Abstract
Background
Persons who inject drugs (PWID) are at risk of invasive infections; however, hospitalizations to treat these infections are frequently complicated by against medical advice (AMA) discharges. This study compared outcomes among PWID who (1) completed a full course of inpatient intravenous (IV) antibiotics, (2) received a partial course of IV antibiotics but were not prescribed any antibiotics on AMA discharge, and (3) received a partial course of IV antibiotics and were prescribed oral antibiotics on AMA discharge.
Methods
A retrospective, cohort study of PWID aged ≥18 years admitted to a tertiary referral center between 01/2016 and 07/2019, who received an infectious diseases consultation for an invasive bacterial or fungal infection.
Results
293 PWID were included in the study. 90-day all-cause readmission rates were highest among PWID who did not receive oral antibiotic therapy on AMA discharge (n = 46, 68.7%), compared with inpatient IV (n = 43, 31.5%) and partial oral (n = 27, 32.5%) antibiotics. In a multivariate analysis, 90-day readmission risk was higher among PWID who did not receive oral antibiotic therapy on AMA discharge (adjusted hazard ratio [aHR], 2.32; 95% confidence interval [CI], 1.41–3.82) and not different among PWID prescribed oral antibiotic therapy on AMA discharge (aHR, .99; 95% CI, .62–1.62). Surgical source control (aHR, .57; 95% CI, .37–.87) and addiction medicine consultation (aHR, .57; 95% CI, .38–.86) were both associated with reduced readmissions.
Conclusions
Our single-center study suggests access to oral antibiotic therapy for PWID who cannot complete prolonged inpatient IV antibiotic courses is beneficial.
Keywords: substance abuse, opioid use disorder, endocarditis, osteomyelitis
We observed a reduction in 90-day readmission rates in PWID with invasive infections who left AMA who were prescribed oral antibiotic therapy, compared with those who were not. Readmission rates among those who received outpatient oral antibiotics were similar to those who completed inpatient IV antibiotic therapy.
The syndemic of opioids, stimulants, and other illicit substances is driving a concurrent epidemic of invasive infections, such as infective endocarditis and osteoarticular infections. Persons who inject drugs (PWID) are 16.3 times more likely to develop invasive Staphylococcus aureus infections than their peers and experience higher readmission and mortality rates for associated endovascular infections [1, 2]. It is estimated that 1 in every 10 invasive Candida infections in the United States is currently attributed to underlying injection drug use (IDU) [3]. In areas heavily affected by the opioid crisis, IDU-associated infective endocarditis incidence is as high as 13.8 per 100 000 persons [4]. However, despite the mounting death toll and expanding financial burden these infections pose, little evidence exists to support best practices for caring for PWID with invasive bacterial and fungal infections.
For non-PWID, invasive infections are generally managed acutely in the hospital, after which patients are discharged on outpatient parenteral antibiotic therapy to complete prolonged courses of intravenous (IV) antibiotics [5, 6]. Due to safety concerns of discharging PWID with central venous catheters, many infectious diseases (ID) specialists recommend that PWID remain in the hospital for 4–6 weeks to complete IV antibiotic therapy for invasive infections [7]. These admissions can be challenging for patients and healthcare professionals alike; even when underlying substance use disorders are treated, patients face significant consequences from prolonged hospital stays including loss of housing, childcare, or employment issues. Additionally, patients may be isolated and become increasingly depressed during the prolonged admission. For these reasons and others, PWID frequently leave against medical advice (AMA) prior to completion of antimicrobial therapy [4]. Among ID specialists, significant controversy exists on antibiotic treatment strategies in these situations. It is not uncommon for PWID who are unable to complete prolonged hospitalizations for IV antibiotics to leave AMA without being offered alternative antimicrobial options due to fears of inefficacy and/or noncompliance [7]. While integrating medications for opioid use disorder (MOUDs) into inpatient care is associated with a decreased risk of AMA discharge [8], prolonged hospitalizations may not be a realistic expectation for many PWID.
