Abstract
Higher cortisol levels due to a hyperactive hypothalamic–pituitary–adrenal axis have been reported in patients with major depressive disorder (MDD). Increased cortisol levels change both the brain morphology and function in MDD patients. The multivariate source-based morphometry (SBM) technique has been applied to investigate neuroanatomical changes in some neuropsychiatric diseases, but not MDD. We aimed to examine the alterations in gray matter (GM) networks and their relationship with serum cortisol levels in first-episode, drug-naïve MDD patients using SBM. Forty-two patients with MDD and 39 controls were recruited via interviews. Morning serum cortisol levels were measured, and high-resolution T1-weighted imaging followed by SBM analysis was performed in all participants. The patients had significantly higher serum cortisol levels than the controls. We found two GM sources, which were significantly different between patients with MDD and controls (prefrontal network, p < .01; insula-temporal network, p < .01). Serum cortisol levels showed a statistically significant negative correlation with the loading coefficients of the prefrontal network (r = − 0.354, p = 0.02). In conclusion, increased serum cortisol levels were associated with reductions in the prefrontal network in the early stage of MDD, and SBM may be a useful approach for analyzing structural MRI data.
Subject terms: Psychology, Medical research
Introduction
Major depressive disorder (MDD) is a common and serious mental disorder that negatively affects activities of daily living and is associated with high societal costs and functional impairment1. In addition, patients who are depressed have a greater risk of self-harm and even suicide2. Regarding MDD pathophysiology, many possible theories have been investigated, including endocrine dysfunction. Studies have shown that the hypothalamus, thyroid, adrenal glands, ovaries, and testes have been linked to depression. Patients with depression have been found to show abnormal responses to thyroid-stimulating hormone and thyrotropin-releasing hormone (TRH), as well as have elevated TRH concentrations in the cerebrospinal fluid and an increased prevalence of antithyroid antibodies3. Depressive symptoms are significantly more frequent or severe in patients with subclinical hypothyroidism than in age- and sex-matched controls4,5. Episodes of mood disorders have been found to be more prevalent during key life periods with important sexual hormonal changes, such as puberty, menopause, and the postpartum period6,7. Higher oxytocin levels are related to fewer depressive symptoms8. In addition, oxytocin and vasopressin are also associated with cortisol levels9.
Cortisol is a stress hormone that is regulated by the hypothalamic–pituitary–adrenal (HPA) axis and is essential for adequate responses to stressful situations. Alterations in the HPA axis function have been consistently demonstrated as pathophysiological mechanisms of MDD10. Patients with MDD have been reported to have cortisol hypersecretion11, reduced glucocorticoid receptor mRNA expression, and decreased glucocorticoid-induced inhibitory feedback to the HPA-axis12. Previous studies in rats have shown that glucocorticoids increase excitotoxic injury in brain structures that have high concentrations of glucocorticoid receptors, consequently impairing neuro-plasticity13,14. According to a meta-analysis, higher levels of cortisol were associated with smaller hippocampal volumes in late-life depression15. A neuroimaging study also demonstrated that a high cortisol level was associated with a reduction in the orbitofrontal cortex thickness16 and hippocampal volume abnormality17. Therefore, elevated cortisol levels may change the brain morphology in individuals with MDD.
Different techniques have been used to assess brain structure abnormalities in MDD patients. The voxel-based morphometry (VBM) approach is a fully automated univariate approach that segments brain images into voxel-wise measures of gray-matter (GM)18 and compares changes in voxels over the brain. However, VBM does not consider the relationships among voxels, and it only detects voxels with a specific predicted effect. Diffusion tensor imaging (DTI) is also a useful magnetic resonance imaging (MRI) technique for quantifying and describing microstructural changes in the white matter. Previously, DTI analysis demonstrated that high cortisol levels in MDD were associated with various white matter injuries, including disruptions of the inferior fronto-occipital fasciculus, uncinate fasciculus, and anterior thalamic radiation19. However, these data could not provide insight into the interregional connectivity among brain regions. Recently, the source-based morphometry (SBM) technique was applied to investigate neuroanatomical changes in individuals with different neuropsychiatric disorders, such as those with schizophrenia or autism disorder, as well as criminal offenders and patients with delusional infestation20–24; however, to date, SBM has not been used to examine individuals with MDD. SBM is a data-driven multivariate approach that combines information across different voxels and identifies unpredicted patterns25. SBM applies independent component analysis (ICA) to a segmented image, arranges voxels into sets that contain similar information25, and obtains common morphological features of the GM concentration among individuals at the network level. Thus, this method is suitable for identifying novel networks. To the best of our knowledge, no previous study has evaluated the relationships between brain networks and serum cortisol levels in patients with MDD.
