Abstract
Background
Chronic inflammation of the brain has a pivotal role in the pathophysiology of major depressive disorder (MDD) and schizophrenia (SCZ). Matrix metalloproteinases (MMPs) are extracellular proteases involved in pro-inflammatory processes and interact with interleukin-6, which is increased in the cerebrospinal fluid (CSF) of patients with MDD and SCZ. However, MMPs in the CSF in patients with MDD and SCZ remain unclear. Therefore, we compared MMPs in the CSF of patients with MDD and SCZ with those of healthy controls (HC).
Methods
Japanese patients were diagnosed with DSM-IV-TR and clinical symptoms were assessed with the Hamilton Rating Scale for Depression for MDD and the Positive and Negative Syndrome Scale for SCZ. CSF was obtained from MDD (n = 90) and SCZ (n = 86) and from age- and sex-matched HC (n = 106). The levels of MMPs in CSF were measured with multiplex bead-based immunoassay.
Results
The levels of MMP-2 in CSF were higher in both MDD and SCZ than HC and were positively correlated with clinical symptomatic scores in MDD, but not in SCZ. Regardless of diagnosis, the levels of MMP-2, -7, and -10 were positively correlated with each other, and the levels of MMP-7 and -10 were higher in MDD, but not in SCZ, compared with HC.
Conclusion
Increased CSF levels of MMP-2 in MDD and SCZ may be associated with brain inflammation. State-dependent alteration of MMP-2 and activation of cascades involving MMP-2, -7, and -10 appeared to have a role in the pathophysiology of MDD.
Keywords: Matrix metalloproteinases, cerebrospinal fluid, major depressive disorder, schizophrenia, brain inflammation
Significance Statement.
Matrix metalloproteinases (MMPs) are extracellular proteases involved in pro-inflammatory processes. This study found that the levels of MMP-2 in cerebrospinal fluid (CSF) was higher in both major depressive disorder (MDD) and schizophrenia (SCZ) and positively correlated with clinical symptomatic scores in MDD, but not in SCZ. Regardless of diagnosis, levels of MMP-2, -7, and -10 were positively correlated with each other, and the levels of MMP-7 and 10 were higher in MDD, but not in SCZ. The increased CSF levels of MMP-2 in MDD and SCZ may be associated with brain inflammation. Additionally, both the state-dependent increase of MMP-2 and the activation of cascades involving MMP-2, -7, and -10 may be involved in the pathophysiology of MDD.
Introduction
Inflammatory processes in body systems and the central nervous system (CNS) have been shown to play a role in the development of major psychiatric disorders. In particular, elevated levels of pro-inflammatory cytokines in the cerebrospinal fluid (CSF) have been demonstrated in major psychiatric disorders such as major depressive disorder (MDD) and schizophrenia (SCZ). In patients with MDD, there were elevated levels of interleukin (IL)-6 and tumor necrosis factor (TNF-α) in CSF compared with healthy controls (HC) (Enache et al., 2019). In patients with SCZ, levels of IL-6 and IL-8 in CSF were elevated compared with HC (Orlovska-Waast et al., 2019).
Matrix metalloproteinases (MMPs), which are zinc-dependent proteases, belong to a family of more than 25 enzymes (Kim and Joh, 2012; Sbardella et al., 2012) and have a number of roles beyond degradation and remodeling of the extracellular matrix, including processing of bioactive molecules such as cell surface receptors and neurotrophic factors (Kim and Joh, 2012; Shibasaki et al., 2016). MMPs are also indispensable for physiological processes related to the regulation of inflammation (Ravanti and Kahari, 2000; Nissinen and Kahari, 2014). MMPs process cytokines, which in turn alter MMP activity (Harkness et al., 2000; Van Lint and Libert, 2007). For instance, IL-6 upregulates MMP-2 production (Silacci et al., 1998; Kossakowska et al., 1999; Mano et al., 2009) and MMP-2 activates TNF-α (McQuibban et al., 2000). Because of the close interaction between cytokines and MMP and the association with major psychiatric disorders, further exploration of the interaction between MMPs and cytokines in the brain may lead to improved elucidation of the pathophysiology of major psychiatric disorders. However, MMPs in CNS of major psychiatric disorders have yet to be measured. Although it is ethically impossible to measure the levels of MMPs in the brain of living humans, the levels of MMPs in CSF are considered to reflect the levels of MMPs in the brain. Therefore, we measured the levels of MMPs in the CSF of patients with MDD and SCZ.
