Abstract
Background
Patients with multiple recurrent Clostridioides difficile infections (rCDI) are treated with fecal microbiota transplantation (FMT), using feces provided by healthy donors. Blastocystis colonization of donors is considered an exclusion criterion, whereas its pathogenicity is still under debate.
Methods
The introduction of molecular screening for Blastocystis sp. at our stool bank identified 2 donors with prior negative microscopies but positive polymerase chain reactions (PCRs). Potential transmission of Blastocystis sp. to patients was assessed on 16 fecal patient samples, pre- and post-FMT, by PCR and subtype (ST) analyses. In addition, clinical outcomes for the treatment of rCDI (n = 31), as well as the development of gastrointestinal symptoms, were assessed.
Results
There was 1 donor who carried Blastocystis ST1, and the other contained ST3. All patients tested negative for Blastocystis prior to FMT. With a median diagnosis at 20.5 days after FMT, 8 of 16 (50%) patients developed intestinal colonization with Blastocystis, with identical ST sequences as their respective donors. Blastocystis-containing fecal suspensions were used to treat 31 rCDI patients, with an FMT success rate of 84%. This success rate was not statistically different from patients transferred with Blastocystis sp.–negative donor feces (93%, 76/82). Patients transferred with Blastocystis sp.–positive donor feces did not report any significant differences in bowel complaints in the first week, after 3 weeks, or in the months following FMT.
Conclusions
We demonstrated the first transmission of Blastocystis ST1 and ST3 from donors to patients by FMT. This did not result in gastrointestinal symptomatology or have any significant effect on rCDI treatment outcomes.
Keywords: fecal microbiota transplantation, Blastocystis sp, Clostridioides difficile, CDI, donor screening
Transmission of Blastocystis by fecal microbiota transplantation (FMT) from colonized donors occurred in 50% of patients. Transfer did not result in development of gastrointestinal symptoms or affect the outcome of the FMT treatment in patients with recurrent Clostridioides difficile infections.
Blastocystis is a genus of a common unicellular intestinal parasite in humans and animals that belongs to the stramenopiles, 1 of the 8 major phylogenetic groups of eukaryotes. It is a diverse genus comprising 17 characterized lineages: the so-called subtypes (ST1 – ST17), of which 9 have been reported to occur in the human gastrointestinal tract [1, 2]. Blastocystis sp. carriage is very common but varies globally, from 0.5% in Japan to 100% in Senegal and 30–50% in Europe [3–6].
The pathogenicity of Blastocystis sp. is uncertain and, in general, it is considered an innocent parasite [7]. The presumed entero-pathogenicity is based on anecdotal case reports and retrospective reviews and is mainly tested in animal models [8, 9]. The symptoms attributed to this organism include nausea, anorexia, abdominal pain, flatulence, and acute or chronic diarrhea [8]. However, outbreaks have never been reported and a human challenge model has not been applied. An association of Blastocystis sp. with irritable bowel syndrome was suggested [10, 11], but could not be confirmed in 2 large cohort studies [4, 12]. Interestingly, Blastocystis sp. is found to be less prevalent in patients with inflammatory bowel disease, a disorder which is associated with a reduced diversity of the gut microbiota [4, 13, 14], and asymptomatic Blastocystis sp. carriers tend to have a more diverse microbiota [4, 15–20]. These observations could indicate that the presence of Blastocystis sp. may reflect a more healthy and diverse state of the gut microbiota.
Patients with multiple recurrent Clostridioides difficile infections (rCDIs) are treated with fecal microbiota transplantation (FMT), prepared with feces of healthy donors. Carriership of Blastocystis sp. by healthy donors is considered an exclusion criterion for donation by several stool banks, including the Netherlands Donor Feces Bank (NDFB) [21–26], resulting in considerable exclusion of donors (30-50%). It is questionable whether this is justified. Therefore, knowledge about the potential side effects and treatment success of cotransplantation of Blastocystis sp. with FMT is warranted. This study reports the cotransmission of Blastocystis sp. from donor to patient, and its influence on the outcomes and health of rCDI patients receiving FMT.
METHODS
Donors and Donor Fecal Suspensions for Fecal Microbiota Transplantation
The NDFB is located within the Department of Medical Microbiology at the Leiden University Medical Center, and started with the treatment of patients with multiple rCDI with FMTs in 2016 [21]. All donors of the NDFB are healthy individuals between the ages of 18 and 50, with normal weight (body mass index, 18.5–25) and no relevant medical history or medication use. All donors are extensively screened and rescreened for disorders associated with a perturbed microbiota and potential transmissible infectious diseases [21].
