Abstract
Background
Childhood adversity is, unfortunately, highly prevalent and strongly associated with later psychopathology. Recent theories posit that two dimensions of early adversity, threat and deprivation, have distinct effects on brain development. The current study evaluated whether violence exposure (threat) and social deprivation (deprivation) were associated with adolescent amygdala and ventral striatum activation, respectively, in a prospective, well-sampled, longitudinal cohort using a pre-registered, open science approach.
Methods
One hundred and sixty-seven adolescents from the Fragile Families and Child Wellbeing Study completed functional magnetic resonance imaging (fMRI) scanning. Prospective longitudinal data from ages 3, 5 and 9 years were used to create indices of childhood violence exposure and social deprivation. We evaluated whether these dimensions were associated with adolescent brain function in response to threatening and rewarding faces.
Results
Childhood violence exposure was associated with decreased amygdala habituation (i.e. more sustained activation) and activation to angry faces in adolescence, whereas childhood social deprivation was associated with decreased ventral striatum activation to happy faces in adolescence. These associations held when adjusting for the other dimension of adversity (e.g., adjusting for social deprivation when examining associations with violence exposure), the interaction of the two dimensions of adversity, gender, internalizing psychopathology, and current life stress.
Conclusions
Consistent with recent theories, different forms of early adversity were associated with region-specific differences in brain activation.
Keywords: early adversity, fMRI
Introduction
Forty-five percent of US children experience at least one adverse childhood experience (ACE) (Sacks and Murphey, 2018). These experiences are potent contributors to the development of mental disorders such as depression and anxiety (CDC, 2019; Sacks and Murphey, 2018). Observations that adverse experiences often co-occur led to a focus on cumulative risk, whereby the number of exposures is the emphasis, rather than the specific type (e.g. Sameroff et al., 2004). This approach yielded numerous findings linking childhood adversities to internalizing problems (e.g. Appleyard et al., 2005). Though this research demonstrated that exposure number relates to outcomes, it combined qualitatively different adversities together. Alternatively, many neuroimaging studies have focused on a single type of adversity, such as emotional neglect (e.g. Maheu et al., 2010), which does not account for the co-occurring nature of adverse experiences.
To address the need to aggregate similar (and disaggregate dissimilar) adversities based on animal models that can inform potential neuroanatomical specificity of the impact of different types of adversities, McLaughlin and Sheridan proposed the Dimensional Model of Adversity and Psychopathology (DMAP) (McLaughlin et al., 2014; Sheridan and McLaughlin, 2014). The DMAP posits that many early adverse experiences are captured by dimensions of threat and deprivation, which include experiences across domains that are qualitatively similar. These dimensions are expected to have differential associations with specific types of alterations in brain development and the anatomical location of these alterations. Threat is conceptualized as an actual or perceived threat of harm to one’s physical integrity and includes experiences such as physical abuse and intimate partner violence. Based on human and animal models, threat experiences are hypothesized to affect the development of neural structures involved in the stress response and fear conditioning in the limbic system (e.g. amygdala) and lead to anxiety (McLaughlin et al., 2014; McLaughlin and Sheridan, 2016). In support of this hypothesis, threatening experiences (child maltreatment and family violence) are associated with increased amygdala reactivity (see Hein and Monk, 2017, for a meta-analysis; McCrory et al., 2011). Deprivation is the absence of biologically expected input (cognitive or social) and includes experiences such as institutionalization and emotional neglect. Deprivation is hypothesized to be associated with alterations in brain regions implicated in executive function and reward processing (e.g. the ventral striatum) and leads to depression (McLaughlin et al., 2014; McLaughlin and Sheridan, 2016). In line with this hypothesis, more social forms of deprivation are linked to alterations in neural circuitry associated with reward processing, particularly blunted ventral striatum reactivity (Mehta et al., 2010; Hanson et al., 2015). Some degree of exposure to both violence exposure and social deprivation is likely. Co-occurring social deprivation may exacerbate the effects of violence exposure. Additionally, social support, the inverse of social deprivation, may serve as a buffer against violence exposure (Sheridan et al., 2018; Sonuga-Barke et al., 2010). Prior work in our sample has demonstrated an interaction effect whereby the impact of violence exposure on cortisol response to a social stressor was exacerbated by high social deprivation (Peckins et al., 2019). Therefore, when examining the unique impacts of violence exposure and social deprivation, it is important to consider their interaction.