Methods and Materials
Participants
Japanese patients were recruited at the National Center of Neurology and Psychiatry (NCNP) Hospital, Tokyo, Japan, through an announcement on the website of NCNP between May 2010 and July 2015. HC were recruited from the community through advertisements in a free local magazine and an announcement on the website of NCNP. All individuals underwent a structured interview with the Mini-International Neuropsychiatric Interview, Japanese version (Sheehan et al., 1998; Otsubo et al., 2005), administered by trained psychologists or psychiatrists. For patients with either MDD or SCZ, a consensus diagnosis was performed according to DSM-IV criteria (American Psychiatric Association, 2000) based on Mini-International Neuropsychiatric Interview, additional unstructured interviews, and information from medical records. Individuals were excluded from this study if they had a history of CNS disease, severe head injury, or substance abuse. From all participants, 1 CSF sample was collected via lumbar puncture. One hundred patients were diagnosed with MDD and 96 patients were diagnosed with SCZ. One-hundred-fifteen healthy participants, with no history of past or current mental disorders, were recruited as HC. Antidepressant doses were converted to imipramine (IMI)-equivalent doses (Inada and Inagaki, 2015). Antipsychotic doses were converted to chlorpromazine (CPZ)-equivalent doses (Inada and Inagaki, 2015). Clinical data were managed with a FileMaker server (FileMaker Inc., Santa Clara, CA). Details regarding participant clinical data are shown in Table 1. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the National Hospital Organization Kure Medical Center and by the ethics committee of the National Center of Neurology and Psychiatry, Japan. Written informed consent was obtained from all participants.
Table 1.
Participant Clinical and Laboratory Data
| HC (n = 106) | MDD (n = 90) | SCZ (n = 86) | P value | |
|---|---|---|---|---|
| Sex, female | 48 (45.3%) | 47 (52.2%) | 34 (39.5%) | .238a |
| Age (y) | 42.6 ± 15.4 (n = 106) | 43.7 ± 11.0 (n = 90) | 40.7 ± 10.4 (n = 86) | .365b |
| HAMD17-items score | 9.9 ± 8.2 (n = 77) | |||
| PANSS total score | 59.9 ± 15.6 (n = 78) | |||
| PANSS positive score | 13.9 ± 4.9 (n = 78) | |||
| PANSS negative score | 16.1 ± 5.2 (n = 78) | |||
| PANSS cognitive or general psychopathology score | 29.9 ± 8.6 (n = 78) | |||
| IMI-equivalent doses (mg/d) | 149.0 ± 131.7 (n = 47) | |||
| CPZ-equivalent doses (mg/d) | 86.8 ± 175.1 (n = 47) | 946.5 ± 950.8 (n = 61) | <.001c*** | |
| CSF levels of Glu (mg/dL) | 61.0 ± 10.7 (n = 104) | 61.5 ± 8.0 (n = 89) | 64.3 ± 14.1 (n = 86) | .112b |
| WCC (/mL) | 4.0 ± 3.0 (n = 104) | 4.2 ± 3.0 (n = 89) | 4.5 ± 4.1 (n = 86) | .660b |
Abbreviations: CPZ, chlorpromazine; Glu, glucose; HAMD, Hamilton Rating Scale for Depression; HC, healthy controls; IMI, imipramine; MDD, major depressive disorder; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia; WCC, white cell count.
Data shown as either mean ± SD and number or number and percent of total (%). ***P < .001.
aComparison between 3 groups by chi-squared test.
bComparison between 3 groups by the Kruskal Wallis test.
cComparison between 2 patients groups by the Mann-Whitney U-test.
