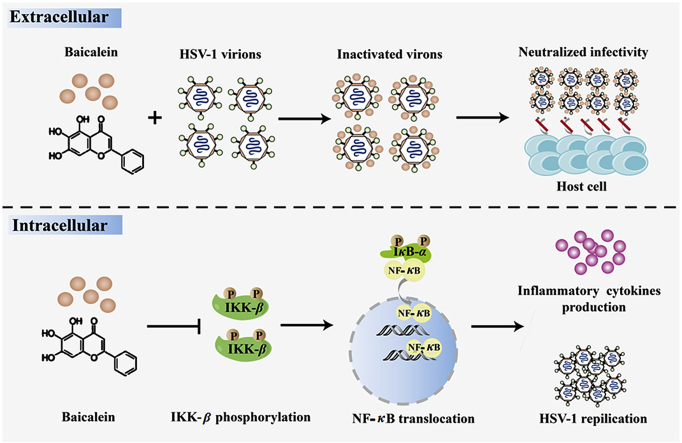
Abstract
Herpes simplex virus type 1 (HSV-1) is a ubiquitous and widespread human pathogen, which gives rise to a range of diseases, including cold sores, corneal blindness, and encephalitis. Currently, the use of nucleoside analogs, such as acyclovir and penciclovir, in treating HSV-1 infection often presents limitation due to their side effects and low efficacy for drug-resistance strains. Therefore, new anti-herpetic drugs and strategies should be urgently developed. Here, we reported that baicalein, a naturally derived compound widely used in Asian countries, strongly inhibited HSV-1 replication in several models. Baicalein was effective against the replication of both HSV-1/F and HSV-1/Blue (an acyclovir-resistant strain) in vitro. In the ocular inoculation mice model, baicalein markedly reduced in vivo HSV-1/F replication, receded inflammatory storm and attenuated histological changes in the cornea. Consistently, baicalein was found to reduce the mortality of mice, viral loads both in nose and trigeminal ganglia in HSV-1 intranasal infection model. Moreover, an ex vivo HSV-1-EGFP infection model established in isolated murine epidermal sheets confirmed that baicalein suppressed HSV-1 replication. Further investigations unraveled that dual mechanisms, inactivating viral particles and inhibiting IκB kinase beta (IKK-β) phosphorylation, were involved in the anti-HSV-1 effect of baicalein. Collectively, our findings identified baicalein as a promising therapy candidate against the infection of HSV-1, especially acyclovir-resistant strain.
Keywords: Anti-HSV-1, Baicalein, Viral inactivation, IKK-β phosphorylation, NF-κB activation, HSV-1 infection
Abbreviations: CC50, 50% cytotoxic concentration; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; dpi, days post-infection; EC50, 50% effective concentration; GB, glycoprotein B; HSV-1, herpes simplex virus types 1; ICP, infected cell polypeptide; IKK-β, IκB kinase beta; IL-1β, interleukin 1 beta; IL-6, interleukin 6; IκB-α, inhibitor of NF-κB alpha; LPS, lipopolysaccharides; MOI, multiplicity of infection; NAC, N-acetyl-l-cysteine; NF-κB, nuclear factor kappa-B; PFU, plaque-forming units; PGA1, prostaglandin A1; p-IKK-β, phosphorylated-IKK beta; p-IκB-α, phosphorylated-IκB alpha; ROS, reactive oxygen species; SI, selectivity index; TG, trigeminal ganglia; TNF-α, tumor necrosis factor alpha
Graphical abstract
Baicalein exerts potent ability against HSV-1 infection and dual mechanisms were disclosed.
Highlights
-
•
Baicalein is highly effective against HSV-1infection ex vivo and in vivo.
-
•
Inactivation of viral particles and suppression of NF-κB activation were involved in the anti-viral effect of baicalein.
-
•
Hence, our work offers experimental basis for baicalein as a potential drug in treating HSV-1 associated diseases.
1. Introduction
Herpes simplex virus type 1 (HSV-1) is a type of neurotropic double-stranded DNA virus belonging to the alpha herpesviridae family. HSV-1 infection is very common, with a high seroprevalence of 50%–90% worldwide1. Although the symptoms of HSV-1 infection commonly manifest as cold sores, it can be particularly severe in some cases, such as corneal blindness, lymphocytic meningitis, viral encephalitis, and even death2, 3, 4. In particular, HSV-1 has become a major cause of infectious blindness in industrialized countries. It is gradually replacing HSV-2 as a causative pathogen of genital herpes in Western and Asian countries5,6. Currently, there is no clinically approved vaccine for preventing HSV-1 infection. Nucleoside analogs, such as acyclovir and penciclovir, have become primary therapeutic agents for HSV-1 infection. These compounds are overwhelmingly successful in a variety of clinical diseases caused by HSV-17,8. Nevertheless, severe side effects and drug-resistance strains often emerge after long-term use of these anti-herpes drugs9. Thus, it is imminent to find novel antiviral compounds against HSV-1 infection.
Flavonoids, as potential antiviral agents, have attracted widespread attention due to its low toxicity10,11. Baicalein, 5,6,7-trihydroxyflavone, is a flavonoid isolated from the root of Scutellaria baicalensis Georgi12. It has been commonly used in South Korea and China to treat cancer and inflammatory diseases13. Till now, multiple biological functions of baicalein have been discovered. It has been shown that baicalein has anti-oxidant, anti-apoptotic, vascular-protective, and neuroprotective properties14, 15, 16. Recently, baicalein rises to be well-known for its inhibitory effect on ferroptosis17, 18, 19. Importantly, growing evidence has suggested that baicalein has non-specific activities against viral infection, including anti-Zika virus20, anti-dengue virus21, anti-chikungunya virus22 and anti-influenza virus23. However, little is known about the inhibitory effect of baicalein against HSV-1 infection. Here, we provide the first evidence that baicalein exerts protective effect against HSV-1 infection ex vivo and in vivo, and the underlying mechanisms are also investigated.
2. Materials and methods
2.1. Chemicals and reagents
Baicalein was obtained from Biopurify Phytochemicals Ltd. (Chengdu, China). N-Acetyl-l-cysteine (NAC) and punicalagin were purchased from Beyotime (Shanghai, China) and TargetMol (Shanghai, China), respectively. Acyclovir, lipopolysaccharides (LPS), and prostaglandin A1 (PGA1) were purchased from Sigma–Aldrich (St Louis, MO, USA). Antibodies for p-IKK-β, IKK-β, IκB-α, p-IκB-α, NF-κB p65, p-NF-κB p65 and Histone H3 were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies for infected cell polypeptide 27 (ICP27), infected cell polypeptide 8 (ICP8) and glycoprotein B (GB) were purchased from Abcam (Cambridge, UK). Antibody for β-actin was obtained from Fude Co., Ltd. (Hangzhou, China).
2.2. Cells and viruses
Vero cells and HaCat human keratinocytes were propagated in culture medium (10% FBS+DMEM). HSV-1/F strain and a recombinant HSV-1-EGFP24 were propagated and titrated by plaque-forming units (PFU) assay as previously described25. Briefly, the confluent Vero cells were infected with serial dilutions of HSV-1, and incubated with overlaid medium (2% carboxymethylcellulose+2% FBS+DMEM). After 72 h incubation, the cells were fixed and stained with crystal violet, and the plaques were counted.
2.3. Cytotoxicity assay
Vero or HaCat cells were seeded in 96-well plates overnight. On the second day, baicalein was added to cells at different concentrations. Cell Counting Kit-8 (CCK-8, Dojindi labs, Kumamoto, Japan) was used to measure the cell viability after 72 h incubation. The 50% cytotoxic concentration (CC50) of baicalein was determined by linear regression analysis.
2.4. In vitro antiviral assay
Cells were treated with different concentrations of baicalein for 24 h before infection. After infection and being washed, cells were treated with baicalein again. Viral titers were titrated by PFU assay at 24 h post infection after three freeze-thaw cycles. The 50% effective concentration (EC50) of baicalein was calculated by linear regression of the viral inhibition curves. Viral inhibition (%) was calculated as Eq. (1):
| Viral inhibition (%) = [1−(Number of plaques)baicalein/(Number of plaques)control] × 100 | (1) |
To identify the stage of HSV-1 life cycle affected by baicalein, cells were treated with baicalein prior or post infection and PFU assay was conducted. Specifically, to investigate the effect of baicalein pretreatment on HSV-1 infection, HaCat cells were incubated with baicalein prior to HSV-1 infection. To determine whether baicalein had any post-entry effects, baicalein was added in the cells after HSV-1 challenge.
2.5. Anti-attachment, anti-penetration, and viral inactivation assay
HaCat cells were treated with baicalein (100 μmol/L) at various stages of HSV-1 (F strain) infection. For attachment assay, HaCat cells were pre-cooled at 4 °C for 1 h, and then inoculated with HSV-1 at multiplicity of infection (MOI) of 1 for 2 h at 4 °C in the presence or absence of baicalein. After incubation, baicalein and unattached virus were washed with ice-cold PBS, and PFU assay was conducted as described above.
For the penetration assay, HaCat cells were pre-chilled at 4 °C for 1 h, and then infected with HSV-1 for 2 h at 4 °C. After attachment, cells were incubated for 1 h at 37 °C in the presence or absence of baicalein, then washed with PBS and treated with citrate buffer (pH 3.0) for 1 min to inactivate non-penetrating virus. Then, PFU assay was performed.
For virus inactivation analysis, HSV-1 (106 PFU) was mixed with baicalein at 37 °C for 2 h, then diluted 100-fold prior to infecting HaCat cells. After an additional 48 h of incubation, the rate of viral inhibition was analyzed by PFU assay as described above.