The NDFB uses standardized procedures for the collection, preparation, and storage of donor fecal suspensions, as described previously [21]. In short, donors deliver stool at the NDFB within 2 hours after defecation. It takes 60 grams of donor feces to prepare 1 fecal suspension. The feces are homogenized with sterile saline with the use of a mortar and pestle, sieved, and centrifuged until an end volume of 200 ml (containing 10% glycerol). Then, 2 cc of the final fecal suspension and 2 grams of the original donor stool are separately aliquoted and stored as quality controls. The fecal suspensions are stored within 6 hours following defecation. Storage is accommodated by a certified, centralized biobanking facility in a dedicated −80°C freezer with connected alarm notification and biobanking information and management system (BIMS SampleNavigator).
Patient Selection and Treatment
Requests for FMT in rCDI patients are carefully evaluated by the working group of the NDFB. Upon approval, the NDFB facilitates FMT by providing ready-to-use fecal suspensions for treatment at the local hospital, as previously described [21]. Patients are preferably pretreated with vancomycin (125–250 mg means 4 times each day) for a minimum of 4 days, followed by 2 liters of macrogol solution (bowel lavage) 1 day prior to FMT. The thawed fecal suspension is slowly infused through a duodenal tube, or via a colonoscopy in selected patients.
Follow-Up
The routine follow-up of patients consists of a standardized questionnaire filled out 3 weeks post-FMT by their local, treating physician and a telephonic interview performed by a member of the NDFB working group at 2 months post-FMT. For this study, an additional telephonic interview was performed in January 2019, between 5 to 33 months post-FMT. In addition, treating physicians were asked to contact the NDFB in case of any adverse events or treatment failures. Success of FMT was defined as the resolution of CDI symptoms without a relapse of CDI within 2 months. A relapse of CDI was defined as the development of diarrhea for at least 2 consecutive days within 2 months following FMT, either in combination with a positive free-feces toxin test or polymerase chain reaction (PCR; proven relapse), or clinical suspicion for CDI (probable). A CDI episode occurring at a later time point than 2 months post-FMT was regarded as a new CDI episode, as proposed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) C. difficile treatment guideline [27]. The development of gastrointestinal and other adverse events was also assessed, including nausea, vomiting, burping, abdominal pain, diarrhea not caused by rCDI, obstipation, hospital admittance, and antibiotic use, and we included an open field for other complaints. In addition, participants were asked to evaluate their defecation pattern post-FMT, compared to pre-FMT (improved, similar, or deteriorated).
Stool samples of patients were collected before and approximately 3 weeks after FMT. Stool samples were preserved until use at −80°C. Patients provided informed consent for the collection of stool samples and outcome data of FMT for research purposes, which was approved by the Medical Ethics Committee at the Leiden University Medical Center (P15.145).
Blastocystis sp. Diagnostics and Typing
Stool samples of the donors were routinely screened for the presence of Blastocystis sp. by direct microscopy of the feces and the Ridley-Allen sedimentation method [28]. These screenings were performed on fresh donor stool (<2 hours after defecation). With the introduction of a specific Blastocystis PCR at our department in 2018, 2 donors were identified with negative microscopies but positive PCRs for Blastocystis sp. In retrospect, all donated fecal samples used to treat patients were tested for the presence of Blastocystis sp. with a specific PCR targeting approximately 360 bp of the small subunit ribosomal RNA gene (see Supplementary Material). Positive samples were subtyped using a sequence analysis, as described previously [29]. Furthermore, 16 available pre- and post-FMT fecal samples of the patients treated by these 2 respective donors were tested with Blastocystis sp. PCR and, when positive, were subsequently subtyped. Patients and donors that were PCR positive for Blastocystis sp. were regarded as Blastocystis sp. colonized.
Statistics
The statistical analysis was performed using SPSS 23.0 statistical software. To test for differences between the prevalence rates of relapses and gastrointestinal symptoms of Blastocystis sp.–positive versus –negative donors and patients, a Chi-square test or Fischer exact was performed in cases of n < 5. An odds ratio (OR) was calculated using logistic regression and presented with a 95% confidence interval (CI). For ordinal data, a linear-by-linear association test was used. In addition, Kaplan-Meier curve and log-rank tests to compare CDI-free survival rates between patients receiving Blastocystis sp.–positive or –negative donor feces were performed. For statistical comparisons, a P value below .05 was considered statically significant.
RESULTS
Blastocystis sp.–Positive Donors
In the period between May 2016 and December 2018, 110 patients were treated with 113 FMTs, using fecal suspensions of 10 donors. In 2 out of 10 donors, Blastocystis sp. testing revealed a negative stool microcopy but, in retrospect, a positive PCR, with cycle quantification values ranging from 18.95 to 25.13 (Table 1). A subtype analysis revealed that 1 donor had Blastocystis ST1 and the other donor had ST3. The Blastocystis ST1 donor carried the Blastocystis for at least 3 donating months, and the second donor carried the Blastocystis ST3 for at least 9 donating months.
Table 1.