This present study examined the associations between neural networks and serum cortisol levels in MDD patients using SBM. The purpose was to determine whether SBM can identify structural brain abnormalities in patients with MDD and whether serum cortisol levels were associated with these alterations. Since antidepressant administration can change brain morphometry26, and serum cortisol levels are unstable throughout the day27, we included only first-episode, drug-naïve MDD patients in this study and measured cortisol levels after awakening, which is when these levels reach their peak.
Results
Baseline demographic data
Demographic information is shown in Table 1. There were no significant differences in age (p = 0.102) and sex (p = 0.2) between the two groups. The MDD group had significantly higher cortisol levels than the healthy subjects (HS) group (p = 0.03).
Table 1.
Demographic characteristics and values of serum cortisol of participants.
| Healthy subjects | MDD patients | p-value | |
|---|---|---|---|
| (n = 39) | (n = 42) | ||
| Age, years; mean ± SD | 43.3 ± 11.6 | 48.1 ± 14.3 | 0.1 |
| Male/female | 26/13 | 21/21 | 0.2 |
| Cortisol level, mean ± SD (nmol/L) | 9.6 ± 3.6 | 12.3 ± 5.1 | 0.03* |
SD, standard deviation, MDD, major depression disorders.
*Significant difference.
VBM analyses
The whole-brain analysis showed no significant differences in the regional GM volume between the patients and HS.
SBM analyses
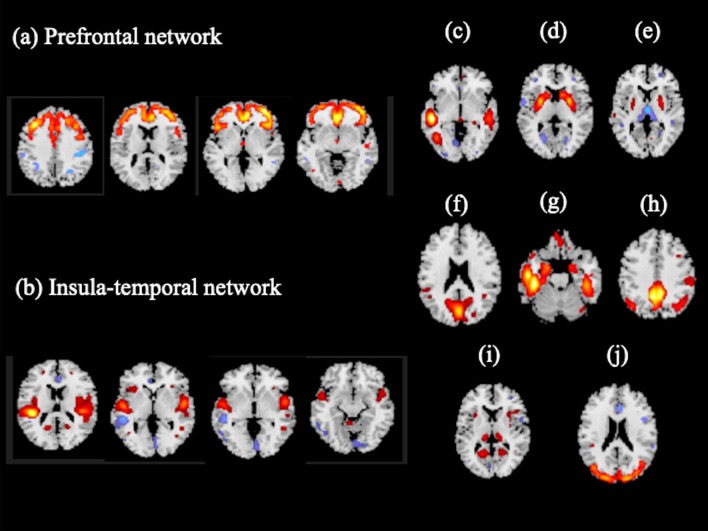
We extracted 17 independent components (ICs). Three of these components were determined to be artifacts based on the criteria that the components contained several sharp edges near the boundary of the brain or primarily appeared in regions that did not contain GM20. These components were excluded from subsequent analyses. Thus, from the remaining 14 ICs, we extracted 10 ICs based on a previous review article regarding depression and anxiety28. Four ICs, which were excluded from subsequent analysis, mainly included cerebellar networks. Of the 10 ICs, 2 components were significantly different between the MDD and HS group. For these 2 components, the mean loading coefficients were significantly lower in the MDD group than in the HS group after Bonferroni correction (Table 2). We called these components the "prefrontal network" (PF network) (Fig. 1a) and “insula-temporal network” (Fig. 1b).
