex, % | 45 | 43 | 44 | 44 | 36 | 42 | .844 |
| Urinary catheter use in past 30 d, % | 26 | 23 | 31 | 33 | 31 | 29 | .695 |
| Age, y, mean (SD) | 71.44 (13.81) | 71.71 (12.62) | 73.61 (12.96) | 77.56 (9.21) | 79.8 (10.46) | 74.34 (11.88) | < .001 |
| Physical self-maintenance scale, mean (SD) | 14.45 (5.06) | 14.01 (4.41) | 13.9 (4.09) | 13.98 (4.13) | 15.17 (4.79) | 14.28 (4.73) | .467 |
| Length of hospital stay, d, mean (SD) | 7.84 (5.61) | 6.58 (5.12) | 7.9 (5.77) | 6.16 (5.3) | 6.9 (4.31) | 7.52 (9.16) | .478 |
| Charlson comorbidity score, mean (SD) | 2.91 (1.89) | 3.39 (2.38) | 2.84 (1.9) | 2.13 (2.2) | 2.47 (1.97) | 2.23 (1.82) | < .001 |
| Black race, % | 96 | 71 | 72 | 0 | 7 | 22 | < .001 |
| Exposure to 1st/2nd-gen cephalosporins, % | 0 | 5 | 11 | 11 | 9 | 16 | .004 |
| Exposure to 3rd/4th-gen cephalosporins, % | 11 | 18 | 23 | 11 | 13 | 11 | .145 |
| Exposure to glycopeptides, % | 4 | 16 | 13 | 9 | 17 | 13 | .2 |
| MRSA colonization at discharge, % | 9 | 11 | 13 | 7 | 12 | 14 | .662 |
| VREfm colonization at discharge, % | 18 | 14 | 33 | 8 | 23 | 15 | .002 |
| VREfc colonization at discharge, % | 5 | 8 | 7 | 6 | 11 | 10 | .67 |
| CipREc colonization at discharge, % | 4 | 10 | 5 | 7 | 6 | 4 | .452 |
One-way analysis of variance was used to compare patient characteristics and antibiotic resistant–organism prevalence at different hospitals.
Abbreviations: CipREc, ciprofloxacin-resistant Escherichia coli; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation; VREfc, vancomycin-resistant Enterococcus faecalis; VREfm, vancomycin-resistant Enterococcus faecium.
DISCUSSION
The lack of effective infection prevention and antibiotic stewardship programs in NFs has been hypothesized to be a major driver of the high rates of antibiotic resistance [16, 17]. However, an overlooked contributor to antibiotic resistance in NFs is the high rates of patients colonized with AROs entering these facilities [8, 18]. Here, we focused on 6 regional NFs where the admission prevalence of ARO colonization was > 50%. To gain insights into the patient populations and transmission pathways mediating the high rates of ARO colonization on admission to these NFs, we integrated genomic analyses, patient transfer data, and clinical information. While our data support a role for patient transfer in regional dissemination of each studied ARO species, in these regional healthcare networks individual patient characteristics—in particular physical disability and antibiotic exposure—were better predictors of ARO colonization than specific transfer facility. We hypothesize that this observation is a consequence of long-term regional endemicity of epidemic ARO lineages making the risk for ARO exposure more even across regional facilities via the constant influx of colonized patients.
MLST analysis of each ARO species revealed that most isolates found in the 6 regional NFs in the current study, and the 12 NFs in a previous study, belonged to epidemic ARO lineages associated with healthcare settings, suggesting that these epidemic lineages have been stably circulating in the region over time. The endemic spread of these lineages across regional networks was further demonstrated by an overall lack of phylogenetic clustering by NF or transfer ACH. We hypothesize that this lack of clustering by facility is a reflection of the high rates of interfacility transmission leading to rapid movement of closely related strains. The rapid intermixing of strains across proximate healthcare facilities is further supported by the relative genetic isolation of ARO strains from NF 1, where the patient transfer pattern and geographical location made it most distant from the other NFs.