Details of Donor to Patient Transfer of Blastocystis Subtypes 1 and 3 by Fecal Microbiota Transplantation
| Donors | Recipients Pre-FMT | Recipients Post-FMT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Donor ID | Subtype of Blastocystis | Blastocystis Cq Value | Feces Collection, Days Pre-FMT | Patient ID | Blastocystis Status Pre-FMT | Feces Collection, Days Post-FMT | Blastocystis Status Post-FMT | Blastocystis Cq Value | Subtype of Blastocystis | Colonization With Blastocystis Due to FMT |
| A | ST1 | 25.13 | 119 | 1 | Neg | 21 | Neg | n/a | n/a | No |
| A | ST1 | 23.57 | 199 | 2 | Neg | 21 | Pos | 25.05 | ST1 | Yes |
| B | ST3 | 24.19 | 43 | 3 | Neg | 20 | Pos | 22.28 | ST3 | Yes |
| B | ST3 | 20.16 | 34 | 4 | Neg | 5 | Neg | n/a | n/a | No |
| B | n/aa | n/a | 66 | 5 | Neg | 18 | Pos | 22.57 | ST3 | Yes |
| B | ST3 | 19.51 | 64 | 6 | Neg | 53 | Pos | 27.64 | ST3 | Yes |
| B | ST3 | 18.95 | 119 | 7 | Neg | 15 | Pos | 27.77 | ST3 | Yes |
| B | ST3 | 20.94 | 124 | 8 | Neg | 20 | Neg | n/a | n/a | No |
| B | ST3 | 19.81 | 140 | 9 | Neg | 48 | Pos | 25.78 | ST3 | Yes |
| B | ST3 | 23.21 | 152 | 10 | Neg | 20 | Neg | n/a | n/a | No |
| B | ST3 | 21.11 | 255 | 11 | Neg | 31 | Neg | n/a | n/a | No |
| B | ST3 | 21.68 | 360 | 12 | Neg | 29 | Neg | n/a | n/a | No |
| B | ST3 | 21.68 | 376 | 13 | Neg | 23 | Neg | n/a | n/a | No |
| B | ST3 | 19.96 | 385 | 14 | Neg | 20 | Pos | 23.86 | ST3 | Yes |
| B | n/ab | n/a | 509 | 15 | Neg | 20 | Neg | n/a | n/a | No |
| B | ST3 | 20.29 | 521 | 16 | Neg | 27 | Pos | 19.56 | ST3 | Yes |
Abbreviations: Cq, cycle quantification; FMT, fecal microbiota transplantation; ID, identification; n/a, not available or not applicable; Neg, negative; Pos, positive; ST, subtypes.
aTransplanted donor feces were not available; samples 6 days prior and 2 days post-FMT were positive with Blastocystis ST3.
bTransplanted donor feces were not available; samples 30 days prior and 3 days post-FMT were positive with Blastocystis ST3.
Patients Treated With Blastocystis sp. Containing Fecal Microbiota Transplantation Suspensions
Donor feces suspensions of Blastocystis sp.–positive donors were used for rCDI treatment of 31 patients; 4 patients were treated with donor feces containing Blastocystis ST1 and 27 with Blastocystis ST2. From 16 of 31 patients, stool samples pre-FMT and post-FMT were available. All fecal samples of the patients tested Blastocystis sp.–negative prior to FMT (Table 1). With a median of 20.5 days (5–53 days) post-FMT, 8 of 16 (50%) patients developed intestinal colonization with Blastocystis: 7 of 14 with ST3 and 1 of 2 with ST1 (Table 1). Patient DNA sequences of part of the Blastocystis small subunit ribosomal RNA region were 100% identical to the sequences of their respective donors.
Patient Follow-Up for Recurrent Clostridioides difficile Infections Treatment
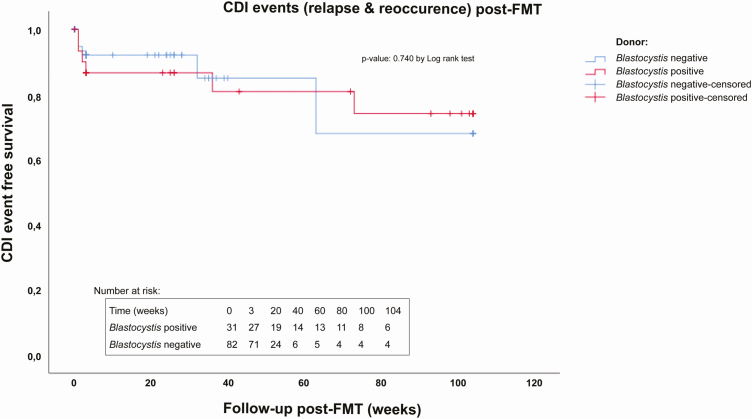
Of the 113 FMTs performed in 110 patients to cure rCDI, 31 FMTs were performed with feces from the Blastocystis sp.–positive donors and 82 with Blastocystis sp.–negative donor feces. Patients treated with Blastocystis sp.–positive donor feces had an FMT success rate (cure without relapse <2 months) of 84% (26/31), whereas treatment with Blastocystis sp.–negative donor feces had a success rate of 93% (76/82). This difference in success rates was not significant (Table 2; Figure 1). Moreover, no significant difference in the numbers of confirmed (3 versus 3) and probable CDI relapses (2 versus 3) was found (OR, 1.5; 95% CI, .14–16.54; P value = 1). Of a total of 11 relapses of CDI, 3 were challenged by antibiotic treatment, whereas 8 (5 in Blastocystis-positive and 3 in Blastocystis-negative treated patients) developed a relapse without antibiotics as a predisposing factor. The ST1 and ST3 Blastocystis sp.–positive donor fecal suspensions were used for the treatment of 4 and 27 rCDI patients, respectively. Treatment with feces of the Blastocystis sp. ST1 donor resulted in a treatment success of 75% (1/4), whereas the ST3 donor had a success rate of 85% (4/27; OR, 0.522; 95% CI, .04–6.36; P value = .525). In addition, no difference was found in the relapse rates between patients with (12.5%, 1/8) or without (0%, 0/8) Blastocystis sp. colonization following FMT with a donor suspension containing Blastocystis sp. (OR, 1.143; 95% CI, .88–1.49; P value = 1).