2.6. Fluorescence and flow cytometry analysis
After infection with HSV-1-EGFP, the cells in 96 wells were incubated with serial dilution of baicalein, and the plates were scanned using BioStack Microplate Stacker (BioTek Instruments, Winooski, VT, USA) at 24 h post-infection. Viral infection (%) was calculated using Eq. (2):
| Viral infection (%)=(Fluorescencebaicalein–Fluorescencecell control)/(Fluorescencevirus−Fluorescencecell control) × 100 | (2) |
Viral plaque inhibition (%) was calculated as described in Section 2.4. For fluorescence microscopy and flow cytometry analysis, the infected HaCat cells were treated with baicalein as describe above, and then detected under fluorescence microscope (IX51, Olympus Co., Tokyo, Japan) or measured by flow cytometry (Epics XL, Beckman Coulter, CA, USA) at 24 and 48 h post-infection.
2.7. Ex vivo antiviral assay
Murine epidermal sheets were employed to evaluate the ex vivo antiviral activity of baicalein. The back skins of newborn mice (C57BL/6) were employed to prepare for epidermal sheets as described in previous report26. Briefly, newborn mice (3–5 days after birth) were sacrificed and the back skins were isolated using a scalpel in an aseptic environment. After incubation with dispase II (Roche Diagnostics, Almere, the Netherlands), the epidermises were gently removed from the underlying dermis. Subsequently, the isolated epidermal sheets were infected with HSV-1-EGFP (MOI = 10). After infection, the epidermal sheets were washed and cultured in medium (DMEM+5% FBS) containing baicalein (100 μmol/L) or acyclovir (50 μmol/L). At 24 h post-infection, the epidermal sheets were stained with DAPI (Beyotime) for fluorescent analysis.
2.8. Determination of intracellular reactive oxygen species (ROS)
The HSV-1 infected HaCat cells were treated with baicalein, acyclovir, or NAC at indicated concentrations. Twenty-four hours post-infection, cells were subsequently incubated with 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Beyotime) for 30 min before the analysis by flow cytometer or fluorescence microscope.
2.9. Detection of nuclear factor kappa-B (NF-κB) nuclear translocation
HaCat cells were seeded in culture dishes for 24 h and infected with HSV-1. Then, cells were treated with baicalein for 24 h and collected for confocal microscopy examination and Western blot assay. NF-κB proteins were probed with primary antibody and the corresponding secondary antibody Alexa Fluor 555 IgG (Life Technology, Gaithersburg, MD, USA) and visualized by a laser confocal microscopy (Zeiss LSM 700; Carl Zeiss, Inc., Thornwood, German). Besides, NF-κB protein expressions in the nucleus and cytoplasm were determined by Western blot assay. Proteins fractions were extracted by Nuclear and Cytoplasmic Extraction reagents kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.10. Animals and treatments
All animal experiments were approved by the Animal Care and Use Committee of Jinan University (Guangzhou, China). BALB/c mice (4-week-old) were obtained from Guangdong Medical Laboratory Animal Center (Guangzhou, China).
In the first batch of animal experiment, mice were intranasally challenged with HSV-1/F strain (1 × 106 PFU) in 20 μL PBS after isoflurane anesthesia. The infected mice were orally administered with baicalein (200 mg/kg/day) or acyclovir (50 mg/kg/day) for 7 consecutive days. Saline (0.9%) were treated to mice in mock and HSV-1 control groups. In addition to daily monitoring of the body weight and survival rate, mouse nose, trigeminal ganglia (TG), and the whole brain from mice were harvested at 3, 5, and 7 days post-infection (dpi) and then immediately stored at −80 °C for determination of virus titers and Western blotting analysis.
In the second batch of animal experiment, the therapeutic effect of baicalein on HSV-1-induced corneal disease was evaluated. Briefly, mice were anesthetized, and the corneas were scratched and infected with HSV-1/F strain (1 × 105 PFU) in 5 μL PBS. After infection, mice were treated with baicalein or acyclovir as described in the first batch of animal experiment. The scoring of eye infection (0–5) was performed in a blinded fashion based on the criteria reported previously27. The criteria are set as following: 0, no symptoms; 1, mild swelling of the eyelids; 2, moderate swelling of the eyelids with some crusting; 3, moderate swelling of the eyelids with >50% crusting; 4, severe crusting; 5, eye completely swollen shut. For virus titer measurement, eye swabs were collected from the tears at 5 and 7 dpi and were titrated by PFU assay. For histopathology analysis, the right eyes of mice were removed at 9 dpi, and then embedded, fixed and sectioned. The sections were stained with hematoxylin and eosin, and imaged at 40× objective using the microscope (PreciPoint M8, Freising, Germany). The thickness of the corneal epithelium was measured using the ViewPoint Software (PreciPoint).
2.11. Determination of HSV-1 titers in the tissues
At indicated days post infection, tissues were collected, homogenized and sonicated in 1 mL DMEM. After centrifugation at 1500 rpm (Centrisart D-16C, Sartorius, Goettingen, Germany) for 10 min, HSV-1 titers in the supernatants were measured by PFU assay.
2.12. Measurement of inflammatory cytokines in the eye tissues
According to the manufacturer's instructions, the production of interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) in eye tissues was detected at 9 dpi by commercial ELISA kits (Thermo Fisher Scientific). The value of absorbance was determined using a microplate reader at 450 nm.
2.13. Western blotting analysis
The total protein levels from cells and tissues were measured by BCA protein assay kit (Pierce, Rockford, IL, USA). Protein samples were separated by SDS-PAGE followed by transferring to polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membranes were probed with primary antibodies and secondary antibodies. The bands were imaged by an ECL system (Tanon 5200, Shanghai, China).
2.14. RT-qPCR
Cell pellets or tissues were extracted using TRIzol reagent (Introvigen, Carlsbad, CA, USA) and RNA concentrations were then determined by a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific). cDNA was synthesized by a cDNA synthesis kit (TransGen Biotech, Beijing, China). Relative expression of mRNA levels was measured using SYBR green (TransGen Biotech) on a CFX Connect™ reverse transcription (RT) machine (Applied Biosystems, Carlsbad, CA, USA) and calculated via the 2−ΔΔCt method. The primer sequence was as follows: HSV-1 infected cell polypeptide 0 (ICP0) (forward, 5′-CCCACTATCAGGTACACCAGCTT-3′; reverse, 5′-CTGCGCTGCGACACCTT-3′); HSV-1 ICP27 (forward, 5′-TGGCGGACATTAAGGACATTG-3′; reverse, 5′-TGGCCGTCAACTCGCAGA-3′), HSV-1 ICP8 (forward, 5′-CCACGCCCACC-GGCTGATGAC-3′; reverse, 5′-TGCTTACGGTCAGGTGCTCCG-3′), HSV-1 GB (forward, 5′-GCCTTCTTCGCCTTTCGC-3′; reverse, 5′-CGCTCGTGCCCTTCTTCTT-3′), Human TNF-α (forward: 5′-ACAAGCCTGTAGCCCATGTT-3′; reverse, 5′-AAAGTAGACCTGCCCAGACT-3′), Human IL-6 (forward: 5′-CAGCCCTGAGAAAGGAGACAT-3′; reverse, 5′-GGTTCAGGTTGTTTTCTGCCA-3′), Human GAPDH (forward: 5′-TGACTTCAACAGCGACACCCA-3′; reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′).
Mouse 18S (forward: 5′-CGGACAGGATTGACAGATTGATAGC-3′; reverse, 5′-TGCCAGAGTCTCGTTCGTTATCG-3′).