We observed that patient clinical characteristics were the primary risk factors for colonization with all 4 AROs on NF admission. Consistent with previous studies, physical disability and exposure to antibiotics were risk factors for colonization with each of the AROs studied, the latter likely driven by selection for resistant organisms (vancomycin/VRE and cephalosporins/MRSA). As antibiotic prescription patterns vary greatly across ACHs, this highlights the potential for regional antibiotic stewardship interventions to impact ARO colonization prevalence [19, 20]. We note that while patient characteristics and antibiotic exposure were dominant risk factors for ARO colonization, we did observe that recent exposure to ACH19 was an independent risk factor for VREfm colonization, suggesting that there were unmeasured facility-level factors that contributed to its association with high VREfm prevalence. This finding supports the premise that high-risk facilities in regional healthcare networks can be identified by performing admission screening at selected sentinel facilities and quantifying the independent contribution of recent facility exposures to ARO colonization risk [21].
We note several strengths in our study. First, the unique parent dataset allowed us to investigate the regional transmission of 4 different AROs simultaneously and assess the commonality of risk factors for colonization with different AROs. Second, the linkage of patient metadata to the curated patient movement data allowed us to empirically discern the risk factors for colonization unique to each organism. Importantly, curated patient transfer data allowed us to accurately assign each patient to their previous ACH, which may be difficult with aggregate data derived from billing records, especially when multiple campuses of the same hospital share the same provider identifier [22].
Our study has several limitations. First, the 6 NFs participating in the parent study were in metropolitan Detroit area within 40 km (25 mi) of each other. The close proximity of NFs, frequent patient transfer, and a lack of data on community exposures make it challenging to delineate with certainty the location where each patient acquired ARO colonization. In particular, it is possible that the patients in our study did not acquire their ARO strains during their most recent hospitalization, but remained persistently colonized following acquisition during a prior healthcare or community exposure [23]. This possibility could account for the lack of clustering of isolates by transfer ACH. Second, we had few data on recent community exposures, leaving unclear the potential role of the community networks in ARO spread. However, we note that most of the ARO lineages observed in study NFs are rarely observed among healthy individuals residing in the community, suggesting that these lineages preferentially spread in healthcare facilities [24–26]. Third, we only sequenced 1 isolate from each patient, potentially not capturing the full genetic diversity of the bacterial population. While our approach for comparing the genetic diversity of AROs between NFs would be unaffected by missing genetic variation unique to each patient’s colonizing population, the failure to capture multiple independent strain acquisitions could reduce our precision.
Together, the integration of genomic and patient transfer analyses in this study provided evidence that ARO burden was driven by epidemic lineages that were endemic across the regional healthcare network. Thus, to effectively disrupt the regional transmission of AROs, healthcare facilities will need to work together to identify high-risk patients and facilities, monitor epidemiological trends, and implement more effective communication strategies to control regional prevalence of these persistent resistance threats [21, 27, 28].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank members of the Mody laboratory for data collection and analysis; members of the Snitkin lab for critical discussion of genomic and epidemiological analyses; the Microbial Systems Molecular Biology Laboratory at the University of Michigan for performing whole-genome sequencing; and Drs Rachel Slayton, Nimalie Stone, Alexander Kallen, Hannah Wolford, and Paul Prabasaj (Centers for Disease Control and Prevention [CDC]) on their insightful feedback on patient transfer analysis.