Consistent with the DMAP, recent neuroimaging studies examining the impact of early adversity on socioemotional outcomes have focused on two neural circuits: threat and reward. Perceiving and responding correctly to stimuli that signal risk of harm, such as angry or fearful faces, involve the amygdala and afford survival advantage (Phelps and LeDoux, 2005). Variability in amygdala activation is linked to the development of maladaptive emotion regulation and psychopathology (e.g. Monk et al., 2008; Hyde et al., 2016). Beyond simply examining reactivity to threat, recent studies have begun to assess amygdala habituation (Rankin et al., 2009), which may be a more reliable indicator of amygdala function (Plichta et al., 2014; Gee et al., 2015) and is associated with anxiety symptoms (Hare et al., 2008). A second critical circuit is reward, which processes stimuli such as food, money and happy faces (Izuma et al., 2008). One key region in this circuit is the nucleus accumbens, which is part of the ventral striatum (Russo and Nestler, 2013) and serves as a node for reward-driven behaviors (Schultz et al., 1992). Variability in ventral striatum activation to rewards is related to internalizing symptoms (Forbes and Dahl, 2012).
We evaluated whether dimensions of early adversity are associated with threat- and reward-related neural activity and whether the neural activation in these regions is in turn associated with anxiety and depression, respectively, in adolescence. To evaluate these hypotheses, we integrated prospective longitudinal data on exposure to adversity and neuroimaging in a sample of adolescents drawn from a nationally representative sample. For the present work, we created a violence exposure and victimization (violence exposure, henceforth) construct to measure threat. We selected the term ‘violence exposure’ because it more fully captured the targeted experiences of the construct. Specifically, violence exposure included child abuse, intimate partner violence and community violence. Based on a prior work linking exposure to violence and alterations in threat processing (McCrory et al., 2011), we examined amygdala habituation and activation to angry and fearful faces, which signal threat. Whereas DMAP’s deprivation dimension refers to material deprivation and extreme global deprivation (e.g. institutional rearing), the latter of which can be associated with lack of both age-expectant cognitive and social inputs, we focused specifically on the deprivation of inherently rewarding components of the social environment (heretofore social deprivation) and their potential impacts on social reward circuitry. In the present study, social deprivation included caregiver neglect, absence of supportive relationships of the caregiver in the home and absence of supportive community relationships. Based on a work that found that deprivation that is social in nature is related to altered reward processing (Hanson et al., 2015), we examined neural response to happy faces, inherently socially rewarding stimuli. Consistent with the DMAP’s predictions, we hypothesized that violence exposure would be associated with anxiety, whereas deprivation would be associated with depressive symptoms, via their respective neural circuits (i.e. threat-related amygdala reactivity and reward-related ventral striatum reactivity, respectively).
The current study had the following four objectives: (i) to establish the association of childhood violence exposure on adolescent amygdala habituation to threat; (ii) to assess the relation between childhood violence exposure and adolescent amygdala activation to threat; (iii) to relate childhood social deprivation to adolescent ventral striatum activation to social reward and (iv) to examine whether activity in these neural regions mediated associations between childhood violence exposure and adolescent anxiety, and childhood social deprivation and adolescent depression. These objectives, the associated variables and the analyses were pre-registered with the open science framework (https://osf.io/54uhy). Data are publicly available through the National Institute of Mental Health (NIMH) database (https://nda.nih.gov/edit_collection.html?id=2106). We hypothesized the following: (i) greater childhood violence exposure would be associated with decreased amygdala habituation to threat; (ii) greater childhood violence exposure would be associated with increased amygdala activation to threat; (iii) greater childhood social deprivation would be associated with decreased ventral striatum reactivity to social reward and (iv) increased amygdala reactivity and decreased habituation to threat would mediate an association between childhood violence exposure and adolescent anxiety, and decreased ventral striatum reactivity to social reward would mediate an association between childhood social deprivation and adolescent depression.
Methods
Participants
The Fragile Families and Child Wellbeing Study (FFCWS) (Reichman et al., 2001) is comprised of a population-based sample of children born in the large US cities, with an oversample of non-marital births (~3:1). FFCWS families were interviewed at the birth of the focal child, and when the child was 1, 3, 5, 9 and 15 years of age. At 15 years, 237 FFCWS adolescents born in Detroit, Toledo or Chicago and their caregivers participated in the Study of Adolescent Neural Development (SAND), a neuroimaging study of the core FFCWS (Supplement S1). Importantly, this sample contains substantial representation of African American youth, as well as adolescents from families living in low-income contexts (S1). 167 adolescents successfully completed functional magnetic resonance imaging (fMRI) scanning (S1). This sample partially overlaps with those in some of our prior work (Hein et al., 2018; Goetschius et al., 2019).
Ethical considerations
Adolescents provided written informed assent, and their caregivers provided written consent for both themselves and their children. The University of Michigan Medical School institutional review board approved this study (HUM00074392).
Procedures
Emotional faces task.
Participants completed an event-related emotional faces task during fMRI acquisition (Hein et al., 2018). Participants viewed fearful, happy, sad, neutral and angry faces. Faces were presented for 250 ms, and 20 trials of each emotion were presented in one of two random orders. Following each face, a black screen appeared for 1500 ms. During this time, participants identified the gender of the actor with a button response. Finally, a second black screen, the baseline, appeared (jittered at 2, 4 and 6 s).