2.15. Statistical analysis
The data are expressed as the mean ± standard deviation (SD) from at least three experiments. Statistical differences were analyzed by Student's t-test or analysis of variance (ANOVA) with Dunnett's multiple comparisons test. Kinetics of mortality was analyzed by Kaplan–Meier curves and log-rank test with Bonferroni adjustment. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Baicalein inhibits HSV-1 replication in vitro
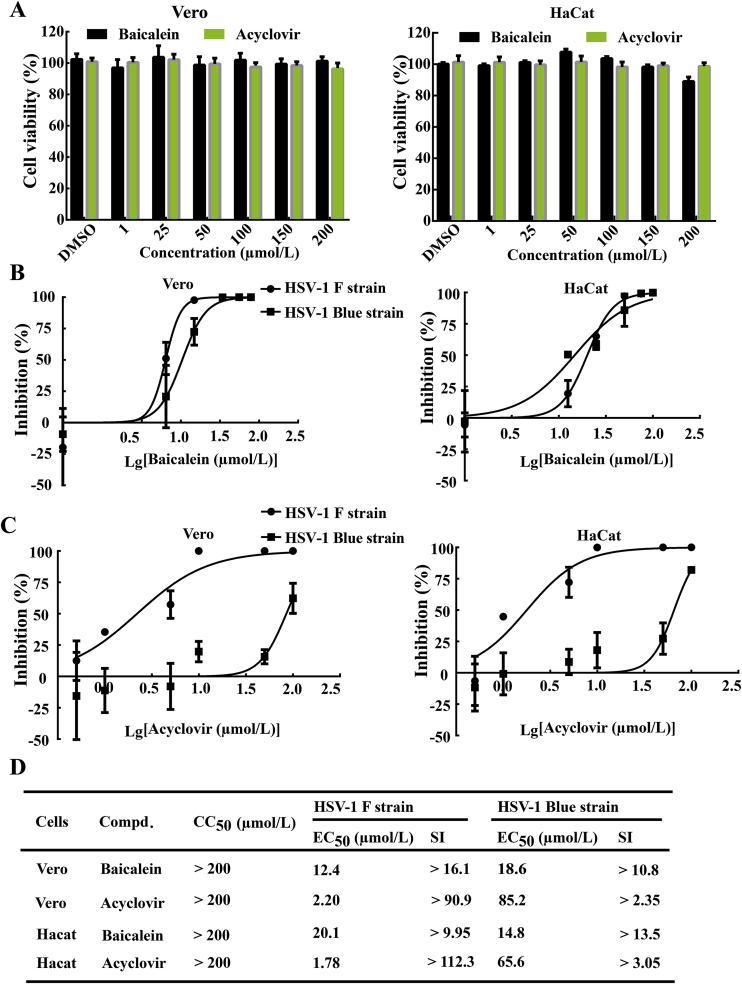
Prior to the determination of the antiviral effect, the cytotoxicity of baicalein was estimated in Vero and HaCat cells by CCK-8 assay after 72 h incubation. As shown in Fig. 1A, baicalein showed no obvious cytotoxicity in cells at concentrations lower than 200 μmol/L. Then, PFU assay was conducted to evaluate the anti-HSV-1 activities of baicalein at non-toxic doses. Vero and HaCat cells were pre-incubated with different concentrations of baicalein for 24 h, and then infected with HSV-1. After infection, the cells were treated with baicalein or acyclovir for another 24 h. As shown in Fig. 1B and C, both baicalein and acyclovir exhibited dose-dependent antiviral activities against HSV-1 F strain. It is worth to note that, when comparing with acyclovir, baicalein presented a lower antiviral efficacy against HSV-1 F strain (Fig. 1D), but showed superiority towards acyclovir-resistant strain (HSV-1/Blue) in both Vero cells (EC50 = 85.2 μmol/L; selectivity index (SI) > 2.35 vs. EC50 = 18.6 μmol/L; SI > 10.8) and HaCat cells (EC50 = 65.6 μmol/L; SI > 3.05 vs. EC50 = 14.8 μmol/L; SI > 13.5).
Figure 1.
Baicalein inhibited HSV-1 viral replication in vitro. (A) Vero and HaCat cells were incubated with baicalein or acyclovir at different concentrations for 72 h, and the cell viability was measured by CCK-8 assay (n = 6). (B) and (C) Vero and HaCat cells were pretreated with different concentrations of baicalein or acyclovir and then infected with HSV-1/F (MOI = 1) or HSV-1/Blue (MOI = 1) at 37 °C for 2 h. After infection, the cells were incubated with baicalein or acyclovir for an additional 24 h, and virus titers were determined by PFU assay (n = 3). (D) CC50 and IC50 of baicalein were calculated by linear regression analysis of the viral inhibition curves. SI was defined as the ratio of CC50 to EC50 (CC50/EC50). Data are presented as mean ± SD.
3.2. Baicalein suppresses viral replication
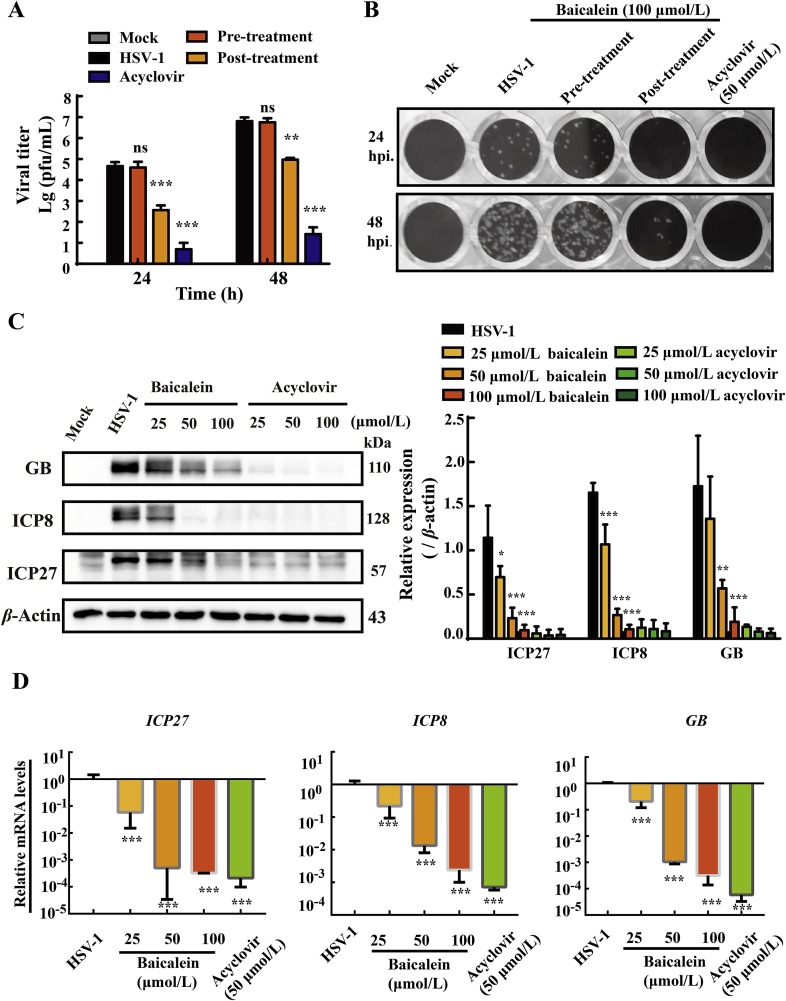
HSV-1 infection begins with viral attachment and fusion with membrane, and ultimately replicate and assemble inside the cell to release. To identify the target stage affected by baicalein during HSV-1 infection cycle, HaCat cells were treated with baicalein (100 μmol/L) for 24 and 48 h prior to or post virus inoculation. As shown in Fig. 2A and B, HSV-1 viral replication in HaCat cells was significantly prohibited by treatment of baicalein for 24 and 48 h after viral infection, compared with HSV-1 group (P < 0.001). By sharp contrast, little inhibitory effect was noticed when baicalein was pretreated (P > 0.05). This observation suggested that baicalein may target post-infection HSV-1 replication, and had a poor prophylactic effect against HSV-1. Upon entry into the cell, HSV-1 replication is temporally controlled by three main kinetic classes, known as the immediate early genes (IE), early genes (E), and late genes (L)28. Hence, we investigated the influences of baicalein on the expressions of these viral genes. The infected HaCat cells were treated with baicalein (25, 50, and 100 μmol/L) or acyclovir (25, 50, and 100 μmol/L) for 12 h post-infection. Protein expressions of ICP27 (immediate-early protein), ICP8 (early protein) and GB (glycoprotein B, late protein) were measured by Western blot assay. Similar to the effect of acyclovir, we observed that baicalein significantly and dose dependently inhibited the protein expressions of ICP27, ICP8 and GB (Fig. 2C). Moreover, the effects of baicalein on viral mRNA transcription were analyzed by RT-qRCR. A remarkable suppression of ICP27, ICP8 and GB mRNA expression was found in baicalein treatment at 12 h post-infection (Fig. 2D). These results demonstrated that baicalein might possess an inhibitory effect on HSV-1 replication.
Figure 2.
Baicalein hampered HSV-1/F gene expression in Hacat cells. (A) Infected HaCat cells were pre-treated or post-treated with baicalein (100 μmol/L) for 24 h, and virus titers were measured by PFU assay at 24 and 48 h post-infection. Acyclovir (50 μmol/L) was added after infection as a positive control (n = 3). (B) Representative images were taken from PFU assay. (C) and (D) HaCat cells were infected with HSV-1/F strain (MOI = 1) for 2 h and then treated with the indicated concentrations of baicalein or acyclovir. At 12 h post-infection, viral mRNA and protein expression levels (ICP27, ICP8, and GB) were determined by Western blot assay and RT-qPCR, respectively (n = 3). Data are presented as SD. ns, P > 0.05; *P < 0.05, **P < 0.01, ***P < 0.001 vs. HSV-1 group.