Table 2.
Follow-Up of Recurrent Clostridioides difficile Infection Fecal Microbiota Transplantation Treatment Success of Patients Transferred With Blastocystis sp.–Positive Versus –Negative Donor Feces
| Patients Outcome | Blastocystis sp.–Positive Donor Feces | Blastocystis sp.–Negative Donor Feces | Significance, OR [95% CI], P value |
|---|---|---|---|
| FMT success rate | 83.9% (26/31) | 92.7% (76/82) | 0.411 [.12, 1.46], P value = .159 |
| Relapses of CDI | 16.1% (5/31) | 7.3% (6/82) | 2.436 [.69, 8.65], P value = .159 |
| New CDI episode, >2 months after FMT | 9.7% (3/31) | 7.3% (6/82) | 1.357 [.32, 5.80], P value = .704 |
| CDI event: relapse or new episode | 25.8% (8/31) | 14.6% (12/82) | 2.029 [.74, 5.88], P value = .165 |
Percentages and final ORs with 95% CIs of the FMT treatment outcome between patients treated with Blastocystis sp.–positive versus –negative donor feces. A χ2 test or Fischer exact test was performed in cases of n < 5.
Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval; FMT, fecal microbiota transplantation; OR, odds ratio.
Figure 1.
Kaplan-Meier curve of CDI event-free survival in patients post-FMT who were treated with Blastocystis sp.–positive versus Blastocystis sp.–negative fecal suspensions. CDI-free survival is defined as survival without a relapse (<2 months post-FMT) or new CDI infection (>2 months post-FMT) within 2 years (104 weeks) after FMT. Follow-up data exceeding 2 years were censored at 104 weeks. Patients suffering from a new CDI event after 104 weeks were counted as having no CDI event. Abbreviations: CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
There were 9 (8.0%, 9/113) patients who experienced a new episode of CDI later than 2 months after FMT, at a median of 4 months (range 63–402 days) post-FMT. All new episodes could be attributed to the initiation of antibiotic treatment shortly before the development of CDI symptoms. The frequency of development of a new initial episode of CDI was not statistically different in patients transferred with Blastocystis sp.–positive feces (9.7%, 3/31), versus Blastocystis sp.–negative feces (7.3%, 6/82; Table 2; Figure 1). Moreover, no statistically significant difference in the development of a new initial CDI episode was found between patients transferred with ST1 (0%, 0/4) and ST3 (11.1% 3/27; OR, 0.889; 95% CI, .78–1.02; P value = 1), or between patients that were demonstrably colonized with Blastocystis post-FMT using Blastocystis-containing donor feces (12.5%, 1/8), versus those demonstrably Blastocystis negative post-FMT (0%, 0/8; OR, 1.143; 95% CI, .88–1.49; P value = 1).
Potential Side Effects Due to Newly Acquired Blastocystis sp. Colonization Following Fecal Microbiota Transplantation
Compared to patients treated with Blastocystis sp.–negative donor feces, patients treated with Blastocystis sp.–positive donor feces did not report significantly more bowel complaints (nausea, abdominal pain, or diarrhea) after 1 week, after 3 weeks, or at the long-term follow-up (median, 35 weeks; range, 10–143 weeks; Table 3). Moreover, no difference in side effects was observed in the subgroup of patients with demonstrable Blastocystis sp. colonization after FMT. Interestingly, a significant difference towards an improvement of the self-evaluated defecation pattern was observed at long-term follow-up in patients receiving Blastocystis sp.–positive donor feces (Table 3).
Table 3.