Recent clinical trials provide evidence supporting transition to oral antibiotic therapy to complete treatment of invasive bacterial infections [9, 10]. However, PWID were either excluded [9] or comprised less than 2% of patients studied [10]. Thus, prolonged courses of IV antibiotics remain the treatment of choice for ID specialists treating PWID [11, 12]. The objective of this study was to compare 3 antibiotic treatment strategies in PWID hospitalized with severe infectious complications of IDU: (1) a full course of inpatient IV antibiotic therapy, (2) a partial course of IV antibiotic therapy without prescription of oral antibiotic therapy on AMA discharge, and (3) a partial course of IV antibiotic therapy with prescription of oral antibiotic therapy on AMA discharge.
METHODS
Data Source and Study Design
We performed a retrospective chart review of PWID with an invasive bacterial infection admitted between 1 January 2016 and 30 July 2019 to Barnes-Jewish Hospital, a 1400-bed academic medical center in St Louis, Missouri, part of the BJC HealthCare system. Electronic medical records were reviewed for complications and readmissions during the 90 days following each index admission.
Inclusion and Exclusion Criteria
Hospital admissions for invasive infections among PWID were identified using International Classification of Diseases (ICD)-10 diagnosis codes as previously described [13]. A total of 1027 visits were identified during the study period as having one of the listed ICD codes. Due to the absence of a specific ICD code for IDU each chart was then individually chart reviewed (including admission notes, progress notes, and consult notes) by an ID physician to determine if the identified infection was related to IDU. A total of 307 qualifying encounters were identified in which the ID consult notes specifically stated that the infection was related to underlying IDU. We defined invasive infections as S. aureus bacteremia, infective endocarditis, epidural abscess, osteomyelitis, and septic arthritis. We only included admissions in which an ID consultation occurred, as this has been associated with improved patient outcomes [14–17].
Variables
Invasive infection type was identified by chart review of the ID specialist documentation. Infecting pathogens were verified by review of culture results, the most common pathogen being S. aureus (Supplementary Table 1). Staphylococcus aureus infection was defined as any infection type where S. aureus was identified from at least 1 sterile site culture. Duration of bacteremia was calculated as the difference in days between the first and last positive blood culture. The number of prior IDU-related infections was defined as the number of infections in the last 3 years captured via chart review prior to the index admission. Diabetes mellitus, hypertension, psychiatric comorbidities, and homelessness were obtained via chart review. Substance use history was captured via urine drug screen results and patient self-report as documented in the electronic medical record. Substance use was not mutually exclusive; patients who used multiple substances were included in each category. Hepatitis B, hepatitis C, and human immunodeficiency virus (HIV) serostatus were obtained via a combination of laboratory result and chart review. Recommended length of IV antibiotics was identified as the duration of therapy specified by the ID specialist. The percentage of IV antibiotic course completed was calculated by dividing the completed IV treatment course in the hospital by the recommended length of IV antibiotics. Surgical source control was defined as receiving a surgical procedure to address invasive infection. Addiction medicine consultation was defined as an inpatient consultation from a board-certified addiction medicine attending physician.
Our primary outcome was all-cause 90-day readmission. A readmission was defined as at least 1 admission to any of the 15 BJC Healthcare hospitals or the additional 20 local non-BJC hospitals in the St Louis metropolitan region within 90 days of the index admission.
Mortality within 90 days after discharge was identified by review of the electronic medical record, local obituaries, or the Social Security Death Index.