Table 2.
Differences in the loading coefficient of independent components.
| Components | Healthy subjects | MDD patients | p-value | Cohen’s d |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Component 2 | 0.43 ± 0.54 | 0.08 ± 0.92 | 0.04 | 0.46 |
| Component 3 | − 0.28 ± 0.74 | − 0.58 ± 0.81 | 0.09 | 0.38 |
| Component 4 prefrontal network | 0.24 ± 0.76 | − 0.25 ± 0.71 | < 0.01* | 0.68 |
| Component 5 insula-temporal network | 0.39 ± 0.74 | − 0.27 ± 0.80 | < 0.01* | 0.84 |
| Component 6 | − 0.19 ± 0.74 | 0.16 ± 0.74 | 0.04 | 0.47 |
| Component 9 | − 0.20 ± 0.87 | − 0.05 ± 0.93 | 0.46 | 0.17 |
| Component 10 | − 0.05 ± 0.84 | − 0.3 ± 0.95 | 0.21 | 0.28 |
| Component 11 | 0.11 ± 0.80 | − 0.18 ± 0.72 | 0.09 | 0.38 |
| Component 12 | − 0.04 ± 0.78 | − 0.26 ± 0.85 | 0.24 | 0.26 |
| Component 14 | − 0.23 ± 0.99 | − 0.41 ± 0.86 | 0.39 | 0.19 |
SD, standard deviation, MDD, Major depression disorders.
*Significant difference after Bonferroni correction.
Figure 1.
Sources discovered by SBM.
Source-based morphometry revealed 10 structural networks. The loading coefficients of the MDD patients in (a) the prefrontal network and (b) the insula temporal network were significantly lower than those of the healthy subjects (prefrontal network: − 0.25 ± 0.71 and 0.24 ± 0.76, respectively, p < 0.01; insula-temporal network: − 0.27 ± 0.80 and 0.39 ± 0.74, respectively, p < 0.01). (c) component 2; (d) component 3; (e) Component 6; (f) Component 9; (g) Component 10; (h) Component 11; (i) Component 12; (j) component 14.
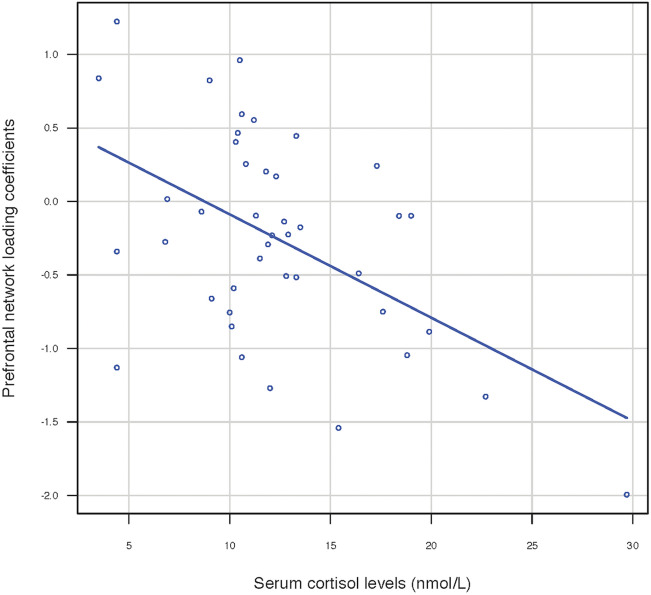
To identify whether serum cortisol levels affected these 2 brain structural networks, we correlated the loading coefficients with the serum cortisol levels for each group. In the MDD group, the serum cortisol levels showed a statistically significant negative correlation with the loading coefficients of the PF network after Bonferroni correction (r = − 0.354, p = 0.0216) (Fig. 2), but the correlation with the insula-temporal network was not significant (r = − 0.294, p = 0.0588). There was no significant correlation in the HS group.