3.3. Baicalein directly inactivates HSV-1 particles
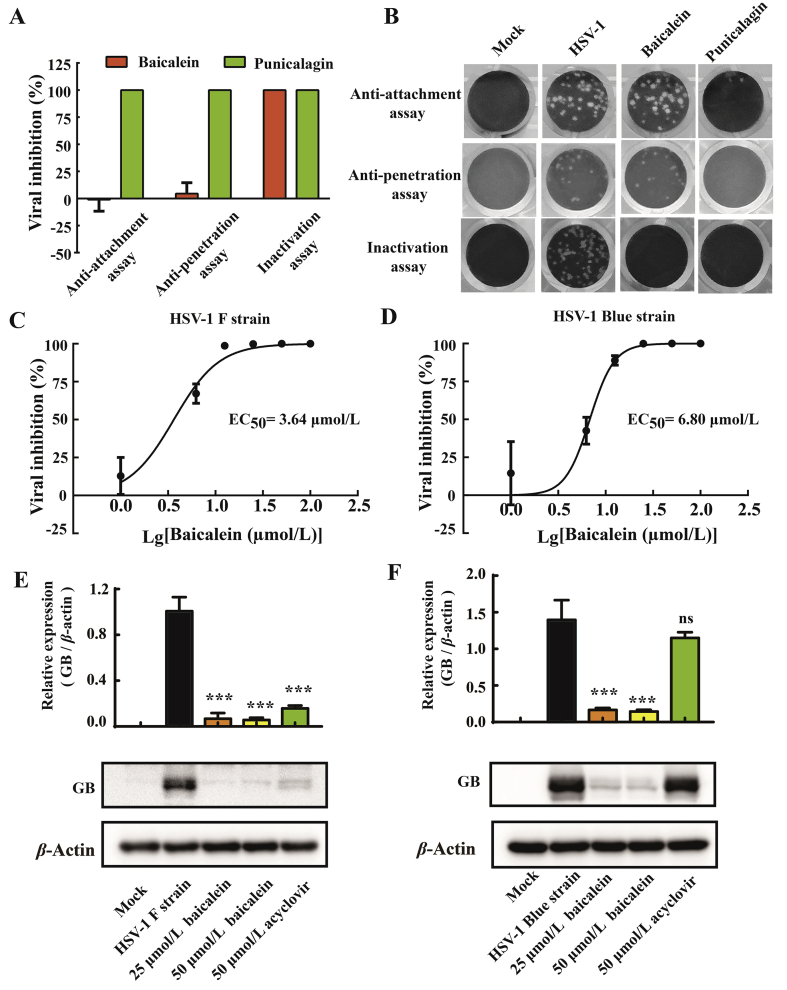
To illustrate whether baicalein could target other stages of HSV-1 infection, the influence of baicalein against viral attachment, penetration, and infectivity of viral particles was studied. Punicalagin, a large polyphenolic compound with anti-attachment, anti-penetration, and virucidal activities against HSV-129,30, was included as a positive control. Our data demonstrated that baicalein exhibited inactivating activity rather than anti-attachment and anti-penetration ability against HSV-1/EGFP infection (Supporting Information Fig. S1). This result was further confirmed by viral plaque assay (Fig. 3A and B), which was consistent with the previous finding that pretreatment of baicalein (100 μmol/L) had no preventive effect (Fig. 2A and B). Importantly, we found that baicalein inactivated HSV-1 virons in a dose-dependent manner with an EC50 value of 3.64 μmol/L against HSV-1/F and an EC50 value of 6.80 μmol/L against HSV-1/Blue (Fig. 3C and D), respectively. In addition, the infectivity of baicalein-pretreated HSV-1 was also investigated by Western blotting analysis. As shown in Fig. 3E and F, when HSV-1/F or HSV-1/Blue was pretreated with 25 or 50 μmol/L baicalein, the expression levels of GB protein were significantly inhibited (P < 0.001). These results indicated that baicalein was an effective virucidal agent against HSV-1 and neutralized the infectivity.
Figure 3.
Baicalein exerted virucidal activity against HSV-1 virions. (A) and (B) HaCat cells were treated with baicalein (100 μmol/L) at various stages of HSV-1 infection (HSV-1/EGFP). For attachment assay, HaCat cells were prechilled at 4 °C for 1 h and then inoculated with HSV-1 (MOI = 1) in the presence or absence of baicalein for another 2 h at 4 °C. After incubation, the unattached virus and baicalein were removed and washed with ice-cold PBS. For penetration assay, HaCat cells were prechilled at 4 °C for 1 h prior to inoculation with HSV-1 for 2 h at 4 °C. After attachment, the cells were incubated at 37 °C for 1 h in the presence or absence of baicalein, and then washed with PBS and treated with citrate buffer (pH 3.0) for 1 min to inactivate the extracellular non-penetrated viruses. For virus inactivation assay, HSV-1 (106 PFU) were mixed with baicalein for 2 h at 37 °C and then infected HaCat cells for an additional 48 h at a 100-fold dilution. Punicalagin (50 μmol/L) was included as a positive control. The rate of viral inhibition was analyzed by PFU assay (n = 3). (C) and (D) HSV-1/F or HSV-1/Blue (106 PFU) was mixed with a series of baicalein concentrations for 3 h at 37 °C, and then diluted 100-fold prior to infecting HaCat cells. The rate of viral inhibition was analyzed by PFU assay at 48 h post-infection. (E) and (F) HSV-1/F or HSV-1/Blue (106 PFU) was pretreated with baicalein (25 and 50 μmol/L) for 3 h at 37 °C prior to infection. Acyclovir (50 μmol/L) was added after infection as a positive control. The protein expression of GB was analyzed by Western blot assay at 12 h post-infection (n = 3). Data are presented as mean ± SD; ns, P > 0.05; ***P < 0.001 vs. HSV-1 group.
3.4. Baicalein restricts HSV-1-EGFP infection in vitro and ex vivo
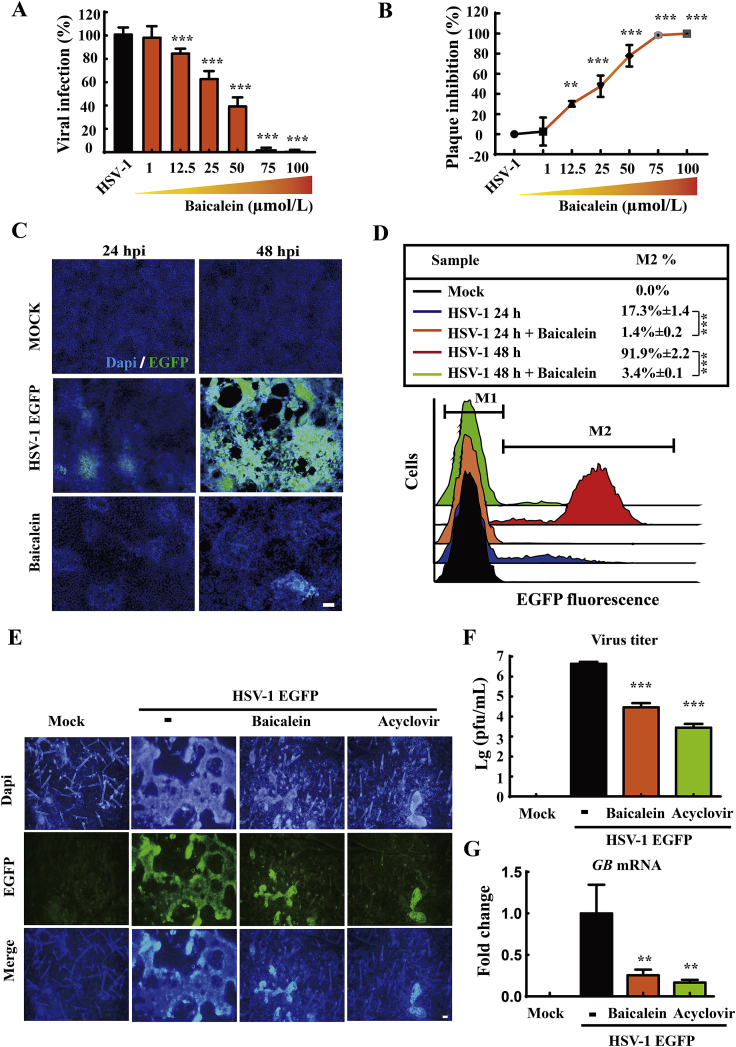
For a more intuitive observation on the anti-HSV-1 effects of baicalein, HaCat cells were infected with EGFP-labeled HSV-1 and treated with different concentrations of baicalein. Viral yields were quantitated at 24 h post-infection by EGFP fluorescence and PFU assay tests. As expected, baicalein significantly reduced viral titers at concentration above 12.5 μmol/L (Fig. 4A and B) comparing with HSV-1 group (P < 0.01). Fluorescence microscopy and flow cytometry were then employed to monitor EGFP fluorescent signaling. The inhibitory effect of baicalein on HSV-1-EGFP infection was evaluated at 24 and 48 h post-infection. As shown in Fig. 4C and D, EGFP signaling was noticeably decreased by the treatment of baicalein (100 μmol/L) at 24 h (P < 0.001) and 48 h post infection (P < 0.001) compared with HSV-1 group.
Figure 4.
Baicalein decreased HSV-1-EGFP infection. (A) and (B) HaCat cells were infected with HSV-1-EGFP (MOI = 1) for 2 h, and then treated with various concentrations of baicalein for 24 h. The rate of viral infection and plaque inhibition were quantified by EGFP fluorescence and PFU assay, respectively (n = 3). (C) and (D) HaCat cells were infected with HSV-1-EGFP (MOI = 1) and treated with baicalein (100 μmol/L) for 24 or 48 h post-infection. The infected cells were analyzed by fluorescence microscopy and flow cytometry (n = 3). Scale bars = 100 μm. (E) Murine epidermal sheets were challenged with HSV-1-EGFP (MOI = 10) for 2 h, and then cultured in the presence of baicalein (100 μmol/L) or acyclovir (50 μmol/L). At 24 h post-infection, the EGFP signal in epidermal sheets was detected by fluorescence microscopy (n = 3). Scale bars = 50 μm. (F) and (G) Virus titers and GB mRNA expression in epidermal sheets were determined by PFU assay and RT-qPCR, respectively (n = 3). Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 vs. HSV-1 group.
In addition, an ex vivo viral infection model was established in murine epidermal sheets to further confirm the anti-HSV-1 efficacy of baicalein according to a previous report26. The isolated epidermal sheets were subjected to HSV-1-EGFP infection for 2 h, and then cultured with baicalein (100 μmol/L) or acyclovir (50 μmol/L). After incubation for 24 h, EGFP fluorescence was examined under immunofluorescence microscopy. As shown in Fig. 4E, the EGFP signals were noticed throughout the basal layer of epidermis in the virus group, which was remarkably blocked by the treatment of baicalein or acyclovir. Furthermore, in comparison with HSV-1 EGFP group, both baicalein and acyclovir treatment significantly reduced viral titer (P < 0.001) and the gene expression of GB (P < 0.01) in epidermal tissues (Fig. 4F and G). The above evidences from HSV-1-EGFP infection offered additional proofs to support the anti-HSV-1 effect of baicalein.