Assessment of Clinical Symptoms
Clinical symptoms of MDD were assessed with a 17-item version of the Hamilton Rating Scale for Depression (HAMD 17-items). HAMD 17-items were scored with the following 5 subscales: core symptom (items 1, 2, 7, 8, 10, 13); sleep (items 4, 5, 6); activity (items 7, 8); psychic anxiety (items 9, 10); and somatic anxiety (items 11, 12, 13) in accordance with a previous report (Seretti et al., 1999). Clinical symptoms of SCZ were assessed with the Positive and Negative Syndrome Scale (PANSS). PANSS was scored with the following 3 subscales: positive scale (7 items); negative scale (7 items); and cognitive or general psychopathology scale (16 items). The assessment of clinical symptoms was performed by a psychiatrist prior to lumbar puncture.
MDD Patients Received Antipsychotics Pharmacotherapy
Current medication in 47 of 90 MDD patients was investigated because the remaining 43 patients had no information about their medication. Seventeen patients received antipsychotics pharmacotherapy: aripiprazole (3–15 mg/d; n = 3), levomepromazine (10–75 mg/d; n = 3), olanzapine (2.5–12.5 mg/d; n = 4), paliperidone (6 mg/d; n = 1), perospirone (4 mg/d; n = 1), quetiapine (25–200 mg/d; n = 7), risperidone (1–3 mg/d; n = 2), or sulpiride (150–300 mg/d; n = 3). Thirty patients did not receive any antipsychotics. There were no statistically significant differences between MDD patients either with or without antipsychotics pharmacotherapy in terms of the HAMD 17-items score (with antipsychotics: 9.8 ± 7.0; without antipsychotics: 9.7 ± 8.5, P = .962). There were no statistically significant differences between MDD patients either with or without antipsychotics pharmacotherapy in terms of IMI-equivalent doses (with antipsychotics: 157.0 ± 143.1 mg/d; without antipsychotics: 149.4 ± 126.3 mg/d, P = .852). Because there were no clinical data other than the above-mentioned details, we could not determine if 17 patients had treatment-resistant depression.
Lumbar Puncture
Lumbar puncture was performed according to procedure guidelines at NCNP (Hattori et al., 2015). Samples were obtained at NCNP between May 2010 and July 2015. After neurologic examination, each participant received local anesthesia followed by a lumbar puncture at L3–4 or L4–5 using an atraumatic pencil point needle (Uniever 22G, 75 mm, Unisis Corp, Tokyo, Japan). The initial 2 mL of CSF was used for routine laboratory tests, including glucose, white cell count (WCC), and total protein (TP). The subsequent 8 mL of CSF was collected in a low-protein adsorption tube (PROTEOSAVE SS 15 mL Conicaltube, Sumitomo Bakelite Co., Japan) and immediately chilled on ice. Then, CSF was centrifuged (4000g × 10 min, 4°C) and the supernatant was dispensed into 0.5-mL aliquots in low-protein adsorption tubes (PROTEOSAVE SS 1.5 mL Slimtube, Sumitomo Bakelite Co.) and stored in a deep freezer (−80°C) until use.
Detection of MMP Levels in CSF
The levels of MMPs in CSF were measured with Bio-Plex Pro Human MMP Assays (Bio-Rad Laboratories, Inc., Hercules, CA) at the National Hospital Organization Kure Medical Center according to the manufacturer’s instructions. As a pilot study, the levels of MMP-1, -2, -3, -7, -8, -9, -10, -12, and -13 in CSF were measured in 10 patients with MDD, 10 patients with SCZ, and 9 HC with a Bio-Plex Pro Human MMP-9-Plex Panel. Pilot data showed that MMP-3, -9, -12, and -13 levels were below the detection limit (data not shown). The levels of MMP-1, -2, -7, -8, and -10 in CSF were measured in an additional 90 patients with MDD, an additional 86 patients with SCZ, and an additional 106 HC with the Bio-Plex Pro Human MMP Custom Panel (5-Plex). For statistical analysis, if the concentration was below the detection limit, the concentration level was presented as 0.