Figure 2.
Correlation of cortisol levels and loading coefficients (Z-scores) of the prefrontal network in MDD patients. Scatter plots of prefrontal network loading coefficients and serum cortisol levels in patients with MDD showed a significant negative linear correlation (r = -0.354, p = 0.02).
Discussion
This study revealed two main findings: (1) The loading coefficients of the PF network and insula-temporal network in patients with MDD were significantly lower than those in HS. (2) In the MDD group, only the loading coefficients of the PF network had a significant negative correlation with serum cortisol levels. This is the first study to use a whole-brain morphometric method using ICA, which showed alterations in the GM volume and a correlation between those alterations and serum cortisol levels in first-episode, drug-naïve MDD patients. Thus, our study suggests that the reductions in the GM volume in the PF network in the early stage of MDD were associated with serum cortisol levels and that prefrontal cortex (PFC) alterations might be considered a potential cause rather than a consequence of MDD.
Additionally, we found significantly higher serum cortisol levels in the MDD group than in the HS group. Higher cortisol levels are considered an important characteristic of the pathophysiology of depression. A previous study showed that increased serum cortisol in patients with depression could accurately distinguish between patients with depression and those without depression29. Depressive states as side effects of increased glucocorticoid levels have been observed in patients with Cushing’s disease30 or accompanying hormone therapy31. Treatment of rodents with corticosterone resulted in similar depressive-like behavioral modifications32. Mifepristone, a glucocorticoid receptor antagonist, might be effective for the treatment of psychotic depression. This result may support hyperactivation of the HPA axis in MDD pathophysiology.
MDD is a debilitating disease that is characterized by at least one discrete depressive episode lasting at least 2 weeks and involves clear-cut changes in mood, interests and pleasure, changes in cognition, and vegetative symptoms. Because it is described as a heterogeneous syndrome, it is inappropriate to delineate the pathophysiology of MDD by abnormalities in the structure and function of a single brain area, but instead by dysregulation of the interaction of multiple brain regions33. Meanwhile, SBM has been used to identify source networks of spatially distinct regions with common covariation among subjects. In turn, these source networks provide the localization of differences in GM concentrations along patterns of voxel-covariation. Notably, such anatomical covariance has been shown to reflect functional connectivity34. By using SBM in this study, we found 2 source networks of the GM that show aberrant patterns of covariance in MDD, as compared to HS. Hence, SBM is particularly suitable for studying the pathophysiology of MDD.
The PFC was considered a major part of the circuits that Williams et al. identified as neural circuit dysfunctions underlying depression and anxiety, including the default mode network (DMN), positive affect circuit, frontoparietal attention circuit, and cognitive control circuit28. The DMN is primarily composed of the medial PFC, posterior cingulate, lateral temporal cortex, hippocampal formation, and angular gyrus35,36. The positive affect circuit consists of the striatal nucleus accumbens and ventral tegmental areas and their projections to the orbitofrontal cortex and medial PFC37. The frontoparietal attention circuit is defined by nodes in the medial superior PFC, anterior insula, anterior inferior parietal lobule, and precuneus38. The cognitive control circuit is composed of the dorsolateral PFC, anterior cingulate cortex, dorsal parietal cortex, and precentral gyrus. In MDD participants, hyperactivation of the DMN has been associated with higher levels of maladaptive, depressive rumination39. Furthermore, the PFC is considered a key node in the frontal-subcortical circuit and frontal-limbic circuit40. These circuits are involved in emotional and cognitive processing and have been proposed as pathogenic factors associated with mood and anxiety disorders41. A resting-state functional MRI study found decreased functional connectivity among the bilateral hippocampus, dorsolateral PFC, and ventral PFC in the frontal-limbic circuit in first-episode, drug-naïve patients with MDD42. Notably, Matsuo et al. provided imaging evidence of prefrontal hyperactivation during working memory tasks in untreated individuals with MDD43. The areas of PFC mentioned above were included in the PF network (Fig. 1a), although some other ICs that were revealed in the present study also encompass a part of the prefrontal components.