Potential Side Effects Due to Newly Acquired Blastocystis sp. Infections After Fecal Microbiota Transplantation
| FMT With Blastocystis sp.–Negative Donor, n = 82 | FMT With Blastocystis sp.–Positive Donor, n = 31 | Blastocystis sp. Colonized Post-FMT, n = 8a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Side Effect | Week 1 | Weeks 2 + 3 | LTFU | Week 1 | Weeks 2 + 3 | LTFU | Week 1 | Weeks 2 + 3 | LTFU |
| Nausea, % yesa | 11.0% (9/69) | 12.2% (10/70) | 35.0% (7/20) | 13.0% (3/23) | 3.2% (1/23) | 12.5% (2/16) | 0.0% (0/8) | 0.0% (0/8) | 0.0% (0/3) |
| Abdominal pain, % yesb | 22.0% (18/70) | 18.3% (15/71) | 27.8% (5/18) | 34.8% (8/23) | 16.1% (5/23) | 25.0% (3/12) | 25.0% (2/8) | 12.5% (1/8) | 33.3% (1/3) |
| Diarrheab | 32.9% (23/70) | 22.0% (18/70) | 35.0% (7/20) | 26.1% (6/23) | 26.1% (6/23) | 25.0% (4/16) | 0.0% (0/8) | 37.5% (3/8) | 33.3% (1/3) |
| Defecation pattern | |||||||||
| Improved | n/a | 16.1% (9/56) | 17.6%c (3/17) | n/a | 13.6% (3/22) | 53.8%c (7/13) | n/a | 12.5% (1/8) | 33.3% (1/3) |
| Similar | n/a | 67.9% (38/56) | 58.8%c (10/17) | n/a | 68.2% (15/22) | 38.5%c (5/13) | n/a | 62.5% (5/8) | 66.7% (2/3) |
| Worsened | n/a | 16.1% (9/56) | 23.5%c (4/17) | n/a | 18.2% (4/22) | 7.7%c (1/13) | n/a | 25.0% (2/8) | 0.0% (0/3) |
The LTFU median duration was 35 weeks, and the range was 10–143 weeks.
Abbreviation: FMT, fecal microbiota transplantation; LTFU, long-term follow-up.
aA subgroup of patients receiving Blastocystis sp.–positive fecal suspensions with proven intestinal colonization of Blastocystis sp. post-FMT.
bPrevalences of nausea, abdominal pain, or diarrhea were not significantly different between the groups, as tested with either a χ2 or Fischer exact test in cases of n < 5.
cA statistically significant difference in the self-evaluated defecation pattern at LTFU between patients that received Blastocystis sp.–positive versus Blastocystis sp.–negative donor feces, as tested by a χ2 linear-by-linear test (P = .043).
DISCUSSION
Healthy stool donors colonized with Blastocystis sp. are usually excluded from FMT donorship [21–26], though the enteropathogenicity of Blastocystis sp. remains debatable [7]. Through a combination of PCR and subtyping techniques of donors and of patient pre-FMT and post-FMT fecal samples, the first human-to-human transmission by FMT of Blastocystis sp. ST1 and ST3 was described. This transmission did not influence the success rate of the FMT to treat rCDI. More importantly, it did not result in gastrointestinal symptomatology of the recipients.
Symptoms attributed to Blastocystis sp. infection that have been described in anecdotal case reports, series, and retrospective cohorts include nausea, anorexia, abdominal pain, flatulence, and acute or chronic diarrhea [8]. The high prevalence of Blastocystis sp. colonization in healthy individuals suggests that Blastocystis sp. does not harm most hosts. As Blastocystis consists of 17 subtypes, initially the idea was raised that the subtype correlated with pathogenicity [30]. Numerous, globally performed studies comparing the subtypes of Blastocystis could not confirm a consistent correlation and could not explain the pathogenicity in some patients [30]. Currently, it is mostly acknowledged that Blastocystis sp. may colonize many hosts, but the infection’s potential depends on the interplay between the virulence of the parasite, the number of infecting parasites present, the duration of infection (acute versus chronic), and host factors like genetics, immune competence, or gut microbiota composition [3, 4, 20, 30, 31]. The 2 identified subtypes in this study, ST1 and ST3, are the most commonly found subtypes in Europe and the Netherlands [3]. In a Dutch study in which the stool samples of 442 patients were evaluated by routine parasitological examination, 107 (24%) stool samples contained Blastocystis sp., of which 40% had Blastocystis ST3 and 21% had Blastocystis ST1 [3]. The sustained colonization with Blastocystis ST1 and ST3 observed in 50% (median, 20.5 days) of Blastocystis-transferred patients in this study did not result in gastrointestinal symptomatology, as determined by patient follow-up questionnaires. In contrast, these Blastocystis sp.-transferred patients evaluated their defecation pattern as being significantly better post-FMT, compared to patients receiving Blastocystis sp.–negative donor feces.