Violence exposure and social deprivation composite scores
Composite scores indexing violence exposure and social deprivation were created using data collected at 3, 5 and 9 years from the FFCWS (S2). For both constructs, experiences that directly and indirectly impacted the focal child were included, allowing for comprehensive capture of adversity in the environment. Violence exposure included abuse from a caregiver, intimate partner violence in the home and violence within the community. Social deprivation included neglect from a caregiver, absence of supportive relationships of the caregiver in the home and absence of supportive relationships within the community. Child abuse and neglect were assessed using subscales of the Parent-Child Conflict Tactics Scale (CTS-PC; Straus et al., 1998). Questions about intimate partner violence, community violence, supportive relationships in the home and neighborhood social cohesion came from prior studies using the FFCWS sample (Zhang and Anderson, 2010; Manuel et al., 2012; Donnelly et al., 2016; Hunt et al., 2017).
We created composite scores using averaging (Song et al., 2013) within a dimension and divided by the number of experiences with a dimension each participant had data for to maximize sample size diversity. We centered violence exposure and social deprivation scores and created an interaction term of the two variables. Violence exposure and social deprivation composite scores were correlated (r (235) = 0.45, P < 0.001), but their multi-collinearity was low (VIF = 1.24).
Analyses
fMRI data analysis.
fMRI data were collected, preprocessed and analyzed using methodology detailed in our prior work and in supplemental materials (Goetschius et al., 2019; S3). In terms of quality assurance, de-spiking is completed in K space and then at the end of preprocessing. Artifact Detection Tools (ART) software (http://www.nitrc.org/projects/artifact_detect) was used to identify volumes with above threshold motion (>2 mm movement or 3.5° rotation). These volumes were used as covariates and removed statistically from individual participant models. Habituation was analyzed using methodology described in our prior work and in supplemental materials (Hein et al., 2018; S3). Briefly, to derive habituation, activation during the first half of the task to specific emotions was contrasted with activation to the second half of the task to the same emotions.
Group analyses
Objectives 1 and 2.
The relations between childhood violence exposure and both adolescent amygdala habituation and activation to threatening (angry and fearful, separately) faces were evaluated using multiple regression analysis in SPM12. For habituation, we regressed the violence exposure composite score onto the contrasts of early > late angry and early > late fearful, while controlling for social deprivation, the interaction of violence exposure and social deprivation, and gender. As some degree of exposure to both violence exposure and social deprivation is likely, we adjusted for social deprivation and the interaction of violence exposure and social deprivation to better isolate associations with violence exposure. For activation, we ran similar analyses, but regressed the violence exposure composite score onto the contrasts of angry > implicit baseline and fearful > implicit baseline. Significance was evaluated for predetermined left and right structural amygdala regions of interest (ROIs) from PickAtlas (Maldjian et al., 2003) at a voxel-wise family-wise error P < 0.05 threshold. Additionally, we corrected for multiple comparisons for both habituation and activation analyses using a Holm–Bonferroni correction for four comparisons: two emotions (angry and fearful) in both amygdalae. Individual parameter estimates for the main effects of amygdala habituation and activation were extracted from anatomical ROIs by averaging across voxels to be used in subsequent structural equation models.
Objective 3.
The relationship between childhood social deprivation and adolescent ventral striatum reactivity to socially rewarding (happy) faces was evaluated using SPM12 multiple regression analysis. We regressed the social deprivation composite score onto the contrast of happy > implicit baseline, while adjusting for violence exposure, the interaction of violence exposure and social deprivation, and gender. As with violence exposure, we adjusted for the interaction of violence exposure and social deprivation to better isolate the main associations with social deprivation. To evaluate the significance for ventral striatum, we used left and right nucleus accumbens structural ROIs from PickAtlas at a voxel-wise family-wise error P < 0.05 threshold. As mentioned above, we corrected for multiple comparisons by using the Holm–Bonferroni correction. This corrected for two comparisons: one emotion (happy) in left and right ventral striatum. Individual parameter estimates for the main effects of both ventral striatum activations were extracted by averaging across voxels.
Objective 4.
To examine whether violence exposure and social deprivation were associated with adolescent anxiety and depression via alterations in threat- and reward-related neural activation, respectively, we evaluated structural equation models that followed from our pre-registration and posited violence exposure and social deprivation were related to latent factors of anxiety and depression created from symptom counts on child and parent report and from clinical interviews and questionnaires (Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS-PL), Children’s Depression Inventory (CDI), Mood and Feelings Questionnaire (MFQ), and Screen for Child Anxiety Related Disorders (SCARED) S4) via their association with threat and reward neural circuitry (Figure 1).
Fig. 1.