In this study, we found that the serum cortisol levels were inversely correlated with the loading coeficients of the PF network. This finding suggested that elevated cortisol levels were associated with reductions in the PF network. Cortisol is known to regulate neuronal apoptosis and neurogenesis44, and increased glucocorticoids affect both structural and functional changes in the nervous system45,46. This finding might be explained by glucocorticoid–glucocorticoid receptor (GR) interactions and regional specificity of GR distribution. Previous animal studies have demonstrated that the cortices containing high concentrations of GR are vulnerable to the neurotoxic effects of glucocorticoids, consequently impairing neuronal plasticity and neurogenesis14,47. In the central nervous system, neurons and glia throughout the brain express GR47, but the hippocampus and frontal cortex have higher levels of GR expression47,48, and the frontal cortex includes the PF network that was identified as being affected in the patients in this study. In a postmortem study, GR mRNA levels showed abnormalities in layers III–VI of the frontal cortex in subjects with depression48, which also supports our results.
A potential limitation of our study is the modest sample size, which can lead to some sampling bias. Moreover, although SBM, a multivariate method, takes into account cross-voxel information and is more robust than univariate voxel-wise approaches, this approach should be used on big data to increase the power20. However, we limited our sample to first-episode and unmedicated MDD patients so that we could exclude the effects of antidepressants and benzodiazepines on the patients’ brain morphometry. In the future, analysis on a larger number of patients should be conducted to confirm our results from the present study.
Conclusion
In the current study, SBM, a multivariate statistical data analysis method, was used to investigate differences in GM networks in first-episode, drug-naïve MDD patients, we found changes in the pattern of GM in the PF network and insular-temporal network. However, only the PF network was associated with serum cortisol levels after awakening. These results support the view that the PFC is one of the target sites for the negative-feedback effects of cortisol on the HPA axis in the early development of MDD. Our study also suggests that SBM is a useful alternative to univariate approaches for analyzing structural MRI data.
Methods
Participants
A total of 42 first-episode and drug-naïve patients with MDD (21 males, 21 females; mean age, 48.1 ± 14.3 years) were recruited and assessed using the fully Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text revision (DSM-IV-TR). Patients were excluded if they met the following criteria: any past DSM-IV-TR Axis I disorder via interviews with a psychiatrist, a history of other medical illnesses, neurological disorders, or use of drugs that may cause depression.
Thirty-nine healthy subjects (HS; 26 males and 13 females; mean age 43.3 ± 11.6 years) were recruited as the HS group via an interview conducted by a psychiatrist using the SCID-I/NP. These subjects were recruited from nearby communities via advertisement and included not only staff at our institution but also their relatives by blood or marriage and close friends. None of the HS had a history of medical or neuropsychiatric illness or a family history of major psychiatric or neurological illness among their first-degree relatives.
Serum cortisol assay
Because cortisol levels change throughout the day, the timing of a cortisol test is important. In the morning, 30–60 min after awakening, cortisol levels are at their highest27. Furthermore, previous studies have shown that patients with MDD exhibit high morning cortisol levels49,50. Therefore, 1 h after participants had awakened (9–10 A.M), blood samples were drawn. All samples were immediately centrifuged and the serum was stored at − 20 °C until further analysis. Cortisol was quantitatively displaced from its binding proteins and measured by a direct radioimmunoassay using a highly specific antibody51.
MRI acquisition
All participants underwent brain MRI. The brain images of the patients with MDD were taken before they had received antidepressant medication or psychotherapy. Therefore, all patients were antidepressant-free at the time of the MRI. The MRI data were obtained on a 3.0 T MR system (Signa EXCITE 3T; GE Healthcare, Waukesha, WI) with an eight-channel brain-phased array coil. The images were acquired by 3D fast spoiled gradient-recalled acquisition (3D-FSPGR). The acquisition parameters were as follows: repetition time/echo time/inversion time, 10/4.1/700 ms; flip angle, 10°; field of view, 24 cm; section thickness, 1.2 mm; and resolution, 0.9 × 0.9 × 1.2 mm. All images were corrected for image distortion due to gradient nonlinearity using “Grad Warp”52 and for intensity inhomogeneity with the “N3” function53.