Unfortunately, a human challenge model to study the presumed enteropathogenicity of Blastocystis sp. has not been described [7]. In our study, the transfer of Blastocystis sp. was accompanied by a healthy donor microbiota. This may not reflect the effects of Blastocystis sp. transfers from individuals with intestinal complaints or a disturbed microbiota to individuals with a healthy microbiota. Interestingly, Blastocystis sp. may not be able to maintain itself in a dysbiotic rCDI microbiota, since we found that none of the rCDI patients carried Blastocystis sp. pre-FMT. Low Blastocystis sp. colonization rates in diseased individuals were previously also reported in patients with active inflammatory bowel disease or hepatic encephalopathy [4, 13, 14, 32]. These diseased individuals and rCDI patients have a perturbed gut microbiota in common. Whether the association between a perturbed microbiota and low Blastocystis sp. colonization results from an absence of Blastocystis sp. or from the inability of Blastocystis to colonize and sustain itself in a dysbiotic gut microbiota composition is an interesting question that merits further research.
In this study, the importance of performing appropriate Blastocystis sp. diagnostics is shown. The NDFB used microscopy on unfixed material and used Ridley-Allen sedimentation to detect Blastocystis sp., in contrast to the more superior techniques, which use microscopy on 2 sodium acetate formalin-fixated stool samples or molecular detection of a single stool sample [3]. Blastocystis sp. colonization of the donors or patients was, therefore, defined by positive PCR, irrespective of microscopic findings. Post-FMT stool samples with a positive Blastocystis sp. PCR were taken more than 2 weeks post-FMT. Together with the relatively low cycle quantification values (high load) found in these rCDI patients post-FMT, this suggests actual Blastocystis colonization instead of Blastocystis passage after FMT.
There is no consensus among FMT centers and stool banks about Blastocystis sp. screening of donors, though published guidelines still recommend screening, especially for immunocompromised patients [24]. Many centers do not screen for Blastocystis sp. and, according to a recent systemic review, only 14.5% of 168 studies reported specific Blastocystis sp. screening [33]. In addition, the method of screening for ova and parasites was often not stated [21–26]. Consequently, we assume that a substantial number of patients has received FMT treatment for rCDI or other diseases in experimental settings, with cotransplantation with Blastocystis sp.
Our study is the first study that indicates that Blastocystis sp. transmission does not result in gastrointestinal symptoms in recipients. In the setting of rCDI, the transmission of Blastocystis ST1 and ST3 via FMT did not result in a significant decrease in the efficacy of FMT, although there was a nonsignificant trend towards an increased rate of CDI events (both relapses and new episodes) in patients treated with Blastocystis sp.–positive donors (8/31) versus Blastocystis sp.–negative donors (12/82). Interestingly, this contrasts with expected outcomes that could have extrapolated from recent metagenomic studies, in which Blastocystis sp. is correlated with a more diverse and healthier microbiota, a general prerequisite of a good donor [4, 15–20]. In a large cohort of 1106 healthy Flemish individuals, Blastocystis sp. carriership was associated with higher microbial diversity, richness, and composition. Tito et al [4] found that the most common subtypes in Europe—ST1, ST2, ST3, and ST4—were all associated with higher diversity, though ST1 and ST3 (which were identified in our study) had lower diversity increases than ST2 and ST4. For FMT treatment of rCDI, super donors have not been detected [34, 35] and all donors display a high cure rate, of around 85% [21]. The role of super-donors could play a more significant role in possible future FMT indications other than rCDI, such as ulcerative colitis, metabolic syndrome, the eradication of multidrug resistant organisms, or hepatic encephalopathy [4, 36, 37].
In this study, only the transfer of Blastocystis ST1 or ST3 was studied. To assess the contribution of Blastocystis sp. transfers to FMT success, it is important to include microbiota data of donors and patients, other subtypes of Blastocystis, and longer-term follow-up, as colonization has been described for up to 6–10 years [38]. An important limitation of this study is voluntary reporting by the treating physicians of late CDI relapses (after 3 weeks) or new CDI episodes (after 2 months) to the NDFB. However, physicians had a low threshold to contact the NDFB, since an excellent relationship was developed during the entire process of the FMT request and treatment of the patient.
In conclusion, to the best of our knowledge we demonstrate the first transmission of Blastocystis ST1 and ST3 from donor to recipient via FMT without the development of gastrointestinal symptoms. This study is an important step towards a possible exemption of Blastocystis sp. (ST1 and ST3) as a donor exclusion criterion in FMT.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. B. and E. J. K. contributed equally to this work. The Netherlands Donor Feces Bank (NDFB) study group is Elisabeth M. Terveer and Karuna E. W. Vendrik (Department of Medical Microbiology, Center for Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands), Rogier Ooijevaar (Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, Vrije Universiteit University Medical Center, Amsterdam, The Netherlands), Emilie van Lingen (Department of Gastroenterology, Haaglanden Medical Center, Den Haag, The Netherlands), Eline Boeije-Koppenol (Department of Medical Microbiology, Center for Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands), Joffrey van Prehn (Department of Medical Microbiology, Center for Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands), Yvette van Beurden (Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, VU University Medical Center, Amsterdam, The Netherlands), Martijn P. Bauer (Department of Internal Medicine, Leiden University Medical Center, Leiden, The Netherlands), Els van Nood (Department of Internal Medicine, Erasmus Medical Center, Rotterdam, The Netherlands), Abraham Goorhuis (Department of Internal Medicine, Amsterdam University Medical Centers, Amsterdam Medical Center, Amsterdam, The Netherlands), Jos F. M. L. Seegers, Marcel G. W. Dijkgraaf (Clinical Research Unit, Amsterdam University Medical Centers, Amsterdam, The Netherlands), Chris J. J. Mulder (Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, VU University Medical Center, Amsterdam, The Netherlands), Christina M. J. E. Vandenbroucke-Grauls (Department of Medical Microbiology & Infection Control, Amsterdam University Medical Centers, VU University Medical Center, Amsterdam, The Netherlands), Hein W. Verspaget (Department of Biobanking and Gastroenterology, Leiden University Medical Center, Leiden, The Netherlands), Ed J. Kuijper (Department of Medical Microbiology, Center for Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands), and Josbert J. Keller (Department of Gastroenterology, Leiden University Medical Center, Leiden, The Netherlands; Department of Gastroenterology, Haaglanden Medical Center, Den Haag, The Netherlands).