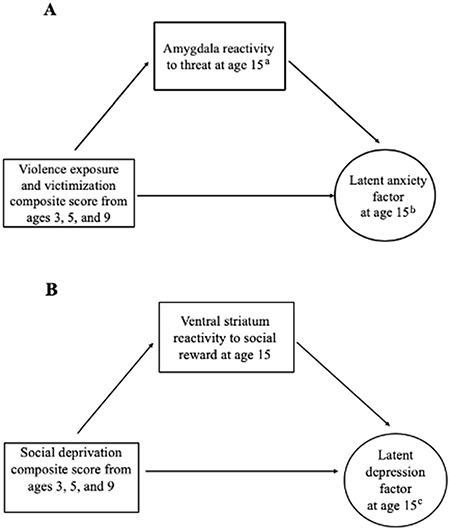
Planned structural equation modeling (SEM) analyses.
Proposed SEMs to test full models. Model A tested mediation models where alterations in amygdala reactivity (habituation and activation) to threat mediate the association between violence exposure and adolescent anxiety. Model B represents a mediation model where alterations in ventral striatum activation to social reward mediate the association between social deprivation and adolescent depression. aBoth amygdala habituation and activation (two separate models). bCreated from symptom counts on child and parent report on anxiety in clinical interviews and questionnaires (S4). cCreated from symptom counts on child and parent report on depression in clinical interviews and questionnaires (S4).
Sensitivity analyses
We ran a number of sensitivity analyses to assess potential confounders. First, given stability in family adversity over time (Gest et al., 1999), we assessed whether any associations found between early adversity and later neural reactivity were related to current stress, by re-running our SPM analyses with adolescent life stress (ALES; S5) as a covariate. Second, due to associations between neural reactivity and internalizing psychopathology, we evaluated whether our results were driven by earlier presentation of internalizing psychopathology as opposed to associations of early adversity with later neural reactivity by re-running our SPM analyses with a composite measure of internalizing symptoms as measured by modified versions of the Child Behavior Checklist (CBCL) (Achenbach, 1999) administered by FFCWS at ages 3, 5 and 9 years (S12). Third, we re-ran our SPM analyses with the number of censored scans as a covariate to assess whether our results were driven by differences in motion during fMRI scanning (S13).
Results
Objective 1
Higher levels of violence exposure were associated with reduced right amygdala habituation to angry faces (Figure 2; S7). This association was specific to angry faces; violence exposure was not related to habituation to fearful faces. It was also not related to habituation to other expressions (evaluated as follow-up to gain clarity on specificity). Using individual parameter estimates extracted from the significant clusters from this analysis, we visualized activation in the first and second halves of the faces task, separating participants into groups based on above or below mean levels of violence exposure (Figure 3, for visualization purposes only). Individuals low on violence exposure demonstrated typical amygdala habituation, with significantly less activation to angry faces in the second half of the task. In contrast, individuals high on violence exposure showed no significant amygdala habituation.
Fig. 2.
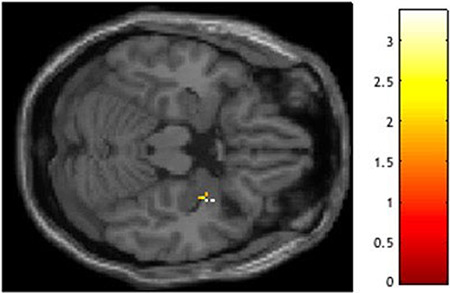
Negative relation between violence exposure and right amygdala habituation to angry faces.
Peak t(160) = 3.36, P = 0.012, XYZ = 34, 4, −20 (coordinates in Montreal Neurological Institute (MNI) space). Violence exposure, social deprivation, the interaction of violence exposure and social deprivation, and gender were entered as regressors in a multiple regression analysis in SPM12. Finding visualized in SPM with a P < 0.05 uncorrected threshold.
Fig. 3.
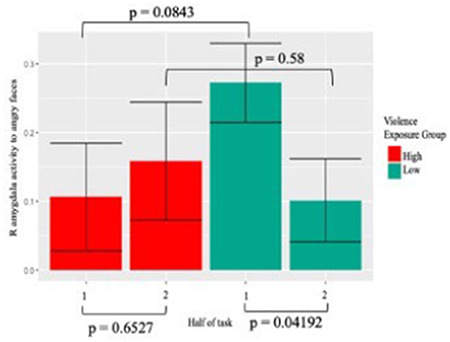
Right amygdala habituation to angry faces in individuals with high and low violence exposure.
In a confirmatory data visualization in RStudio (2015), participants high (above the mean) on violence exposure demonstrate less right amygdala habituation to angry faces than participants low (below the mean) on violence exposure. t-tests were used to compare activation between first and second halves of the task within groups and to compare activation within a half between groups.
Objective 2
Higher levels of violence exposure were associated with reduced left amygdala activation to angry, but not fearful, faces; this finding did not survive correction for multiple comparisons (S7). Activation for the angry > fear contrast was not significant.