A radiologist (S.K., 22 years of experience in neuroradiology) who reviewed the conventional MRI data (including T2-weighted images) reported no gross abnormalities, such as infarcts, hemorrhages, or brain tumors, in any of the study participants.
Image processing for SBM
SBM is a multivariate technique that takes advantage of independent component analysis (ICA). SBM takes into account information across different voxels and identifies unpredicted, naturally occurring patterns of covariance across brain regions20. Preprocessing of the images was identical to the procedures adopted for classical VBM analyses, which were introduced in our previous paper54.
A fully automatic technique for the computational analysis of differences in regional brain volume throughout the brain was conducted using the SPM12 software program (Statistical Parametric Mapping 12; Institute of Neurology, London, UK)55,56. The 3D-FSPGR images in the native space were spatially normalized, segmented into GM, white matter, and cerebrospinal fluid images, and modulated using the Diffeomorphic Anatomical Registration through Exponential Lie Algebra (DARTEL) toolbox in SPM1257. DARTEL was proposed by Ashburner as an alternative method for normalization in the SPM package55. To preserve the GM and white matter volumes within each voxel, we modulated the images using the Jacobian determinants derived from the spatial normalization by DARTEL. The resulting modulated GM images were smoothed using an 8-mm full-width at half-maximum Gaussian kernel.
The SBM analysis methods have been described in detail elsewhere20. For image processing, GIFT toolbox (http://icatb.sourceforge.net) was used20. We estimated the number of ICs by using the minimum description length (MDL) principle. The MDL found 17 reliable ICs. Next, we performed ICA using a neural network algorithm (Infomax) that attempts to minimize the mutual information of the network outputs to identify naturally grouping and maximally independent sources58. The ICA was repeated 20 times in ICASSO 84 (http://research.ics.aalto.fi/ica/icasso/), and the resulting components were clustered to ensure the consistency and reliability of the results. Reliability was quantified using a quality index, Iq, which ranges from 0 to 1 and reflects the difference between intra-cluster and extra-cluster similarity59. All 10 components extracted from the GM images were found to be associated with an Iq > 0.97, indicating highly stable ICA decomposition. SBM involves converting each GM volume into a vector.
As a result, we obtained a matrix where the 81 rows represented the 81 subjects (39 HS and 42 MDD) and each column indicated a voxel. This matrix was decomposed into two matrices by the ICA. The first matrix was named the "mixing matrix" and was composed of a subject per row and an IC per column. The mixing matrix involved "loading coefficients", which demonstrated how each structural component contributed to the 81 subjects and, thus, contained information about the relationship between each subject and each component. The second matrix is named the source matrix, and it specifies the relation between the ICs and the voxels. For GM volume component visualization, the source matrix was reshaped back to a three-dimensional image, scaled to unit standard deviations (Z maps) and thresholded at Z > 2.5.
Statistical analyses
All statistical analyses were performed using EZR software version 1.35 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)60, which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). All the results were corrected for family-wise error (FWE).
We applied a two-tailed t-test to assess the differences in age and the Mann–Whitney U test to assess the differences in serum cortisol levels between HS and patients with MDD. A χ2 test was used for sex comparisons.
In the VBM analysis, statistical analyses were performed using SPM8 software. Morphological changes in the GM were assessed using a two-sample t-test across diagnostic groups. Age, sex, and total intracranial volume were included as covariates of no interest in all analyses as confounding variables. The differences in GM volume between the patients with MDD and HS were assessed at the whole-brain level. This analysis yielded statistical parametric maps (SPMs [t]) based on a voxel-level height threshold of p < 0.001. We used cluster-level FWE corrections. The significance level was set at an FWE-corrected p < 0.05.