Statistical Analysis
Data are shown as mean ± SD. Tests for normality were performed with Shapiro-Wilk and Kolmogorov-Smirnov tests, and data were analyzed with nonparametric tests. A Kruskal-Wallis test was used to evaluate significant differences in parameters (clinical and/or laboratory values) among the groups (HC, MDD, and SCZ). A Bonferroni test was performed as a post-hoc test. Significant differences in parameters between patients with MDD and SCZ were evaluated with the Mann-Whitney U-test. The chi-square test was used for categorical variables. A linear regression analysis was performed to evaluate possible associations between the levels of MMPs in CSF and clinical parameters, clinical symptoms, and diagnosis, controlled for age and sex. Statistical significance was defined as a 2-tailed P < .05. All statistical analyses were performed with SPSS version 22.0 for Windows (IBM Japan Corporation, Tokyo, Japan).
Results
Clinical and Laboratory Data
Sex, mean age, and levels of glucose and WCC in CSF did not significantly differ among the 3 groups (HC, MDD, and SCZ; Table 1).
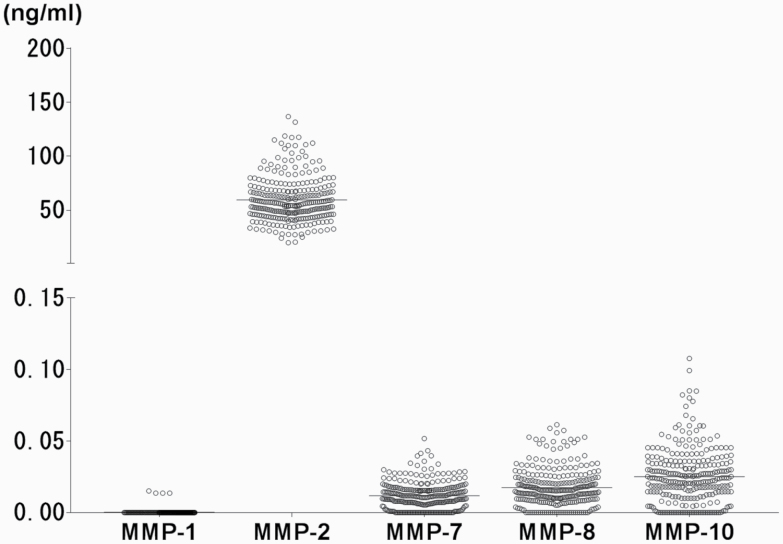
In all CSF samples, MMP-2 was detectable (59.5 ± 20.5 ng/mL; Figure 1). However, the levels of MMP-1 were below the detection limit. The levels of MMP-7, -8, and -10 in CSF were detectable at a much lower level (11.8 ± 9.4 pg/mL, 17.3 ± 13.0 pg/mL, 25.1 ± 19.0 pg/mL, respectively) than that of MMP-2 (Figure 1).
Figure 1.
Scatter plot of cerebrospinal fluid (CSF) levels of matrix metalloproteinases (MMP)-1, -2, -7, -8, and -10 from all participants. The horizontal bars represent mean values.
Association Between Levels of MMPs in CSF and Characteristics Across All Participants
Correlation coefficients were calculated between the levels of MMPs in CSF and age with univariate linear regression analysis. There was a significant positive correlation between the levels of MMPs in CSF, except for MMP-8, and age (MMP-2: nonstandardized coefficient β = 0.368, P < .001; MMP-7: nonstandardized coefficient β = 0.216, P < .001; MMP-8: nonstandardized coefficient β = 0.013, P = .836; MMP-10: nonstandardized coefficient β = 0.274, P = .002). The mean levels of MMPs in CSF, except for MMP-8, of males were significantly higher than those of females (MMP-2: 63.6 ± 21.3 vs 54.6 ± 18.6 ng/mL, P < .001; MMP-7: 12.8 ± 9.6 vs 10.6 ± 9.0 pg/mL, P = .043; MMP-8: 17.3 ± 11.6 vs 17.3 ± 14.6 pg/mL, P = .999; MMP-10: 28.2 ± 18.9 vs 21.4 ± 18.5 pg/mL, P = .003, respectively). These correlations suggest age- and sex-related differences in the levels of MMP-2, -7, and -10 in CSF, which were confirmed by linear regression analysis (Figure 2B; Table 2).