Objective 3
Higher levels of social deprivation were associated with reduced bilateral ventral striatum activation specifically to happy faces (Table 1, Figure 4), but only activation in the right hemisphere remained significant with control for multiple comparisons.
Table 1.
Social deprivation is associated with reduced bilateral ventral striatum activation to happy faces
| Contrast | Side | Negative association with social deprivation, t(161) | P-value (FWE) | X | Y | Z |
|---|---|---|---|---|---|---|
| Happy > baseline | L | 2.52 | 0.037 | −14 | 2 | −12 |
| Happy > baseline | R | 2.97 | 0.016 | 16 | 6 | −14 |
Notes: Family-wise error (FWE) correction is based on structurally defined nucleus accumbens regions of interest; coordinates are in MNI space. Only right side finding survives Holm–Bonferroni correction for multiple comparisons. L, left; R, right.
Fig. 4.
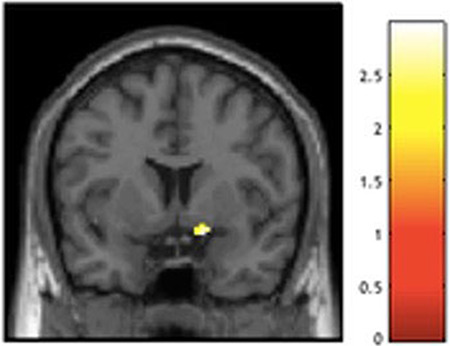
Negative relation between social deprivation and right ventral striatum activation to happy faces.
Peak t(161) = 2.97, P = 0.016, XYZ = 16, 6, −14 (coordinates in MNI space). Violence exposure, social deprivation, the interaction of violence exposure and social deprivation, and gender were entered as regressors in a multiple regression analysis in SPM12. Finding visualized in SPM with a P < 0.05 uncorrected threshold.
Objective 4
In total, 17.7% of participants met full criteria for a current depressive and/or anxiety disorder (S6). We originally fit two latent factors: adolescent anxiety and depression (S8). We evaluated the appropriateness of models testing neural mediation of early adversity and adolescent anxiety and depression by first evaluating the associations between these variables (S9). The direct paths from violence exposure and social deprivation to anxiety and depression were not significant (c paths). However, as important indirect effects can exist in the absence of this direct path (Hayes, 2013), we examined whether violence exposure and social deprivation predicted neural regions (a path) that overlapped with those related to anxiety and depression (b path). Amygdala regions associated with anxiety and depression did not overlap anatomically with those associated with violence exposure (S9). Ventral striatum activation to happy faces was not significantly associated with anxiety or depression.
Our latent factors had less than ideal model fit (e.g. non-significant factor loadings) and were not associated with any predictors. Therefore, we also tried assessing potential mediation using composite variables of adolescent anxiety and depression, and they too were not predicted by the model (S9). Thus, in the absence of neural regions that were related to both the predictors (violence exposure and social deprivation) and the outcomes (anxiety and depression), we did not have sufficient evidence of mediation or indirect effects to warrant additional analyses. Since we did not test for those effects, and to make sure that any associations were not due to internalizing psychopathology, we re-ran all analyses related to violence exposure and social deprivation and neural activity adjusting for both adolescent internalizing psychopathology and adolescent stress and found that all findings remained significant (S11).
Sensitivity analyses
Sensitivity analyses assessing childhood internalizing psychopathology and number of censored scans as confounders both indicated that our amygdala habituation and ventral striatum activation results were robust, but that amygdala activation results were not. Specifically, when adjusting for childhood internalizing psychopathology, violence exposure was still associated with decreased amygdala habituation to angry faces and social deprivation was still associated with decreased ventral striatum activation to happy faces. However, our finding that violence exposure was associated with decreased activation to angry faces became non-significant. When adding the number of censored scans as a covariate, the same pattern was observed.
Discussion
The present study evaluated whether two dimensions of early adversity, violence exposure and social deprivation, were associated with variation in activity in specific hypothesized neural circuits. Utilizing an open-science framework, we tested four pre-registered aims Consistent with our hypothesis, childhood violence exposure related to decreased right amygdala habituation to angry faces in adolescence (i.e. more sustained amygdala activation). Also, in line with our hypothesis, childhood social deprivation was associated with decreased right ventral striatum activation to socially rewarding stimuli (happy faces) in adolescence. These associations remained when adjusting for broadband internalizing psychopathology and adolescent current life stress. In contrast to our hypothesis, childhood violence exposure was associated with ‘decreased’ left amygdala reactivity to angry faces in adolescence (but this did not survive correction for multiple comparisons and was not robust to sensitivity analyses adjusting for childhood internalizing psychopathology and the number of censored scans), and we did not find evidence for an indirect effect between early adversity, neural reactivity and internalizing psychopathology. Importantly, this study used a well-sampled cohort with substantial representation of understudied youth—African American adolescents and adolescents from lower SES families—and tested important hypotheses from a prominent model of the neural embedding of adversity.