Acknowledgements. The authors thank Patricia E. Broekhuizen–van Haaften for her excellent technical support.
Financial support. This work was supported by the Netherlands Organization for Health Research and Development, Netherlands Organization for Health Research and Development (ZonMW) Verspreidings-en implementatie impuls number 1708810011).
Potential conflicts of interest. E. M. T., J. J. K., and E. J. K. report grants from ZonMW, Netherlands Society of Gastroenterology, during the conduct of this study, and an unrestricted research grant from Vedanta, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Netherlands Donor Feces Bank (NDFB) Study Group:
Elisabeth M Terveer, Karuna E W Vendrik, Rogier Ooijevaar, Lingen Emilie van, Eline Boeije-Koppenol, Joffrey van Prehn, Yvette van Beurden, Martijn P Bauer, Els van Nood, Abraham Goorhuis, Jos F M L Seegers, Marcel G W Dijkgraaf, Chris J J Mulder, Christina M J E Vandenbroucke-Grauls, Hein W Verspaget, Ed J Kuijper, and Josbert J Keller
References
- 1. Stensvold CR, Suresh GK, Tan KS, et al. . Terminology for Blastocystis subtypes–a consensus. Trends Parasitol 2007; 23:93–6. [DOI] [PubMed] [Google Scholar]
- 2. Tan KS. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 2008; 21:639–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bart A, Wentink-Bonnema EM, Gilis H, et al. . Diagnosis and subtype analysis of Blastocystis sp. in 442 patients in a hospital setting in the Netherlands. BMC Infect Dis 2013; 13:389. doi:10.1186/1471-2334-13-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tito RY, Chaffron S, Caenepeel C, et al. . Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019; 68:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Safadi D, Gaayeb L, Meloni D, et al. . Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect Dis 2014; 14:164. doi:10.1186/1471-2334-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horiki N, Maruyama M, Fujita Y, Yonekura T, Minato S, Kaneda Y. Epidemiologic survey of Blastocystis hominis infection in Japan. Am J Trop Med Hyg 1997; 56:370–4. [DOI] [PubMed] [Google Scholar]
- 7. Andersen LO, Stensvold CR. Blastocystis in health and disease: are we moving from a clinical to a public health perspective? J Clin Microbiol 2016; 54: 524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sohail MR, Fischer PR. Blastocystis hominis and travelers. Travel Med Infect Dis 2005; 3:33–8. [DOI] [PubMed] [Google Scholar]
- 9. Moe KT, Singh M, Howe J, et al. . Experimental Blastocystis hominis infection in laboratory mice. Parasitol Res 1997; 83:319–25. [DOI] [PubMed] [Google Scholar]
- 10. Rostami A, Riahi SM, Haghighi A, Saber V, Armon B, Seyyedtabaei SJ. The role of Blastocystis sp. and Dientamoeba fragilis in irritable bowel syndrome: a systematic review and meta-analysis. Parasitol Res 2017; 116:2361–71. [DOI] [PubMed] [Google Scholar]
- 11. Poirier P, Wawrzyniak I, Vivares CP, Delbac F, El Alaoui H. New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLOS Pathogens 2012; 8:e1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krogsgaard LR, Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin Gastroenterol Hepatol 2015; 13:507–13.e2. [DOI] [PubMed] [Google Scholar]
- 13. Rossen NG, Bart A, Verhaar N, et al. . Low prevalence of Blastocystis sp. in active ulcerative colitis patients. Eur J Clin Microbiol Infect Dis 2015; 34:1039–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen AM, Stensvold CR, Mirsepasi H, et al. . Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand J Gastroenterol 2013; 48:638–9. [DOI] [PubMed] [Google Scholar]
- 15. Andersen LO, Bonde I, Nielsen HB, Stensvold CR. A retrospective metagenomics approach to studying Blastocystis. FEMS Microbiol Ecol 2015; 91. doi:10.1093/femsec/fiv072 [DOI] [PubMed] [Google Scholar]
- 16. Audebert C, Even G, Cian A, et al. . Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep 2016; 6:25255. doi:10.1038/srep25255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forsell J, Bengtsson-Palme J, Angelin M, Johansson A, Evengard B, Granlund M. The relation between Blastocystis and the intestinal microbiota in Swedish travellers. BMC Microbiology 2017; 17:231. doi:10.1186/s12866-017-1139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iebba V, Santangelo F, Totino V, et al. . Gut microbiota related to Giardia duodenalis, Entamoeba spp. and Blastocystis hominis infections in humans from Cote d’Ivoire. J Infect Dev Countr 2016; 10:1035–41. [DOI] [PubMed] [Google Scholar]