3.5. Baicalein exerts anti-HSV-1 activity independent of its antioxidant property
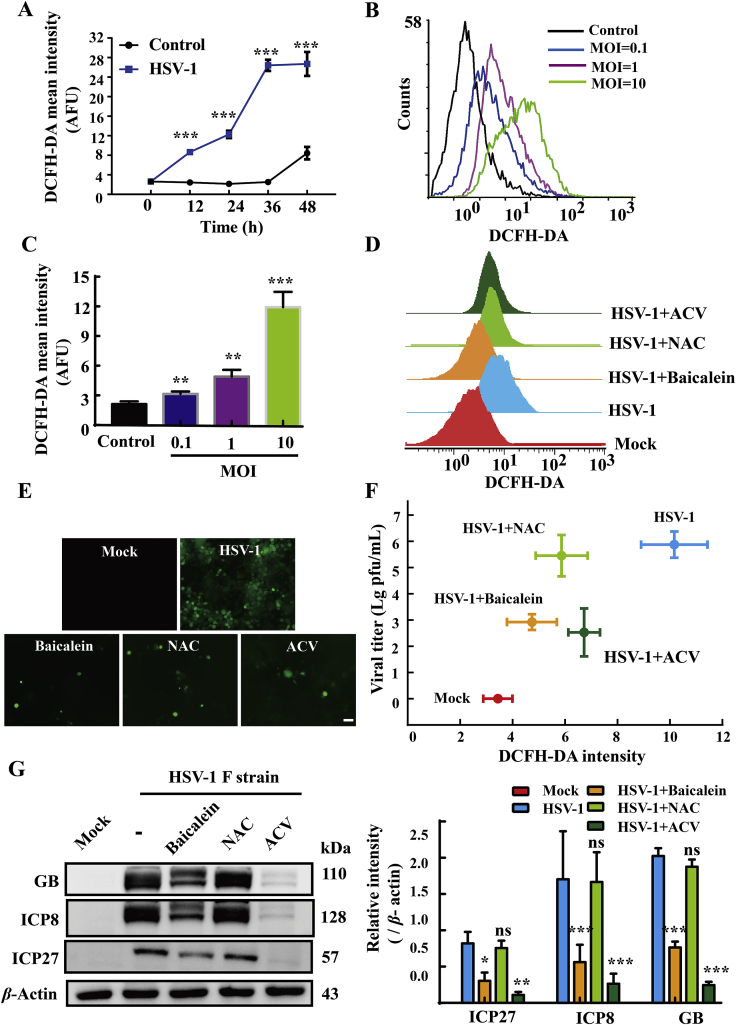
A growing body of evidences indicated that HSV-1-induced oxidative stress plays a crucial role in its replication31, 32, 33. In view of the well-known antioxidant property of baicalein34, 35, 36, we doubted whether this property contributed to its anti-HSV-1 effect. HaCat cells were infected or mock-infected with HSV-1 at different MOI (0.1, 1 and 10), and the intracellular ROS level at various time points was measured by flow cytometry following DCFH-DA staining. Our data demonstrated that HSV-1 infection induced a robust production of ROS in time- and MOI-dependent manners (Fig. 5A–C), which was significantly diminished by baicalein (100 μmol/L) and acyclovir (50 μmol/L) (Fig. 5D and E). Similarly, treatment with 10 mmol/L NAC, a common anti-oxidative agent, also decreased the levels of ROS in virus-infected cells (flow cytometry in Fig. 5D, microscopy in Fig. 5E). Nevertheless, this anti-oxidant agent did not inhibit the synthesis of ICP27, ICP8 and GB protein (P > 0.05) compared to virus-infected cells (Fig. 5G). What's more, no significant reduction of viral titer was found in the NAC-treated cells (P > 0.05). A weak correlation between viral titer and ROS level was found in NAC treatment and virus infection control groups (Fig. 5F). These observations indicated that the antiviral effect of baicalein is unlikely to be mediated by its antioxidant property in our experimental setting.
Figure 5.
The anti-HSV-1 activity of baicalein was independent of its antioxidant activity. (A) HaCat cells were infected with HSV-1/F (MOI = 1) for 2 h, and the level of intracellular ROS was analyzed by flow cytometry at various time points (n = 3). (B) and (C) HaCat cells were infected with HSV-1/F at different MOIs, and the level of intracellular ROS was measured at 12 h post infection by flow cytometry (n = 3). (D) and (E) Cells were infected with HSV-1 (MOI = 1), and then treated with baicalein (100 μmol/L), acyclovir (50 μmol/L), or NAC (10 mmol/L) for 24 h. The production of intracellular ROS was measured by flow cytometry and visualized by fluorescence microscopy (n = 3). (F) The scatter graph showed the correlation of virus titers and intracellular ROS in the infected HaCat cells (MOI = 1) treated with baicalein, acyclovir, or NAC virus titers and intracellular ROS production were quantified by PFU assay and DCFH-DA staining, respectively (n = 3). (G) The protein expressions of ICP27, ICP8, GB were analyzed by Western blot assay at 12 h post-infection (n = 3). Data are presented as mean ± SD. ns, P > 0.05; ∗P < 0.05, *∗P < 0.01, ***P < 0.001 vs. HSV-1 group.
3.6. IKK-β dephosphorylation and NF-κB inactivation contribute to the anti-HSV-1 effect of baicalein
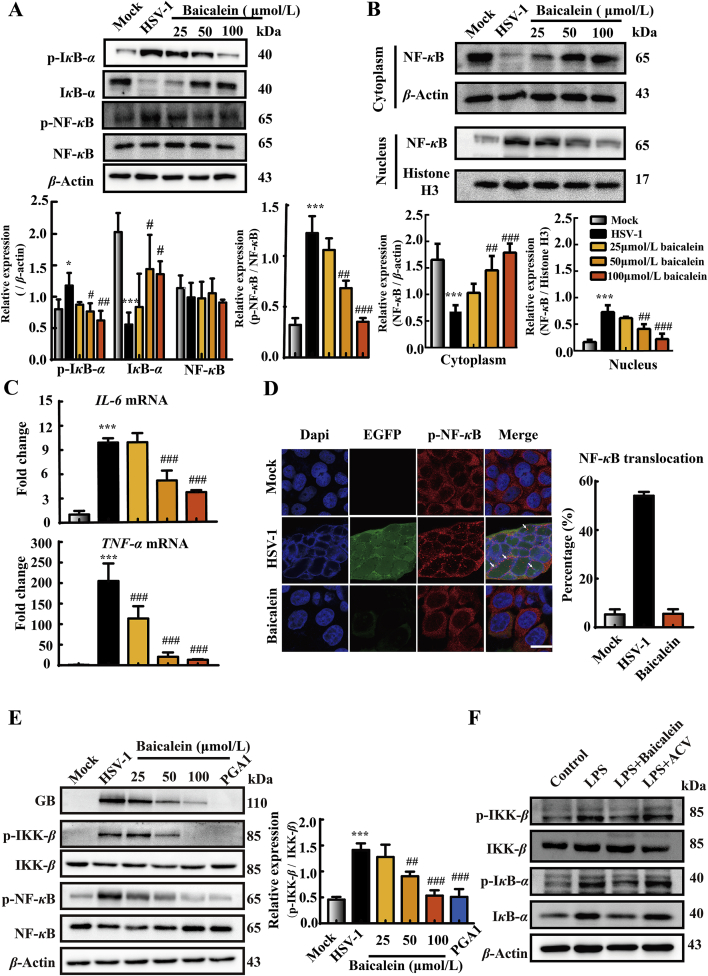
Persistent NF-κB activation was found to be essential during early HSV-1 replication37. Moreover, HSV-1 infection could induce the degradation of IκB-α, an endogenous NF-κB inhibitor, which enhanced its viral gene expression and subsequence replication38. Hence, in HSV-1 infected HaCat cells, we inspected whether baicalein affected the activation of NF-κB. We noticed that HSV-1 enhanced NF-κB (P < 0.001) and IκB-α (P < 0.05) phosphorylation, and increased IκB-α degradation (P < 0.001) at 12 h post-infection when compared with mock group (Fig. 6A). By contrast, these changes were blocked by baicalein (25, 50, 100 μmol/L) in a dose-dependent manner. To provide further evidences on the inhibitory effect of baicalein on NF-κB activation, NF-κB nuclear translocation was analyze by Western blot assay. Results revealed that baicalein dose dependently decreased NF-κB translocation (Fig. 6B). Coincidentally, the transcriptional expressions of TNF-α and IL-6, two downstream genes regulated by NF-κB activation, were significantly declined in baicalein-treated cells compared with the infected cells (Fig. 6C). Moreover, immunofluorescence experiments confirmed that baicalein (100 μmol/L) obviously inhibited NF-κB nuclear translocation and effectively suppressed EGFP-HSV-1 replication in HaCat cells (Fig. 6D).
Figure 6.