In the analysis of loading coefficients calculated from the SBM, we performed the following analyses: (a) an intra-network comparison between the MDD and HS groups and (b) a linear correlation between intra-network connectivity and cortisol levels. The loading coefficients were transformed to Z-scores using Fisher’s z-transformation. The Z-scores in SBM allowed the identification of sources that exhibit group differences (patients vs. HS) or particular relationships with other variables of interest (e.g., cortisol levels). To compare the intra-network differences, we compared the loading coefficients in each component using two-tailed t-tests. Spearman’s rank correlation was applied to identify associations between serum cortisol levels and the loading coefficients (Z-scores). All the results were thresholded at p < 0.05 corrected with a Bonferroni correction.
Ethics statement
This study protocol was approved by the Institutional Review Board at the University of Occupational and Environmental, Japan and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent after being given a detailed description of the study.
Acknowledgements
This study was supported by a Health and Labor Research Grant in Japan (#1401010101).
Author contributions
L.H.N., S.K., and R.Y. planned and designed the study and wrote the manuscript. L.H.N., S.K., K.W., K.S., N.I., O.A., and Y.K. analyzed the data. A.K., T.S., A.I., and R.Y. recruited the study participants and collected the clinical data. All authors discussed the results and contributed to the final manuscript.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brody, D. J., Pratt, L. A. & Hughes, J. P. Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief. 1–8 (2018). [PubMed]
- 2.Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J. R. Soc. Med. 2014;107:194–204. doi: 10.1177/0141076814522033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fountoulakis KN, et al. Peripheral thyroid dysfunction in depression. World. J. Biol. Psychiatry. 2006;7:131–137. doi: 10.1080/15622970500474739. [DOI] [PubMed] [Google Scholar]
- 4.Gulseren S, et al. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch. Med. Res. 2006;37:133–139. doi: 10.1016/j.arcmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Chueire VB, Romaldini JH, Ward LS. Subclinical hypothyroidism increases the risk for depression in the elderly. Arch. Gerontol. Geriatr. 2007;44:21–28. doi: 10.1016/j.archger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv. Nurs. Sci. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Zelkowitz P, et al. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm. Behav. 2014;66:351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Garcia FD, et al. Autoantibodies reacting with vasopressin and oxytocin in relation to cortisol secretion in mild and moderate depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:118–125. doi: 10.1016/j.pnpbp.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Vreeburg SA, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatry. 2009;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 11.Verduijn J, et al. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Transl. Psychiatry. 2015;5:e649. doi: 10.1038/tp.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepgul N, Cattaneo A, Zunszain PA, Pariante CM. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med. 2013;11:28. doi: 10.1186/1741-7015-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madalena KM, Lerch JK. The effect of glucocorticoid and glucocorticoid receptor interactions on brain, spinal cord, and glial cell plasticity. Neural. Plast. 2017;2017:8640970. doi: 10.1155/2017/8640970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorrells SF, Munhoz CD, Manley NC, Yen S, Sapolsky RM. Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology. 2014;100:129–140. doi: 10.1159/000367849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol. Psychiatry. 2017;82:339–350. doi: 10.1016/j.biopsych.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, et al. Relationship between the cortical thickness and serum cortisol levels in drug-naive, first-episode patients with major depressive disorder: a surface-based morphometric study. Depress. Anxiety. 2015;32:702–708. doi: 10.1002/da.22401. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe R, et al. Relationship between the hippocampal shape abnormality and serum cortisol levels in first-episode and drug-naive major depressive disorder patients. Depress. Anxiety. 2017;34:401–409. doi: 10.1002/da.22604. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, et al. Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Br. J. Psychiatry. 2016;208:585–590. doi: 10.1192/bjp.bp.114.155689. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 2009;30:711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grecucci A, Rubicondo D, Siugzdaite R, Surian L, Job R. Uncovering the social deficits in the autistic brain. A source-based morphometric study. Front. Neurosci. 2016;10:388. doi: 10.3389/fnins.2016.