Statistical Analysis
Categorical variables are presented as frequencies and percentages. Continuous variables are presented as means with ranges. Comparisons were made between the groups using analysis of variance, Fisher’s exact tests, and post hoc analysis, as appropriate. We plotted unadjusted Kaplan-Meier estimates to describe the survival distribution for time to readmission and used these numbers to calculate the number needed to treat (NNT) to prevent 1 readmission. Univariate and multivariate regression analyses were performed to compare patient demographic characteristics and covariates between groups. We calculated odds ratios (ORs) with 95% confidence intervals (CIs) to determine predictors for completion of antibiotic therapy and 90-day readmission. A multivariate Cox proportional hazards model was generated using significant covariates from prefiltering univariate analysis (P < .20), which included HIV serostatus, prior IDU-related infections, surgical source control, addiction medicine consultation, and antibiotic treatment group, as follows: (1) those who received a full course of inpatient IV antibiotic therapy (completed inpatient IV), those who received a partial course of IV antibiotic therapy without prescription of oral antibiotic therapy on AMA discharge (partial IV, no oral), and (3) those who received a partial course of IV antibiotic therapy with prescription of oral antibiotic therapy on AMA discharge (partial IV, partial oral). In addition, homelessness and type of infection and infecting pathogen were included initially as clinically relevant variables. Covariates were assessed for violation of the proportional hazards assumption and assessed using log-negative-log survival plots. Backwards stepwise regression was used. Hazard ratios (HRs) and 95% CIs were calculated and reported. Descriptive statistics were calculated using SPSS version 26 (IBM Corporation). Figures were created using GraphPad Prism 9 (San Diego, CA). This study was approved by the Washington University Institutional Review Board.
RESULTS
Patient Characteristics
A total of 307 PWID admitted with invasive infections were identified during the study period. Fourteen patients died during the index inpatient encounter and were excluded as the impact of antibiotic treatment strategy on readmissions could not be assessed, leaving 293 patients for study inclusion (Table 1). All patients were offered standard-of-care inpatient IV antimicrobial regimens. Decisions regarding oral antibiotic therapy for patients leaving AMA were made by the ID consult team and offered on a case-by-case basis. An oral antibiotic with activity against the causative pathogen was identified in all but 1 case. One hundred and forty-three (48.8%) patients completed inpatient IV antibiotic therapy, 83 (28.3%) were treated with partial oral antimicrobial therapy after requesting to leave prior to completion of inpatient IV antibiotic therapy, and 67 (22.9%) received no oral antibiotic prescription prior to or within 48 hours of leaving AMA or eloping. Mean recommended length of therapy specified by an ID specialist was 6 weeks and did not significantly differ between groups (P = .952). The mean percentage of IV antibiotic therapy completed was found to be significant across the 3 treatment groups (P < .001), as those completing inpatient IV antimicrobial therapy completed a mean of 100% of the recommended antibiotic course. However, the mean percentage of IV antimicrobial completion for those not completing inpatient IV antimicrobials (either discharged with or without partial oral therapy) was not significantly different at 14.5 days for the “partial IV, partial oral” group and 12.5 days for the “partial IV, no oral” group (P = .082). A total of 139 (47.4%) patients were seen by addiction medicine physicians, with 69 (23.5%) patients prescribed buprenorphine-naloxone, 77 (26.2%) prescribed methadone, and 1 (0.3%) prescribed extended release naltrexone.