Baicalein suppressed HSV-1-induced NF-κB activation. HaCat cells were infected with HSV-1/F (MOI = 1) and then treated with baicalein (25, 50, and 100 μmol/L) for 12 h. (A) The protein expressions of p-IκB-α, IκB-α, p-NF-κB, NF-κB and β-actin were determined by Western blot assay (n = 3). (B) NF-κB nuclear translocation was analyzed by separation of nuclear and cytosolic fractions and immunoblotting with anti-NF-κB, anti-β-actin and anti-histone H3 antibody (n = 3). (C) The mRNA levels of IL-6 and TNF-α were determined by RT-qPCR (n = 3). (D) HaCat cells were infected with HSV-1-EGFP (MOI = 1) and then treated with baicalein (100 μmol/L) for 12 h. The nuclear translocation of NF-κB was observed using immunofluorescence microscopy. Scale bars = 20 μm. The white arrows indicated the nuclear translocation of NF-κB protein and the quantification analysis were from 100 to 150 cells in three independent fields. (E) HaCat cells were infected with HSV-1/F (MOI = 1) and then treated with baicalein (25, 50, and 100 μmol/L) or PGA1 (50 μmol/L) for 12 h. The protein expressions of GB, p-IKK-β, IKK-β, p-NF-κB, NF-κB and β-actin were determined by Western blot assay (n = 3). (F) HaCat cells were treated with LPS (100 ng/mL) and baicalein (100 μmol/L) or acyclovir (50 μmol/L) for 3 h. The protein expressions of p-IκB-α, IκB-α, p-IKK-β, IKK-β, and β-actin were analyzed by Western blot assay (n = 3). Data are presented as mean ± SD. *P < 0.05, ***P < 0.001 vs. Mock group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. HSV-1 group.
The degradation of IκB-α is mostly dependent on the activity and phosphorylation of IKK-β37,39. As shown in Fig. 6E, we observed that there was a remarkable augment in the phosphorylation of IKK-β in HSV-1-infected cells (P < 0.001), when comparing with mock group. In comparison, the treatment with PGA1, an IKK inhibitor, effectively blocked the phosphorylation of IKK-β (P < 0.001) and suppressed GB expression in virus-infected cells. This result was consistent with earlier reports38,40. Notably, baicalein treatment presented a similar effect as PGA1 (50 and 100 μmol/L, P < 0.01). Simultaneously, we confirmed the effect of baicalein on the phosphorylation of IKK-β induced by LPS (100 ng/mL). We found that LPS induced both the phosphorylation of IKK-β and IκB-α, and the degradation of IκB-α in HaCat cells, which were prevented by baicalein treatment (100 μmol/L). However, acyclovir (50 μmol/L) showed no impact on these proteins (Fig. 6F). These findings collectively supported that the suppression of IKK-β and inactivation of NF-κB were involved in the anti-HSV-1 effect of baicalein.
3.7. Baicalein decreases HSV-1-induced mortality and viral loads in intranasal infection mouse model
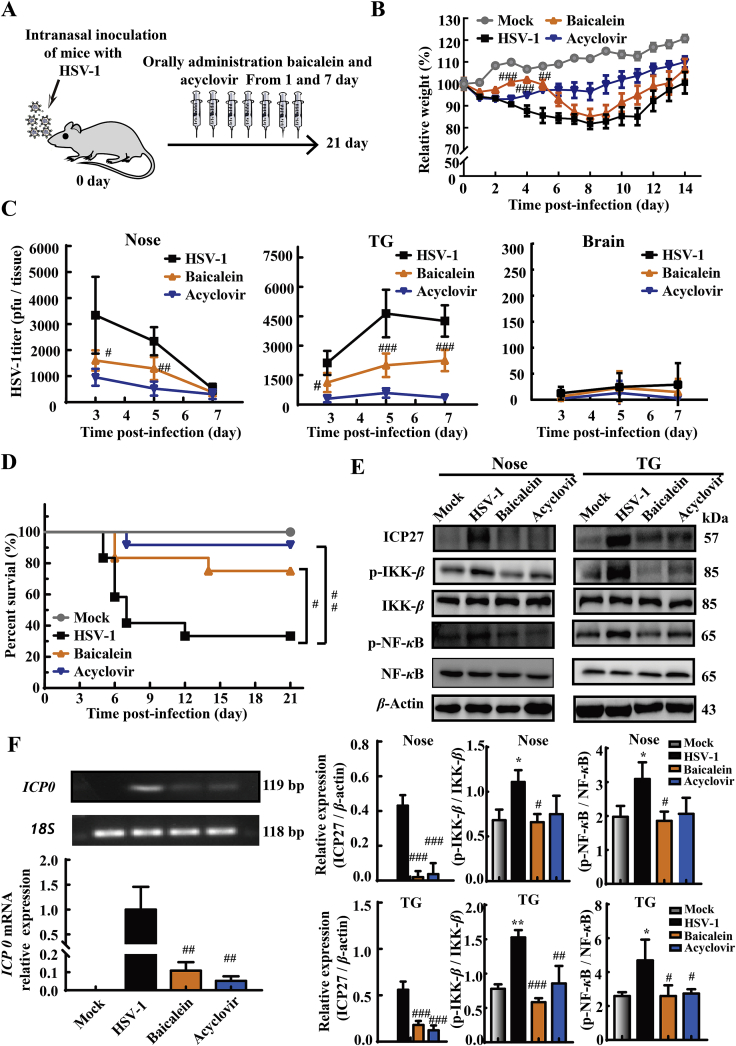
In addition to the in vitro anti-HSV-1 evidences of baicalein, we sought to obtain the in vivo proofs using intranasal infection mouse model. The animals were intranasally inoculated with HSV-1 and administrated with baicalein (200 mg/kg/day) or acyclovir (50 mg/kg/day) for 7 consecutive days (Fig. 7A). Compared with HSV-1 infected mice, baicalein treatment significantly increased the body weight from 3 to 5 dpi (P < 0.01, Fig. 7B), when the infected mice began to succumb to death (Fig. 7D). Of note, the final survival rate (by 21 dpi) of mice after baicalein treatment was significantly higher than HSV-1 infected mice (75% vs. 33.3%, Fig. 7D). Mouse nose, TG, and brain tissues were subsequently harvested to measure the virus titers at 3, 5, and 7 dpi. As shown in Fig. 7C, both baicalein and acyclovir treatments significantly decreased the viral titers in nose (3 and 5 dpi, P < 0.05) and TG (3, 5, and 7 dpi, P < 0.05). However, only a few viral particles were measured in the brains of all groups. This result was consistent with previous studies that the virus infected through intranasal inoculation normally just spread to TG and could hardly reach the brain41. Meanwhile, PCR results indicated that baicalein remarkably reduced the mRNA levels of ICP0 (P < 0.01), an IE gene transcribed by HSV-1 in TG tissues (Fig. 7F). Moreover, baicalein decreased the protein expression of viral ICP27 (P < 0.001) and reduced the phosphorylation of NF-κB (P < 0.05) and IKK-β (P < 0.05) in the nose and TG tissues (Fig. 7E). These data indicated the in vivo prohibiting effect of baicalein on HSV-1 infection and its influence on NF-κB activation.
Figure 7.
Baicalein reduced HSV-1-induced lethality and tissue viral loads of mice. Mice were intranasally challenged with HSV-1/F strain (1 × 106 PFU), and then treated with baicalein (200 mg/kg/day) or acyclovir (50 mg/kg/day) for 7 consecutive days. Saline (0.9%) was used in Mock and HSV-1 groups. (A) The schema picture illustrated the protocol of baicalein and acyclovir treatment. (B) The relative body weight of mice was monitored for 14 consecutive days after infection (n = 12). (C) The infectious virions in the nose, TG, and whole brain of mice were measured by PFU assay at the 3, 5, and 7 dpi (n = 5). (D) The survival rates of mice were monitored for 21 consecutive days (n = 12). (E) Protein expressions of ICP27, p-IKK-β, IKK-β, p-NF-κB, NF-κB and β-actin in nose and TG tissues were determined at 5 dpi by Western blot assay (n = 3). (F) The expression level of ICP0 mRNA in TG tissues was analyzed by RT-qPCR at 5 dpi (n = 4–5). Data are shown as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 vs. Mock group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. HSV-1 group.