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harenski CL, Harenski KA, Calhoun VD, Kiehl KA. Source-based morphometry reveals gray matter differences related to suicidal behavior in criminal offenders. Brain Imaging Behav. 2018;14:1–9. doi: 10.1007/s11682-018-9957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf R, et al. Source-based morphometry reveals distinct patterns of aberrant brain volume in delusional infestation. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:112–116. doi: 10.1016/j.pnpbp.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Caprihan A, et al. Source-based morphometry analysis of group differences in fractional anisotropy in schizophrenia. Brain Connect. 2011;1:133–145. doi: 10.1089/brain.2011.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: The use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapping. 2009;30:711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dusi N, Barlati S, Vita A, Brambilla P. Brain structural effects of antidepressant treatment in major depression. Curr. Neuropharmacol. 2015;13:458–465. doi: 10.2174/1570159X1304150831121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hucklebridge F, Clow A, Evans P. The relationship between salivary secretory immunoglobulin A and cortisol: neuroendocrine response to awakening and the diurnal cycle. Int. J. Psychophysiol. 1998;31:69–76. doi: 10.1016/S0167-8760(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 28.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–480. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Y, et al. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J. Nerv. Ment. Dis. 2019;207:271–276. doi: 10.1097/NMD.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 30.Sonino N, Fava GA, Raffi AR, Boscaro M, Fallo F. Clinical correlates of major depression in Cushing’s disease. Psychopathology. 1998;31:302–306. doi: 10.1159/000029054. [DOI] [PubMed] [Google Scholar]
- 31.Brown ES, Vera E, Frol AB, Woolston DJ, Johnson B. Effects of chronic prednisone therapy on mood and memory. J. Affect. Disord. 2007;99:279–283. doi: 10.1016/j.jad.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 34.Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 35.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 37.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. USA. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J. Psychosom. Res. 2002;53:647–654. doi: 10.1016/S0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 41.Marchand WR. Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct. Funct. 2010;215:73–96. doi: 10.1007/s00429-010-0280-y. [DOI] [PubMed] [Google Scholar]
- 42.Geng H, et al. Disrupted structural and functional connectivity in prefrontal-hippocampus circuitry in first-episode medication-naive adolescent depression. PLoS ONE. 2016;11:e0148345. doi: 10.1371/journal.pone.0148345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuo K, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol. Psychiatry. 2007;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 44.Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. The influence of glucocorticoids on neuronal survival and synaptic function. Biomol. Concepts. 2012;3:495–504. doi: 10.1515/bmc-2012-0012. [DOI] [PubMed] [Google Scholar]
- 45.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 46.Anacker C, et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology. 2013;38:872–883. doi: 10.1038/npp.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-X. [DOI] [PubMed] [Google Scholar]
- 48.Webster MJ, Knable MB, Ogrady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol. Psychiatry. 2002;7:985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- 49.Bridges PK, Jones MT. The diurnal rhythm of plasma cortisol concentration in depression. Br. J. Psychiatry. 1966;112:1257–1261. doi: 10.1192/bjp.112.493.1257. [DOI] [PubMed] [Google Scholar]
- 50.Herbert J. Cortisol and depression: three questions for psychiatry. Psychol. Med. 2013;43:449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 51.Heckmann M, Wudy SA, Haack D, Pohlandt F. Reference range for serum cortisol in well preterm infants. Arch. Dis. Child. Fetal. Neonatal. Ed. 1999;81:F171–174. doi: 10.1136/fn.81.3.F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jovicich J, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 54.Igata R, et al. PCLO rs2522833-mediated gray matter volume reduction in patients with drug-naive, first-episode major depressive disorder. Transl. Psychiatry. 2017;7:e1140. doi: 10.1038/tp.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Ashburner J. SPM: a history. Neuroimage. 2012;62:791–800. doi: 10.1016/j.neuroimage.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashburner J. Computational anatomy with the SPM software. Magn. Reson. Imaging. 2009;27:1163–1174. doi: 10.1016/j.mri.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural. Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 59.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.