Table 1.
Comparison of 293 Persons Who Inject Drugs Admitted With Invasive Infections, by Antibiotic Treatment Received
| Completed Inpatient IV ( n = 143) | Partial IV, No Oral (n = 67) | Partial IV, Partial Oral (n = 83) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, mean (range), years | 40 (20–71) | 38 (20–71) | 39 (26–61) | .398 |
| Female gender | 65 (45.5) | 40 (59.7) | 40 (48.2) | .152 |
| African American race | 62 (43.4) | 25 (37.3) | 32 (38.6) | .425 |
| Homeless prior to admission | 16 (11.2) | 11 (16.4) | 12 (14.5) | .548 |
| Substance use historya | ||||
| No. of prior IDU-related infections (range) | 1.83 (0–7) | 2.25 (0–9) | 2.22 (0–16) | .295 |
| Heroin or fentanyl | 129 (90.2) | 59 (88.1) | 74 (89.2) | .892 |
| Cocaine | 32 (22.4) | 18 (26.9) | 25 (30.1) | .424 |
| Methamphetamine | 30 (21.0) | 24 (35.8) | 21 (25.3) | .072 |
| Benzodiazepine | 4 (2.8) | 2 (3.0) | 2 (2.4) | .975 |
| Comorbidities | ||||
| Hypertension | 19 (13.3) | 11 (16.4) | 5 (6.0) | .118 |
| Diabetes mellitus | 13 (9.1) | 2 (3.0) | 6 (7.2) | .281 |
| Psychiatric comorbidity | 13 (9.1) | 5 (7.5) | 13 (10.6) | .195 |
| Hepatitis C infection | 86 (60.1) | 49 (73.1) | 29 (65.1) | .186 |
| HIV infection | 3 (2.1) | 6 (9.0) | 3 (3.6) | .063 |
| Infection characteristics | ||||
| Infective endocarditis | 97 (67.8) | 38 (56.7) | 31 (37.3) | <.001 |
| Osteomyelitis | 32 (22.4) | 23 (34.3) | 41 (49.4) | .001 |
| Septic arthritis | 14 (9.8) | 16 (23.9) | 16 (19.3) | .018 |
| Epidural abscess | 15 (10.5) | 11 (16.4) | 8 (9.6) | .371 |
| Isolated bacteremia | 13 (9.1) | 3 (4.5) | 9 (10.8) | .364 |
| Staphylococcus aureus infection | 56 (60.8) | 53 (79.1) | 53 (63.9) | .030 |
| Duration of bacteremia, mean (range), days | 2.7 (0–16) | 2.3 (0–16) | 2.0 (0–16) | .238 |
| Admission characteristics | ||||
| Recommended length of IV antibiotic therapy, mean (SD), days | 41.0 (16) | 40.8 (13) | 40.0 (15) | .952 |
| % planned IV antibiotic course completed in the hospital, mean (range) | 100 (100–100)b | 30.2 (2.3–95.2) | 37.5 (2.2–97.6) | <.001 |
| Received surgical source control | 78 (54.5) | 15 (22.4) | 34 (41.0) | <.001 |
| Addiction medicine consultation | 85 (59.4) | 16 (23.9) | 38 (45.8) | <.001 |
| Outcomes | ||||
| Readmission | 45 (31.5) | 46 (68.7) | 27 (32.5) | <.001 |
| Length of stay for readmission, mean (range), days | 16.7 (1–90) | 14.1 (1–69) | 5.56 (1–16) | <.001 |
| Death within 90 days after discharge | 7 (4.9) | 3 (4.4) | 2 (2.4) | .489 |
Data are presented as n (%) unless otherwise indicated. Seventy-three (24.9%) patients had more than 1 type of invasive infection.
Abbreviations: HIV, human immunodeficiency virus; IDU, injection drug use; IV, intravenous.
aSubstance use history defined as per Methods. Individuals with more than 1 substance were included for each substance.
bReference category. % = column percentages.
Predictors of 90-Day Readmissions
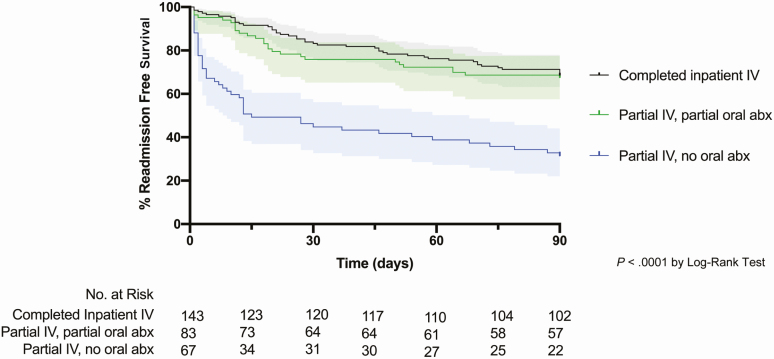
The 90-day readmission rates were highest among those who left prior to completion of IV antimicrobials and did not receive an oral antibiotic at discharge (partial IV, no oral) (P < .0001) (Table 1). The log-rank test for equality indicated a significant difference between the antibiotic treatment strategies observed in this cohort, with both completion of IV antibiotics (completed inpatient IV) and partial completion of IV antibiotics followed by prescription of oral antibiotic therapy on AMA discharge (partial IV, partial oral) associated with increased readmission-free survival in an unadjusted Kaplan-Meier survival curve (P < .0001) (Figure 1). Within the 2 groups who left prior to completing IV antibiotic therapy, the absolute risk reduction of 90-day readmission for partial oral antibiotic therapy (partial IV, partial oral) compared with no oral antibiotic therapy (partial IV, no oral) was 35.7% with an NNT of 3 (95% CI, 2–6).