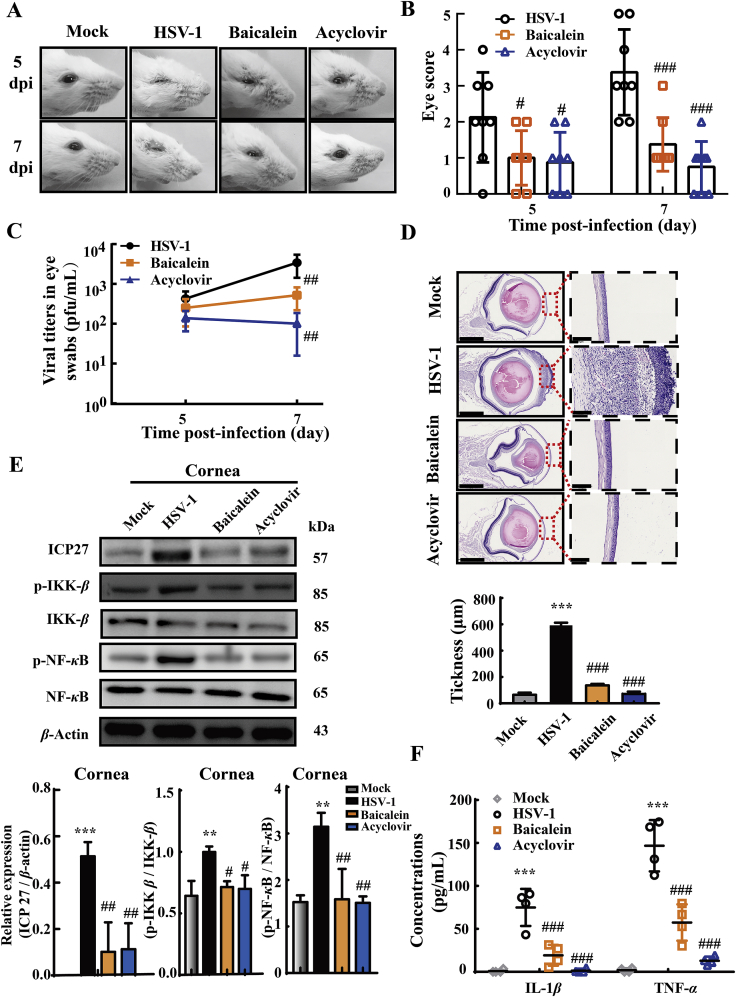
3.8. Baicalein ameliorates the symptoms of corneal diseases induced by HSV-1 infection
Apart from intranasal infection model, the therapeutic effect of baicalein was also investigated in corneally infected mice. Mice were challenged with HSV-1 by corneal inoculation, and treated with baicalein or acyclovir as described in Fig. 7A. We visually examined the status of eyes and scored the severity of eye infection in all groups at 5 and 7 dpi. A sign of eye infection was noticed at 5 dpi (scoring 2.13 ± 1.26) and the symptoms worsened at 7 dpi (scoring 3.38 ± 1.19) in virus-infected mice (Fig. 8A and B), In contrast, HSV-1-caused eye symptoms were significantly alleviated by baicalein treatment (5 dpi: scoring 1.00 ± 0.76, P < 0.05; 7 dpi: scoring 1.38 ± 0.74, P < 0.001). Moreover, by determining the virus titers secreted from the tears, we observed that both baicalein and acyclovir treatments obviously reduced the viral titers at 7 dpi (P < 0.01, Fig. 8C). When compared with mock group, the histological sections showed that the corneas were significantly thickened in HSV-1 infected mice (P < 0.001), with an obvious loss of corneal epithelium cells and a remarkable infiltration of inflammatory cells in the stroma (Fig. 8D). These histological changes were reversed to some extent by the administration of baicalein or acyclovir. Similar to the observations from intranasal infected mice, baicalein treatment also reduced the protein expression of ICP27 (P < 0.01) and decreased the phosphorylation of NF-κB (P < 0.01) and IKK-β (P < 0.05, Fig. 8E). Meanwhile, like acyclovir, baicalein effectively lowered the production of IL-1β (P < 0.001) and TNF-α (P < 0.001) in the infected eye tissues (Fig. 8F). These data demonstrated that baicalein efficiently ameliorated HSV-1 caused ocular disease, which enriched in vivo anti-virus evidence of baicalein.
Figure 8.
Baicalein ameliorated HSV-1-associated corneal disease pathologies. Mice were corneally inoculated with HSV-1/F and orally administrated with baicalein (200 mg/kg/day) or acyclovir (50 mg/kg/day) for 7 consecutive days. (A) Representative photographs were taken from the right eyes of mice. (B) Ocular disease scores were calculated according the criteria: 0, no symptoms; 1, mild swelling of the eyelids; 2, moderate swelling of the eyelids with some crusting; 3, moderate swelling of the eyelids with >50% crusting; 4, severe crusting; 5, eye completely swollen shut (n = 8). (C) The secreted virus titers were measured from the right eyes of mice at 5 and 7 dpi (n = 5). (D) Representative corneal histology sections were taken from the right eyes of mice at 9 dpi and corneal thickness were assessed by histology (n = 4). Scale bars = 1 mm. (E) The protein expressions of ICP27, p-IKK-β, IKK-β, p-NF-κB, NF-κB and β-actin in mouse corneal tissues were determined by Western blot assay at 9 dpi (n = 3). (F) The levels of IL-1β and TNF-α in eye tissue lysate were measured by ELISA at 9 dpi (n = 4). Data are shown as mean ± SD. ∗∗P < 0.01, ∗∗∗P < 0.001 vs. Mock group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. HSV-1 group.
4. Discussion
To date, there are no licensed vaccines to prevent HSV-1 infections. Long-term use of anti-herpetic drugs, such as acyclovir, has led to the emergence of drug-resistant strains and several side effects42. Therefore, the discovery of new drug remains a priority in renovating anti-HSV strategies. The present study discovers the significant in vitro activity of baicalein against HSV-1 infection, even in acyclovir-resistant strain. By using both ocular and intranasal infection in mice, we also observed that baicalein could decrease viral loads in tissues and confer a protection against HSV-1 in vivo. Moreover, baicalein significantly reduced HSV-1-EGFP infection in cultured murine epidermal sheets. Although a Korean group reported that baicalein, mistakenly named it as baicalin by the author, showed a potent inhibitory effect on HSV-1 KOS strain in vitro43, the in vivo effect and antiviral mechanism were still elusive. Undoubtedly, these in vitro, in vivo and ex vivo data underscore the potentiality of baicalein against HSV-1 infection.
To decipher the anti-HSV-1 mechanism, we discovered that pretreatment of baicalein failed to suppress viral replication in host cells, while post-treatment showed an obvious inhibitory effect. This implied that baicalein probably targeted on the post-entry stage of virus infection. Earlier studies have demonstrated that baicalein can inhibit NF-κB activation induced by various pathological factors44,45. The effect of NF-κB on cell survival and viral replication is well documented. Evidences have suggested that sustained activation of NF-κB is required for viral replication46,47. Besides, a relationship between NF-κB inactivation and the anti-HSV effects of some molecules has been established48, 49, 50. In this study, we found that baicalein impeded NF-κB activation by inhibiting IKK-β and IκB-α phosphorylation. The reduced IκB-α degradation eventually hinders viral replication. In fact, an in silico analysis has already indicated baicalein as a potential inhibitor of human IKK-β51. Therefore, the suppression of IKK-β phosphorylation and resultant diminished NF-κB activation contributes to the protective effect of baicalein on HSV-1 infection. Nevertheless, how baicalein affects the phosphorylation of IKK-β requires further exploration. In additional to affecting NF-κB activation, our results also revealed direct inactivation of HSV-1 particles by baicalein. The dual mechanism might interweave the anti-HSV-1 effect of baicalein.
Previous researches31, 32, 33 have reported that the production of ROS is critical to HSV-1 replication after infection. In view of the reported antioxidant activity of baicalein34, 35, 36, we surveyed whether its anti-HSV-1 activity is linked with the antioxidant property. Notably, the production of ROS upon HSV-1 infection was significantly inhibited by baicalein treatment. Nevertheless, the blockage of ROS by NAC had no rescue effects on viral replication, which is supported by a previous report52. These observations indicate that the antiviral activity of baicalein is independent of its antioxidant activity, and host ROS production may be an outcome of the HSV-1 replication.
Owing to poor pharmacokinetic and pharmacodynamic properties, many antiviral compounds effective in vitro were lacking considerable antiviral activity in animal study and clinical trial. Our study shows that oral administration of baicalein presented a considerable anti-HSV-1 capacity in mice. Moreover, in a clinical study, oral administration of baicalein has been shown to possess relatively favorable pharmacokinetic profiles without any serious side effects53, demonstrating a strong potential to develop baicalein as an HSV-1 drug in clinic. As baicalin is the main in vivo metabolite of baicalein after oral intake, whether baicalin contributes to the anti-HSV-1 activity of baicalein in vivo deserves further investigation.
5. Conclusions
Our research indicates that baicalein is highly effective in combating HSV-1 infection. Dual mechanisms were involved in its antivirus effect, namely the inactivation of free viral particles to neutralize the infectivity and the suppression of NF-κB activation, which is distinct from that of acyclovir. Hence, this work offers experimental basis for baicalein as a potential drug in treating HSV-1 infection and related diseases.
Acknowledgments
This study was partly supported by National Natural Science Foundation of China (Grant Nos. U1801284, 81573675, 81622050, 81873209 and 81673709), National Key Research and Development Program of China (Grant No. 2017YFC1700404), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant No. 2017BT01Y036, China) and GDUPS (2019, China), the Guangdong Science and Technology Foundation (Grant No. 2017A030306004), the Program of Hong Kong Scholar (XJ2016017, China), Science and Technology Program of Guangzhou (Grant No. 201903010062, China), the Youth Top-notch Talent Support Program of Guangdong Province (Grant No. 2016TQ03R586, China). We also thank Professor Yaolan Li (Jinan University, Guangzhou, China) and Yifei Wang (Jinan University) for providing HSV-1 standard F strain and HSV-1 Blue strain, respectively.
Author contributions
Rong-Rong He, Yi-Fang Li and Zhuo Luo designed the research. Zhuo Luo, Xiu-Ping Kuang, Qing-Qing Zhou, Chang-Yu Yan, Wen Li, and Hai-Biao Gong performed the experiments and carried out data analysis. Zhuo Luo wrote the manuscript and Rong-Rong He, Yi-Fang Li, Wei-Xi Li, Hiroshi Kurihara revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data related to this article can be found at https://doi.org/10.1016/j.apsb.2020.06.008.
Contributor Information
Wei-Xi Li, Email: liweixi1001@163.com.
Yi-Fang Li, Email: liyifang706@jnu.edu.cn.