Disclaimer. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Financial support. This work was supported by the CDC (contract number BAA 2016-N-17812 to E. S. S.); the National Institutes of Health (grant numbers R01 AG041780 and K24 AG050685 to L. M.); the Canadian Institutes of Health Research fellowship (grant number 201711MFE-396343-165736 to J. W.); the Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program (to J. W.); and the National Science Foundation Graduate Research Fellowship Program (grant number DGE 1256260 to Z. L.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–44. [DOI] [PubMed] [Google Scholar]
- 2. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post–acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med 2015; 175:295–6. [DOI] [PubMed] [Google Scholar]
- 3. Jenq GY, Tinetti ME. Post–acute care: who belongs where? JAMA Intern Med 2015; 175:296–7. [DOI] [PubMed] [Google Scholar]
- 4. Dumyati G, Stone ND, Nace DA, Crnich CJ, Jump RLP. Challenges and strategies for prevention of multidrug-resistant organism transmission in nursing homes. Curr Infect Dis Rep 2017; 19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKinnell JA, Singh RD, Miller LG, et al. The SHIELD Orange County Project: multidrug-resistant organism prevalence in 21 nursing homes and long-term acute care facilities in southern California. Clin Infect Dis 2019; 69:1566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantey J, Min L, Cassone M, Gibson KE, Mody L. Changing dynamics of colonization in nursing facility patients over time: reduction in methicillin-resistant Staphylococcus aureus (MRSA) offset by increase in vancomycin-resistant Enterococcus (VRE) prevalence. Infect Control Hosp Epidemiol 2019; 40:1069–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahvecioglu D, Ramiah K, McMaughan D, et al. Multidrug-resistant organism infections in US nursing homes: a national study of prevalence, onset, and transmission across care settings, October 1, 2010–December 31, 2011. Infect Control Hosp Epidemiol 2014; 35:S48–55. [DOI] [PubMed] [Google Scholar]
- 8. Mody L, Foxman B, Bradley S, et al. Longitudinal assessment of multidrug-resistant organisms in newly admitted nursing facility patients: implications for an evolving population. Clin Infect Dis 2018; 67:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drinka P, Niederman MS, El-Solh AA, Crnich CJ. Assessment of risk factors for multi-drug resistant organisms to guide empiric antibiotic selection in long term care: a dilemma. J Am Med Dir Assoc 2011; 12:321–5. [DOI] [PubMed] [Google Scholar]
- 10. CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 11. Donker T, Reuter S, Scriberras J, et al. Population genetic structuring of methicillin-resistant Staphylococcus aureus clone EMRSA-15 within UK reflects patient referral patterns. Microb Genom 2017; 3:e000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanza VF, de Toro M, Garcillán-Barcia MP, et al. Plasmid flux in Escherichia coli ST131 sublineages, analyzed by Plasmid Constellation Network (PLACNET), a new method for plasmid reconstruction from whole genome sequences. PLoS Genet 2014; 10:e1004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raven KE, Reuter S, Reynolds R, et al. A decade of genomic history for healthcare-associated Enterococcus faecium in the United Kingdom and Ireland. Genome Res 2016; 26:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Hal SJ, Ip CLC, Ansari MA, et al. Evolutionary dynamics of Enterococcus faecium reveals complex genomic relationships between isolates with independent emergence of vancomycin resistance. Microb Genom 2016; 2. doi:10.1099/mgen.0.000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mody L, Krein SL, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med 2015; 175:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mody L, Crnich C. Effects of excessive antibiotic use in nursing homes. JAMA Intern Med 2015; 175:1339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen CC, Pogorzelska-Maziarz M, Herzig CTA, et al. Infection prevention and control in nursing homes: a qualitative study of decision-making regarding isolation-based practices. BMJ Qual Saf 2015; 24:630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stone ND, Lewis DR, Lowery HK, et al. Importance of bacterial burden among methicillin-resistant Staphylococcus aureus carriers in a long-term care facility. Infect Control Hosp Epidemiol 2008; 29:143–8. [DOI] [PubMed] [Google Scholar]
- 19. MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infect Control Hosp Epidemiol 2008; 29:203–11. [DOI] [PubMed] [Google Scholar]
- 20. Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53:1100–10. [DOI] [PubMed] [Google Scholar]
- 21. Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb Mortal Wkly Rep 2015; 64:826–31. [PMC free article] [PubMed] [Google Scholar]
- 22. et al. Hospitals. 2019. Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Hospitals.html. Accessed 2 August 2019.
- 23. Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol 2012; 50:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–71. [DOI] [PubMed] [Google Scholar]
- 25. Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 2013; 34:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McBride SM, Fischetti VA, Leblanc DJ, Moellering RC Jr, Gilmore MS. Genetic diversity among Enterococcus faecalis. PLoS One 2007; 2:e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans CT, Jump RL, Krein SL, et al. Setting a research agenda in prevention of healthcare-associated infections (HAIs) and multidrug-resistant organisms (MDROs) outside of acute care settings. Infect Control Hosp Epidemiol 2018; 39:210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mody L, Washer L, Flanders S. Can infection prevention programs in hospitals and nursing facilities be integrated? From silos to partners. JAMA 2018; 319:1089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.