Figure 1.
Kaplan-Meier survival curve of PWID admitted with invasive bacterial infections stratified by type of antimicrobial therapy. Abbreviations: abx, antibiotics; IV, intravenous; PWID, persons who inject drugs.
Univariate analysis identified AMA discharge without partial oral antibiotic therapy (partial IV, no oral) as a significant predictor of 90-day readmissions (OR, 4.77; 95% CI, 2.55–8.92), along with a history of prior IDU-associated infections (OR, 1.99; 95% CI, 1.18–3.36). Factors identified as protective from 90-day readmissions were multidisciplinary management with an addiction medicine specialist (OR, .39; 95% CI, .24–.64) and surgical source control (OR, .38; 95% CI, .23–.62) (Table 2).
Table 2.
Univariate Analysis of Variables Associated With 90-Day Readmission Among Persons Who Inject Drugs With Invasive Infections
| 90-Day Readmission | |||
|---|---|---|---|
| Variable | OR | 95% CI | P |
| Patient demographic characteristics | |||
| Age >50 years | 1.09 | .59–2.02 | .452 |
| Female | 1.23 | .77–1.96 | .230 |
| African American | .79 | .49–1.27 | .334 |
| Homeless | .92 | .46–1.83 | .475 |
| Substance use history | |||
| Prior IDU-related infection | 1.99 | 1.18–3.36 | .006 |
| Heroin or fentanyl | .69 | .32–1.46 | .217 |
| Cocaine | 1.53 | .90–2.60 | .075 |
| Methamphetamine | 1.32 | .78–2.25 | .184 |
| Benzodiazepine | .49 | .09–2.45 | .307 |
| Comorbidities | |||
| Hypertension | 1.47 | .72–2.98 | .188 |
| Psychiatric comorbidity | .79 | .37–1.73 | .355 |
| Diabetes mellitus | .73 | .28–1.86 | .334 |
| Hepatitis C infection | .88 | .54–1.43 | .343 |
| HIV infection | 2.14 | .66–6.92 | .158 |
| Infection characteristics | |||
| Infective endocarditis | 1.27 | .79–2.04 | .190 |
| Septic arthritis | .60 | .31 –1.18 | .093 |
| Isolated bacteremia | 1.41 | .62–3.21 | .269 |
| Osteomyelitis | .69 | .42–1.15 | .095 |
| Epidural abscess | .91 | .44–1.89 | .475 |
| Bacteremia | 1.02 | .64–1.64 | .516 |
| Staphylococcus aureus infection | .89 | .55–1.47 | .378 |
| Admission characteristics | |||
| Addiction medicine consult | .39 | .24–.64 | <.001 |
| Antibiotic treatment group | |||
| Completed inpatient IV | Reference | ||
| Partial IV, partial oral | 1.05 | .58–1.83 | .491 |
| Partial IV, no oral | 4.77 | 2.55–8.92 | .004 |
| Received surgical procedure | .38 | .23–.62 | <.001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; IV, intravenous; OR, odds ratio.
Adjusting for infection type and infecting pathogen, multivariable analysis identified a significantly lower readmission rate associated with surgical source control (adjusted HR [aHR], .57; 95% CI, .37–.87) and addiction medicine consultation (aHR, .57; 95% CI, .38–.86). Prior admission for IDU-related infections was associated with worse outcomes (aHR, 1.55; 95% CI, .99–2.41), as was AMA discharge without oral antibiotics (partial IV, no oral) (aHR, 2.32; 95% CI, 1.41–3.81). Patients who had received only partial IV antibiotic therapy and were discharged with oral antibiotics (partial IV, partial oral) did not have a significant difference in readmission rates compared with a reference group of patients completing inpatient IV antibiotic therapy (completed inpatient IV) (aHR, .99; 95% CI, .62–1.62) (Table 3).