Rong-Rong He, Email: rongronghe@jnu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Looker K.J., Garnett G.P. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81:103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzich J.B., Cliffe A.R. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology. 2018;522:81–91. doi: 10.1016/j.virol.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S.P., Chandy M.L., Shanavas M., Khan S., Suresh K.V. Pathogenesis and life cycle of herpes simplex virus infection-stages of primary, latency and recurrence. J Oral Maxillofac Surg Med Pathol. 2016;28:350–353. [Google Scholar]
- 4.Yan C., Luo Z., Li W., Li X., Dallmann R., Hiroshi Kurihara H. Disturbed Yin–Yang balance: stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1. Acta Pharm Sin B. 2020;10:383–398. doi: 10.1016/j.apsb.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooq A.V., Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khadr L., Harfouche M., Omori R., Schwarzer G., Chemaitelly H., Abu-Raddad L.J. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2018;68:757–772. doi: 10.1093/cid/ciy562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De-Clercq E. Antivirals: past, present and future. Biochem Pharmacol. 2013;85:727–744. doi: 10.1016/j.bcp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 8.De-Clercq E. A 40-year journey in search of selective antiviral chemotherapy. Annu Rev Pharmacol Toxicol. 2011;51:1–24. doi: 10.1146/annurev-pharmtox-010510-100228. [DOI] [PubMed] [Google Scholar]
- 9.Piret J., Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di-Sotto A., Di-Giacomo S., Amatore D., Locatelli M., Vitalone A., Toniolo C. A polyphenol rich extract from Solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus type 1 activity in vitro. Molecules. 2018;23:2066. doi: 10.3390/molecules23082066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mifsud E.J., Hayden F.G., Hurt A.C. Antivirals targeting the polymerase complex of influenza viruses. Antivir Res. 2019;169:104545. doi: 10.1016/j.antiviral.2019.104545. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q., Chen X.Y., Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull (Beijing) 2016;61:1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li-Weber M. New therapeutic aspects of flavones: the anti-cancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X.J., Liu S., Xing J.P., Liu Z.Q., Song F.R. Effect of type 2 diabetes mellitus on flavonoid pharmacokinetics and tissue distribution after oral administration of Radix Scutellaria extract in rats. Chin J Nat Med. 2018;16:418–427. doi: 10.1016/S1875-5364(18)30075-X. [DOI] [PubMed] [Google Scholar]
- 15.Chen B., Luo M., Liang J., Zhang C., Gao C.F., Wang J. Surface modification of PGP for a neutrophil-nanoparticle co-vehicle to enhance the anti-depressant effect of baicalein. Acta Pharm Sin B. 2018;8:64–73. doi: 10.1016/j.apsb.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan W.J., Ma X.C., Gao X.Y., Xue X.H., Zhang S.Q. Latest research progress in the correlation between baicalein and breast cancer invasion and metastasis. Mol Clin Oncol. 2016;4:472–476. doi: 10.3892/mco.2016.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M.T. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Stockwell B.R., Angeli J.P.F., Bayir H., Bush A.I., Conrad M., Dixon S.J. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q., Li Q.Q., Jia J.N., Sun Q.Y., Zhou H.H., Jin W.L. Baicalein exerts neuroprotective effects in FeCl3-induced post-traumatic epileptic seizures via suppressing ferroptosis. Front Pharmacol. 2019;10:638. doi: 10.3389/fphar.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oo A., Teoh B.T., Sam S.S., Bakar S.A., Zandi K. Baicalein and baicalin as Zika virus inhibitors. Arch Virol. 2019;164:585–593. doi: 10.1007/s00705-018-4083-4. [DOI] [PubMed] [Google Scholar]
- 21.Moghaddam E., Teoh B.T., Sam S.S., Lani R., Hassandarvish P., Chik Z. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oo A., Rausalu K., Merits A., Higgs S., Vanlandingham D., Bakar S.A. Deciphering the potential of baicalin as an antiviral agent for Chikungunya virus infection. Antivir Res. 2018;150:101–111. doi: 10.1016/j.antiviral.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Jin J., Chen Y., Wang D., Ma L., Guo M., Zhou C. The inhibitory effect of sodium baicalin on oseltamivir-resistant influenza A virus via reduction of neuraminidase activity. Arch Pharm Res. 2018;41:664–676. doi: 10.1007/s12272-018-1022-6. [DOI] [PubMed] [Google Scholar]
- 24.Benboudjema L., Mulvey M., Gao Y., Pimplikar S.W., Mohr I. Association of the herpes simplex virus type 1 Us11 gene product with the cellular kinesin light-chain-related protein PAT1 results in the redistribution of both polypeptides. J Virol. 2003;77:9192–9203. doi: 10.1128/JVI.77.17.9192-9203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris J., Stuart P.M., Rogge M., Potter C., Gupta N., Yin X.T. Recurrent herpetic stromal keratitis in mice, a model for studying human HSK. J Vis Exp. 2012;70 doi: 10.3791/4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahn E., Thier K., Petermann P., Knebel-Mörsdorf D. Ex vivo infection of murine epidermis with herpes simplex virus type 1. J Vis Exp. 2015;102 doi: 10.3791/53046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinert L.S., Lopušná K., Winther H., Sun C., Thomsen M.K., Nandakumar R. Sensing of HSV-1 by the cGAS–STING pathway in microglia orchestrates antiviral defence in the CNS. Nat Commun. 2016;7:13348. doi: 10.1038/ncomms13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De-Mello C.P.P., Bloom D.C., Paixão I.C. Herpes simplex virus type-1: replication, latency, reactivation and its antiviral targets. Antivir Ther. 2016;21:277–286. doi: 10.3851/IMP3018. [DOI] [PubMed] [Google Scholar]
- 29.Lin L.T., Chen T.Y., Chung C.Y., Noyce R.S., Grindley T.B., McCormick C. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein–glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 2011;85:4386–4398. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L.T., Chen T.Y., Lin S.C., Chung C.Y., Lin T.C., Wang G.H. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:187. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Dosal R., Horan K.A., Rahbek S.H., Ichijo H., Chen Z.J., Mieyal J.J. HSV infection induces production of ROS, which potentiate signaling from pattern recognition receptors: role for S-glutathionylation of TRAF3 and 6. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z., Xu X., Leng X., He M., Wang J., Cheng S. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch Virol. 2017;162:603–610. doi: 10.1007/s00705-016-3130-2. [DOI] [PubMed] [Google Scholar]
- 33.To E.E., Vlahos R., Luong R., Halls M.L., Reading P.C., King P.T. Endosomal NOX2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat Commun. 2017;8:69. doi: 10.1038/s41467-017-00057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh D.E., Liu L.T., Lin C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 35.Tian Y., Li X., Xie H., Wang X., Xie Y., Chen C. Protective mechanism of the antioxidant baicalein toward hydroxyl radical-treated bone marrow-derived mesenchymal stem cells. Molecules. 2018;23:223. doi: 10.3390/molecules23010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang K.A., Zhang R., Piao M.J., Chae S., Kim H.S., Park J.H. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol Ind Health. 2012;28:412–421. doi: 10.1177/0748233711413799. [DOI] [PubMed] [Google Scholar]
- 37.Santoro M.G., Rossi A., Amici C. NF-κB and virus infection: who controls whom. EMBO J. 2003;22:2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amici C., Rossi A., Costanzo A., Ciafrè S., Marinari B., Balsamo M. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J Biol Chem. 2006;281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 39.Amaya M., Keck F., Bailey C., Narayanan A. The role of the IKK complex in viral infections. Pathog Dis. 2014;72:32–44. doi: 10.1111/2049-632X.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amici C., Belardo G., Rossi A., Santoro M.G. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J Biol Chem. 2001;276:28759–28766. doi: 10.1074/jbc.M103408200. [DOI] [PubMed] [Google Scholar]
- 41.Shivkumar M., Milho R., May J.S., Nicoll M.P., Efstathiou S., Stevenson P.G. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J Virol. 2013;87:10477–10488. doi: 10.1128/JVI.01748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y.C., Feng H., Lin Y.C., Guo X.R. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1–6. doi: 10.1038/ijos.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyu S.Y., Rhim J.Y., Park W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch Pharm Res. 2005;28:1293–1301. doi: 10.1007/BF02978215. [DOI] [PubMed] [Google Scholar]
- 44.Seo M.B., Lee S.K., Jeon Y.J., Im J.S. Inhibition of p65 nuclear translocation by baicalein. Toxicol Res. 2011;27:71–76. doi: 10.5487/TR.2011.27.2.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Ma J., Wang K.S., Mi C., Wang Z., Piao L.X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol Rep. 2016;36:2771–2776. doi: 10.3892/or.2016.5108. [DOI] [PubMed] [Google Scholar]
- 46.Hiscott J., Kwon H., Génin P. Hostile takeovers: viral appropriation of the NF-κB pathway. J Clin Invest. 2001;107:143–151. doi: 10.1172/JCI11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao J., He S., Minassian A., Li J., Feng P. Recent advances on viral manipulation of NF-κB signaling pathway. Curr Opin Virol. 2015;15:103–111. doi: 10.1016/j.coviro.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu M., Chen Y., Chu Y., Song S., Yang N., Gao J. Zinc ionophores pyrithione inhibits herpes simplex virus replication through interfering with proteasome function and NF-κB activation. Antivir Res. 2013;100:44–53. doi: 10.1016/j.antiviral.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Chen X., Wang Z., Yang Z., Wang J., Xu Y., Tan R. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antivir Res. 2011;92:341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutluay S.B., Doroghazi J., Roemer M.E., Triezenberg S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373:239–247. doi: 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sala E., Guasch L., Iwaszkiewicz J., Mulero M., Salvadó M.J., Bladé C. Identification of human IKK-2 inhibitors of natural origin (part II): in silico prediction of IKK-2 inhibitors in natural extracts with known anti-inflammatory activity. Eur J Med Chem. 2011;46:6098–6103. doi: 10.1016/j.ejmech.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Chen D., Su A., Fu Y., Wang X., Lv X., Xu W. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antivir Res. 2015;123:27–38. doi: 10.1016/j.antiviral.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Li M., Shi A., Pang H., Xue W., Li Y., Cao G. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol. 2014;156:210–215. doi: 10.1016/j.jep.2014.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.