- 19. Nash AK, Auchtung TA, Wong MC, et al. . The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017; 5(1):153. doi:10.1186/s40168-017-0373-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nieves-Ramirez ME, Partida-Rodriguez O, Laforest-Lapointe I, et al. . Asymptomatic intestinal colonization with protist Blastocystis is strongly associated with distinct microbiome ecological patterns. mSystems 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terveer EM, van Beurden YH, Goorhuis A, et al. . How to: establish and run a stool bank. Clin Microbiol Infect 2017; 23:924–30. [DOI] [PubMed] [Google Scholar]
- 22. Panchal P, Budree S, Scheeler A, et al. . Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep 2018; 20:14. doi:10.1007/s11894-018-0619-8 [DOI] [PubMed] [Google Scholar]
- 23. Woodworth MH, Carpentieri C, Sitchenko KL, Kraft CS. Challenges in fecal donor selection and screening for fecal microbiota transplantation: a review. Gut Microbes 2017: 1–13. doi:10.1080/19490976.2017.1286006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cammarota G, Ianiro G, Gasbarrini A; European Fecal Microbiota Transplantation Working Group Faecal microbiota transplantation in clinical practice. Gut 2018; 67:196–7. [DOI] [PubMed] [Google Scholar]
- 25. Jørgensen SMD, Hansen MM, Erikstrup C, Dahlerup JF, Hvas CL. Faecal microbiota transplantation: establishment of a clinical application framework. Eur J Gastroenterol Hepatol 2017; 29:e36–45. [DOI] [PubMed] [Google Scholar]
- 26. Goldenberg SD, Batra R, Beales I, et al. . Comparison of different strategies for providing fecal microbiota transplantation to treat patients with recurrent Clostridium difficile infection in two English hospitals: a review. Infect Dis Ther 2018; 7:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20(Suppl 2):1–26. [DOI] [PubMed] [Google Scholar]
- 28. Allen AV, Ridley DS. Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol 1970; 23:545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dagci H, Kurt Ö, Demirel M, et al. . Epidemiological and diagnostic features of Blastocystis infection in symptomatic patients in Izmir province, Turkey. Iran J Parasitol 2014; 9:519–29. [PMC free article] [PubMed] [Google Scholar]
- 30. Kurt O, Dogruman Al F, Tanyuksel M. Eradication of Blastocystis in humans: really necessary for all? Parasitology International 2016; 65:797–801. [DOI] [PubMed] [Google Scholar]
- 31. Tan TC, Ong SC, Suresh KG. Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol Res 2009; 105:1283–6. [DOI] [PubMed] [Google Scholar]
- 32. Yildiz S, Doğan İ, Doğruman-Al F, et al. . Association of enteric protist Blastocystis spp. and gut microbiota with hepatic encephalopathy. J Gastrointestin Liver Dis 2016; 25:489–97. [DOI] [PubMed] [Google Scholar]
- 33. Lai CY, Sung J, Cheng F, et al. . Systematic review with meta-analysis: review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther 2019; 49:354–63. [DOI] [PubMed] [Google Scholar]
- 34. Barnes D, Ng K, Smits S, Sonnenburg J, Kassam Z, Park KT. Competitively selected donor fecal microbiota transplantation: butyrate concentration and diversity as measures of donor quality. J Pediatr Gastroenterol Nutr 2018; 67:185–7. [DOI] [PubMed] [Google Scholar]
- 35. Budree S, Wong WF, Tu E, et al. . Do specific bacteria drive clinical cure in fecal microbiota transplantation for Clostridium difficile infection? Clinical, microbial and metabolomic characterization of universal FMT donors. Gastroenterology 2017; 152:S349-S. [Google Scholar]
- 36. Davido B, Batista R, Dinh A, et al. . Fifty shades of graft: how to improve the efficacy of faecal microbiota transplantation for decolonization of antibiotic-resistant bacteria. Int J Antimicrob Agents 2019; 53:553–6. [DOI] [PubMed] [Google Scholar]
- 37. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol 2019; 9:2. doi:10.3389/fcimb.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scanlan PD, Stensvold CR, Rajilić-Stojanović M, et al. . The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol 2014; 90:326–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.