Our result that childhood violence exposure was associated with reduced adolescent amygdala habituation specifically to angry faces was consistent with predictions of the DMAP about threat exposure and altered fear processing. The finding that violence exposure related to both reduced amygdala habituation and less amygdala activation may seem paradoxical. However, Figure 3 clarifies the overall pattern: whereas adolescents who were exposed to less violence started with a high level of activation and then habituated, adolescents with greater violence exposure started with less activation and then maintained the same level. Thus, the former group habituated more and showed more activation overall, whereas the latter group did not habituate and showed blunted overall activation. High-violence environments may lead to an adaptive response of lower initial amygdala activation or rapid habituation.
The finding that greater violence exposure was associated with ‘less’ amygdala activation is inconsistent with maltreatment (see Hein and Monk, 2017 for a meta-analysis) and other recent research (Gerin et al., 2019). However, maltreatment has also been linked to decreased amygdala activation to social rejection in adolescents (Puetz et al., 2016). Further, recent work with a somewhat comparable low-SES sample found that adversity measured prospectively in early childhood, including parental harshness, related to lower amygdala reactivity to threatening faces in adulthood (Gard et al., 2017). Similarly, a broad index of childhood family adversity was prospectively associated with reduced amygdala reactivity to faces in adults (Holz et al., 2017). Our sample and those samples studied by Gard and colleagues (2017) and Holz and colleagues (2017) were all at higher risk of experiencing chronic forms of early adversity and measured adversity prospectively. As with literature examining the influence of early adversity on cortisol reactivity, exposure to more short-term or isolated forms of childhood stress may be associated with increased stress reactivity, whereas sustained early adversity may be associated with blunted stress response (Fries et al., 2005; Hannibal and Bishop, 2014). Notably, this finding did not survive correction for multiple comparisons and was not robust to sensitivity analyses adjusting for childhood internalizing psychopathology and the number of censored scans. Together, this suggests that violence exposure was weakly associated with reduced left amygdala activation. Further work in diverse samples is necessary, particularly to inform whether divergent findings on the associations of early adversity and amygdala reactivity are due to timing or chronicity of adversity.
The finding that social deprivation was associated with reduced ventral striatum activation to happy faces extends DMAP by focusing exclusively on social deprivation and social reward, as opposed to experiences that may be associated with lack of both cognitive and social input (e.g. institutional rearing). It also extends (Hanson et al., 2015), which linked emotional neglect with decreased ventral striatum activation to monetary rewards. Lowered activity in this region may reflect adaptation to a context marked by lower levels of exposure to positive social interaction. However, given that our task is not typically used to study individual differences in neural reactivity to reward, it will be important to replicate using tasks designed to study reward reactivity, such as the monetary incentive delay task (Knutson et al., 2000).
Our multi-informant, multi-method measures of anxiety and depression were related to amygdala habituation and activation, but in slightly different regions than was threat exposure. The association of internalizing psychopathology with amygdala reactivity is consistent with a large body of prior work (Wiggins and Monk, 2013). It is possible that early experiences such as violence exposure and social deprivation impact specific sub-regions of the amygdala and ventral striatum and that these are distinct from sub-regions that are associated with internalizing psychopathology. Another possibility is that we were unable to find associations due to poor factor loadings of our measures or because of low rates of anxiety and depression in the sample. Further work will need to evaluate the extent to which regions of limbic and reward circuitry associated with early adversity are also associated with psychopathology.
The current study had the following limitations. Due to the population-based sampling methodology, as opposed to convenience samples selected for their ability to participate in neuroimaging data collection, a significant portion of our participants were ineligible to participate in scanning for reasons such as having braces and being too large to comfortably fit in an fMRI scanner. Despite this limitation, our sample size was significantly greater than many neuroimaging studies and had substantial representation of African American adolescents and adolescents from families living in low-income contexts, populations that are often underrepresented in neuroimaging research and populations key to understanding associations between adversity and brain and behavior because of inequalities in exposure to adversity in our society. A second limitation is that our study is correlational, and therefore, it is not possible to identify causal variables. Third, the relatively modest rates of internalizing conditions reduced the ability of the present study to detect associations between early adversity and internalizing psychopathology. Fourth, we examined violence exposure and social deprivation dimensionally while controlling for their overlap, which differs from looking at specific types of exposures (e.g. physical abuse) that might be high on one dimension and low on the other. Fifth, in order to have prospective, longitudinal data, we were limited to data previously collected by FFCWS and chose use constructs developed in prior papers, which resulted in imperfect measures of our constructs. Sixth, our SAND sample includes a small portion of the full FFCWS sample, and thus, some selection bias is possible. Seventh, we used baseline instead of neutral faces, and therefore, task-related reactivity could be related to non-emotion processing aspects of the task. We did not use neutral faces because of prior work suggesting that neutral faces can be interpreted as ambiguous and could signal threat, particularly in those with anxiety (e.g. Yoon and Zinbarg, 2008). Eighth, our finding that violence exposure was associated with reduced amygdala activation was less robust; it did not survive adjustment for the number of censored scans during the fMRI task, or adjustment for internalizing psychopathology in childhood.