Table 3.
Unadjusted and Adjusted Cox Proportional Hazards for 90-Day Readmissions Among Persons Who Inject Drugs With Invasive Infections
| Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P | |
| Addiction medicine consult | |||||
| No | Reference | Reference | |||
| Yes | .49 (.34–.72) | <.001 | .57 (.38–.86) | .007 | |
| Surgical source control | |||||
| No | Reference | Reference | |||
| Yes | .44 (.29–.66) | <.001 | .57 (.37–.87) | .009 | |
| Prior IDU infections | |||||
| No | Reference | Reference | |||
| Yes | 1.67 (1.10–2.58) | .016 | 1.55 (.99–2.41) | .051 | |
| Antibiotic treatment group | |||||
| Completed inpatient IV | Reference | Reference | |||
| Partial IV, partial oral | .92 (.57–1.48) | .730 | .99 (.62–1.62) | .995 | |
| Partial IV, no oral | 3.17 (1.97–5.12) | <.001 | 2.32 (1.41–3.82) | .001 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; IDU, injection drug use; IV, intravenous.
aAdjusted for infection type and infecting pathogen.
Reasons for 90-Day Readmissions
Supplementary Table 2 identifies reasons for 90-day readmissions stratified by antimicrobial treatment group. Readmissions for new sequelae of the previously identified infection were not significantly different between the partial oral antibiotic group (partial IV, partial oral) and those who completed inpatient IV antibiotic therapy (completed inpatient IV). For many PWID in the inpatient IV antibiotic group, surgical source control was previously recommended but did not occur due to comorbid diagnoses or other reasons. This is consistent with our finding that surgical management was protective against 90-day readmissions (Table 2). All treatment groups had readmissions for infections secondary to new microorganisms, likely representing ongoing IDU and the high rate of infectious complications among PWID. More detailed descriptions are provided in Supplementary Table 3. Consultation with addiction medicine was noted to be protective against readmission with a new microorganism (P < .001).
DISCUSSION
Our study has 2 key findings. First, we observed a reduction in 90-day all-cause readmission rates in patients who left AMA who were prescribed oral antibiotic therapy (partial IV, partial oral), compared with those who were not (partial IV, no oral). Second, patients who received outpatient oral antibiotics (partial IV, partial oral) had similar readmission rates compared with those who competed their course of inpatient IV antibiotic therapy (completed inpatient IV). The frequent exclusion and/or underrepresentation of PWID in clinical trials has led to many content experts and guidelines excluding PWID from treatment recommendations. The result is limited data and generally conservative patient management with parenteral antibiotics. Investigations of oral antibiotics for infective endocarditis first began in the 1950s, followed by treatment success in osteomyelitis in the 1960s and 1970s [18–21]. Given the recent randomized controlled trials reported by Iversen et al [9] and Li et al [10] demonstrating that oral antibiotics can be used effectively to treat endocarditis and osteomyelitis, access to oral antibiotic therapies for PWID with invasive infections appears to be a viable treatment option. With an NNT of 3 to prevent 90-day readmission, our data provide real-world evidence that access to oral antibiotics for PWID who leave AMA is safe and is associated with improved outcomes compared with not receiving oral antibiotics, with similar readmission rates compared with those completing IV antibiotic therapy.
Inpatient care for PWID with invasive infections should include care of the underlying substance use disorder [22]. In our study, treatment of underlying opioid use disorder (OUD) had a similar protective effect against readmission as adequate surgical source control, which is a central principle of infectious diseases. The inpatient encounter for an IDU-associated infection represents a critical opportunity to discuss harm-reduction techniques including safer injection practices, cleaning injection sites, and access to clean needles. For patients who inject opioids, MOUDs should be offered and initiated early in the hospital course. We have previously found that MOUD is an independent predictor of retention in inpatient care [8]. However, even when MOUDs are offered, hospital stays of 4–6 weeks for parenteral therapy can cause significant social challenges.