Conclusion
Two dimensions of early adversity—violence exposure and social deprivation—were associated with adolescent amygdala habituation and ventral striatum activation, respectively, in a large, well-sampled cohort of adolescents. That dimensions of adversity were parseable and were associated with different hypothesized alterations in brain reactivity suggests that qualitatively different forms of early adversity impact different regions of the brain and should be considered separately when evaluating the mechanisms linking early adversity to later mental health outcomes. By evaluating dimensions of early adversity using prospective longitudinal data with a well-sampled cohort, this study provides a key step toward understanding the neural mechanisms linking early adversity to later socioemotional function.
Supplementary Material
Acknowledgements
We are immensely grateful to the families for participating and research staff for making the study possible.
Contributor Information
Tyler C Hein, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA; Serious Mental Illness Treatment Resource and Evaluation Center, Office of Mental Health and Suicide Prevention, Department of Veterans Affairs, Ann Arbor, MI 48109, USA.
Leigh G Goetschius, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA.
Vonnie C McLoyd, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA.
Jeanne Brooks-Gunn, Teachers College, Columbia University, New York, NY 10027, USA.
Sara S McLanahan, Department of Sociology, Princeton University, Princeton, NJ 08544, USA.
Colter Mitchell, Survey Research Center of the Institute for Social Research, University of Michigan, Ann Arbor, MI 48106-1248, USA; Population Studies Center of the Institute for Social Research, University of Michigan, Ann Arbor, MI 48106-1248, USA.
Nestor L Lopez-Duran, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA.
Luke W Hyde, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA; Survey Research Center of the Institute for Social Research, University of Michigan, Ann Arbor, MI 48106-1248, USA.
Christopher S Monk, Department of Psychology, University of Michigan, Ann Arbor, MI 48109-1043, USA; Department of Sociology, Princeton University, Princeton, NJ 08544, USA; Neuroscience Graduate Program, University of Michigan, Ann Arbor, MI 48109, USA; Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109, USA.
Funding
This work was supported by National Institutes of Health [R01MH103761 to C.S.M., T32HD007109 to V.C.M. and C.S.M. and S10OD012240] and a Doris Duke Fellowship for the Promotion of Child Well-Being to T.C.H.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest
None declared.
References
- Achenbach T.M. (1999). The child behavior checklist and related instruments. In M.E. Maruish (Ed.), The Use of Psychological Testing for Treatment Planning and Outcomes Assessment (p. 429–66). Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Appleyard K., Egeland B., Dulmen M.H., Alan Sroufe L. (2005). When more is not better: the role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry, 46(3), 235–45. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). About the CDC-Kaiser ACE study, [WWW]. Available : https://www.cdc.gov/violenceprevention/acestudy/about.html [June 9, 2019].
- Donnelly L., McLanahan S., Brooks-Gunn J., et al. (2016). Cohesive neighborhoods where social expectations are shared may have positive impact on adolescent mental health. Health Affairs (Project Hope), 35(11), 2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Hesse J., Hellhammer J., Hellhammer D.H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30(10), 1010–16. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. (2012). Research review: altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Waller R., Shaw D.S., Forbes E.E., Hariri A.R., Hyde L.W. (2017). The long reach of early adversity: parenting, stress, and neural pathways to antisocial behavior in adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(7), 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., McEwen S.C., Forsyth J.K., et al. (2015). Reliability of an fMRI paradigm for emotional processing in a multisite longitudinal study. Human Brain Mapping, 36(7), 2558–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin M.I., Viding E., Pingault J.B., et al. (2019). Heightened amygdala reactivity and increased stress generation predict internalizing symptoms in adults following childhood maltreatment. Journal of Child Psychology and Psychiatry, 60(7), 752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gest S.D., Reed M.G.J., Masten A.S. (1999). Measuring developmental changes in exposure to adversity: a life chart and rating scale approach. Development and Psychopathology, 11(1), 171–92. [DOI] [PubMed] [Google Scholar]
- Goetschius L.G., Hein T.C., Mattson W.I., et al. (2019). Amygdala-prefrontal cortex white matter tracts are widespread, variable, and implicated in amygdala modulation in adolescents. NeuroImage, 191, 278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal K.E., Bishop M.D. (2014). Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Physical Therapy, 94(12), 1816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. (2015). Blunted ventral striatum development in adolescent reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78, 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. 2013. New York: Guilford.