Discharges AMA are independently associated with increases in 30-day readmissions and mortality [23]. The prevalence of AMA discharge is understandably higher among PWID, with national rates as high as 30% [24]. For PWID there is significant stigma surrounding the term AMA. Many physicians may express more bias against those who leave AMA, perceiving them as less engaged with their healthcare, positing that it seems illogical to offer patients “second best” advice, while others hold onto a misconception that medications cannot be prescribed for an AMA discharge [25]. In many settings PWID may be uninsured and require financial assistance for oral antibiotics, a situation that is often challenging to overcome if patients are discharged AMA. The term “unplanned discharge” or “incomplete discharge” has recently been suggested as a more patient-centered term for AMA discharges in this population [26]. Several patients in this cohort discharged on oral antibiotic therapy re-presented to care soon after discharge when they found the cost of filling the prescription provided was prohibitive. Considering potential oral antibiotic options early in a hospital stay may help overcome some of these issues. When using oral antibiotics for severe infections, care should be given to maximizing bioavailability, tolerability, and affordability. Oral antibiotics should not be used simply to expedite discharges in settings where they would not be similarly considered for non-PWID, but instead should be offered with careful planning and with close follow-up. These results demonstrate that a decision to leave prior to completion of inpatient IV antibiotic therapy by PWID admitted with an invasive bacterial or fungal infection should not be viewed as a rejection of all medical care.
We believe a holistic, patient-centered approach to the care of PWID with invasive infections is needed. Prolonged hospital stays in this high-risk patient group could result in losing employment and housing [27]. Elements to consider include an assessment of a patient’s social situation occurring early in a patient’s hospital course, consultation with addiction medicine specialist, with MOUDs, when present. Treatment of OUD should occur during hospitalization and linkage to ongoing care should be arranged at the time of discharge. Longitudinal access to health navigators and coaches to aid PWID with accessing support services is seen as increasingly important [28]. These individuals help ensure that patients continue to engage with the outpatient healthcare system and improve medication adherence. Multidisciplinary teams have been shown to exert a positive impact on the care of non-PWID with infective endocarditis, and it is clear that similar comprehensive management strategies should be offered to PWID with analogously complex invasive infections [29, 30].
Limitations to our findings are that results may not be generalizable to other institutions. The PWID at our hospital had access to addiction medicine consultations, and PWID with OUD had access to MOUDs, although services for persons who inject methamphetamines are more limited. All patients received an ID consultation, which is known to improve outcomes [15, 16]. The PWID who left AMA were offered close outpatient ID follow-up, providing the opportunity to adjust antibiotic therapy for patients who were intolerant or unable to afford certain recommended antibiotics. Our analyses were not sufficiently powered to explore whether certain conditions were safer than others to rapidly transition patients from IV to oral antibiotics.
In conclusion, our single-center study supports the concept of holistic management of PWID, including providing MOUDs, offering oral antibiotics for patients who leave AMA, and engaging in shared decision making with patients about the benefits of IV versus oral antibiotics. We believe that additional studies specifically evaluating the role and impact of health navigators, case managers, therapists, and addiction medicine providers are needed to identify key bundle components while containing costs. We believe that this would be best accomplished by funding prospective clinical trials including adaptive trial designs exploring implementation of evidence-based practices and pragmatic antimicrobial options within the PWID population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (grant numbers KL2TR002346, CRTCUL1RR024992, and T32AI007172) in addition to the National Institute on Drug Abuse (grant number K12 DA041449–02) and the Barnes Jewish Hospital Foundation (grant number 4479).