- Hein T.C., Mattson W.I., Dotterer H.L., et al. (2018). Amygdala habituation and uncinate fasciculus connectivity in adolescence: a multi-modal approach. NeuroImage, 183, 617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein T.C., Monk C.S. (2017). Research review: neural response to threat in children, adolescents, and adults after child maltreatment–a quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58(3), 222–30. [DOI] [PubMed] [Google Scholar]
- Holz N.E., Boecker-Schlier R., Buchmann A.F., et al. (2017). Ventral striatum and amygdala activity as convergence sites for early adversity and conduct disorder. Social Cognitive and Affective Neuroscience, 12(2), 261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T.K., Slack K.S., Berger L.M. (2017). Adverse childhood experiences and behavioral problems in middle childhood. Child Abuse & Neglect, 67, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. (2016). Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical Psychological Science, 4(3), 527–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–94. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Maheu F.S., Dozier M., Guyer A.E., et al. (2010). A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive, Affective & Behavioral Neuroscience, 10(1), 34–49. doi: 10.3758/CABN.10.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–39. [DOI] [PubMed] [Google Scholar]
- Manuel J.I., Martinson M.L., Bledsoe-Mansori S.E., Bellamy J.L. (2012). The influence of stress and social support on depressive symptoms in mothers with young children. Social Science & Medicine, 75(11), 2013–20. [DOI] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., et al. (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21(23), R947–R948. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A. (2016). Beyond cumulative risk: a dimensional approach to childhood adver-sity. Current Directions in Psychological Science, 25(4), 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M.A., Gore-Langton E., Golembo N., Colvert E., Williams S.C., Sonuga-Barke E. (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience, 22(10), 2316–25. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins M.K., Roberts A.G., Hein T.C., et al. (2019). Violence exposure and social deprivation is associated with cortisol reactivity in urban adolescents. Psychoneuroendocrinology, 104426. doi: 10.1016/j.psyneuen.2019.104426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron, 48(2), 175–87. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Grimm O., Morgen K., et al. (2014). Amygdala habituation: a reliable fMRI phenotype. NeuroImage, 103, 383–90. [DOI] [PubMed] [Google Scholar]
- Puetz V.B., Viding E., Palmer A., et al. (2016). Altered neural response to rejection-related words in children exposed to maltreatment. Journal of Child Psychology and Psychiatry, 57(10), 1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C.H., Abrams T., Barry R.J., et al. (2009). Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory, 92(2), 135–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman N.E., Teitler J.O., Garfinkel I., McLanahan S.S. (2001). Fragile families: sample and design. Children and Youth Services Review, 23(4-5), 303–26. [Google Scholar]
- RStudio Team (2015). RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; http://www.rstudio.com/. [Google Scholar]
- Russo S.J., Nestler E.J. (2013). The brain reward circuitry in mood disorders. Nature Reviews Neuroscience, 14(9), 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff A., Seifer R., McDonough S.C. (2004). Contextual contributors to the assessment of infant mental health. Handbook of Infant, Toddler, and Preschool Mental Health Assessment, 61–76. [Google Scholar]
- Sacks V., Murphey D. (2018, February 12). The prevalence of adverse childhood experiences, nationally, by state, and by race or ethnicity. Retrieved December 12, 2018, from https://www.childtrends.org/publications/prevalence-adverse-child-hood-experiences-nationally-state-race-ethnicity.
- Schultz W., Apicella P., Scarnati E., Ljungberg T. (1992). Neuronal activity in monkey ventral striatum related to the expectation of reward. Journal of Neuroscience, 12(12), 4595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A. (2014). Dimensions of early experience and neural development: deprivation and threat. Trends in Cognitive Sciences, 18(11), 580–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., McLaughlin K.A., Winter W., Fox N., Zeanah C., Nelson C.A. (2018). Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nature Communications, 9(1), 1–8. doi: 10.1038/s41467-018-04381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.K., Lin F.C., Ward S.E., Fine J.P. (2013). Composite variables: when and how. Nursing Research, 62(1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga‐Barke E.J., Schlotz W., Kreppner J. (2010). V. Differentiating developmental trajectories for conduct, emotion, and peer problems following early deprivation. Monographs of the Society for Research in Child Development, 75(1), 102–24. doi: 10.1111/j.1540-5834.2010.00552.x [DOI] [PubMed] [Google Scholar]
- Straus M.A., Hamby S.L., Finkelhor D., Moore D.W., Runyan D. (1998). Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse & Neglect, 22(4), 249–70. [DOI] [PubMed] [Google Scholar]
- Wiggins J.L., Monk C.S. (2013). A translational neuroscience framework for the development of socioemotional functioning in health and psychopathology. Development and Psychopathology, 25(4 pt 2), 1293–309. [DOI] [PubMed] [Google Scholar]
- Yoon K.L., Zinbarg R.E. (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117(3), 680. [DOI] [PubMed] [Google Scholar]
- Zhang S., Anderson S.G. (2010). Low-income single mothers’ community violence exposure and aggressive parenting practices. Children and Youth Services Review, 32(6), 889–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