Potential conflicts of interest. D. K. W. was a sub-investigator for an industry research study for Pfizer Inc, and reports consulting fees from Centene Corp and Pursuit Vascular Inc, and advisory board fees from Carefusion/BD and PDI Inc, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jackson KA, Bohm MK, Brooks JT, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs—six sites, 2005–2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leahey PA, LaSalvia MT, Rosenthal ES, Karchmer AW, Rowley CF. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis 2019; 6:ofz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barter DM, Johnston HL, Williams SR, Tsay SV, Vallabhaneni S, Bamberg WM. Candida bloodstream infections among persons who inject drugs—Denver metropolitan area, Colorado, 2017–2018. MMWR Morb Mortal Wkly Rep 2019; 68:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meisner JA, Anesi J, Chen X, Grande D. Changes in infective endocarditis admissions in Pennsylvania during the opioid epidemic. Clin Infect Dis 2020; 71:1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 6. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61:e26–46. [DOI] [PubMed] [Google Scholar]
- 7. Rapoport A, Fischer L, Santibanez S, Beekmann S, Polgreen P, Rowley C. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5:ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marks LR, Munigala S, Warren DK, et al. A comparison of medication for opioid use disorder treatment strategies for persons who inject drugs with invasive bacterial and fungal infections. J Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019; 380:415–24. [DOI] [PubMed] [Google Scholar]
- 10. Li HK, Rombach I, Zambellas R, et al. ; OVIVA Trial Collaborators Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serota DP, Niehaus ED, Schechter MC, et al. Disparity in quality of infectious disease vs addiction care among patients with injection drug use-associated Staphylococcus aureus bacteremia. Open Forum Infect Dis 2019; 6:ofz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serota DP, Barocas JA, Springer SA. Infectious complications of addiction: a call for a new subspecialty within infectious diseases. Clin Infect Dis 2020; 70:968–72. doi: 10.1093/cid/ciz804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis 2019; 68:1935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spec A, Olsen MA, Raval K, Powderly WG. Impact of infectious diseases consultation on mortality of cryptococcal infection in patients without HIV. Clin Infect Dis 2017; 64:558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto S, Iwata K. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 47:432; author reply: 3. [DOI] [PubMed] [Google Scholar]
- 16. Mejia-Chew C, O’Halloran JA, Olsen MA, et al. Effect of infectious disease consultation on mortality and treatment of patients with Candida bloodstream infections: a retrospective, cohort study. Lancet Infect Dis 2019; 19:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green JH. Cloxacillin in treatment of acute osteomyelitis. Br Med J 1967; 2:414–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feigin RD, Pickering LK, Anderson D, Keeney RE, Shackleford PG. Clindamycin treatment of osteomyelitis and septic arthritis in children. Pediatrics 1975; 55:213–23. [PubMed] [Google Scholar]
- 20. Quinn EL, Colville JM, Cox F Jr, Truant J. Phenoxymethyl penicillin (penicillin V) therapy of subacute bacterial endocarditis. J Am Med Assoc 1956; 160:931–6. [DOI] [PubMed] [Google Scholar]
- 21. Gray IR, Tai AR, Wallace JG, Calder JH. Treatment of bacterial endocarditis with oral penicillins. Postgrad Med J 1964; 40SUPPL:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Serota DP, Vettese T. New answers for old questions in the treatment of severe infections from injection drug use. J Hosp Med 2019; 14:E1–7. [DOI] [PubMed] [Google Scholar]
- 23. Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med 2010; 25:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ti L, Ti L.. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health 2015; 105:e53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med 2013; 8:574–7. [DOI] [PubMed] [Google Scholar]
- 26. Seval N, Eaton E, Springer SA. beyond antibiotics: a practical guide for the infectious disease physician to treat opioid use disorder in the setting of associated infectious diseases. Open Forum Infect Dis 2020; 7:ofz539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus 2019:1–7. doi: 10.1080/08897077.2019.1671942 [DOI] [PubMed] [Google Scholar]
- 28. Jack HE, Oller D, Kelly J, Magidson JF, Wakeman SE. Addressing substance use disorder in primary care: the role, integration, and impact of recovery coaches. Subst Abus 2018; 39:307–14. [DOI] [PubMed] [Google Scholar]
- 29. Kaura A, Byrne J, Fife A, et al. Inception of the “endocarditis team” is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart 2017; 4:e000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis 2019; 6:ofz308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.