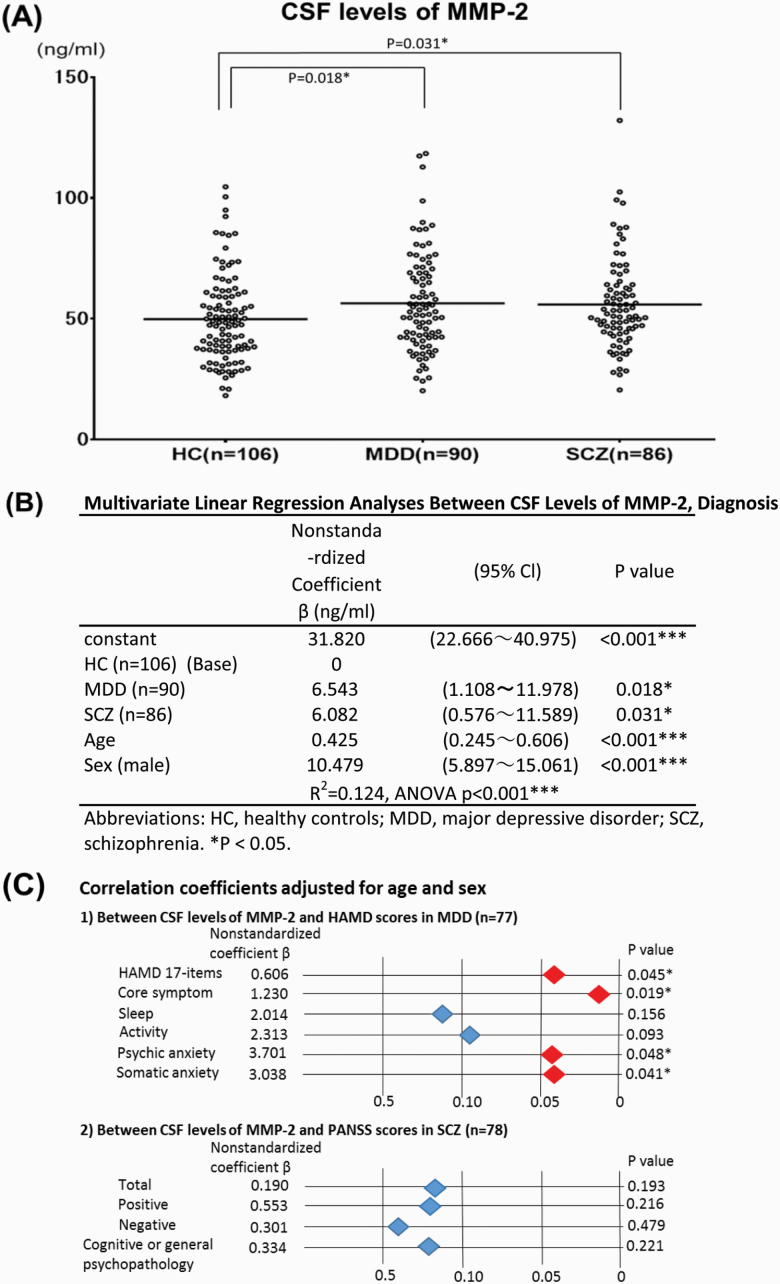
Figure 2.
(A) Scatter plot of cerebrospinal fluid (CSF) levels of matrix metalloproteinases (MMP)-2 in healthy controls (HC), and in major depressive disorder (MDD) and schizophrenia (SCZ). The horizontal bars represent mean values adjusted for age and sex. *P < .05. (B) Cerebrospinal fluid (CSF) levels of MMP-2 in patients with major depressive disorder (MDD) and schizophrenia (SCZ) were significantly higher than those of healthy controls (HC). Linear regression analysis adjusted for age and sex. *P < .05. (C) Correlation between cerebrospinal fluid (CSF) levels of MMP-2 and clinical symptomatic scores in major depressive disorder (MDD) and schizophrenia (SCZ). The correlation coefficients were calculated using linear regression analysis and adjusted for age and sex. *P < .05. (1) Between CSF levels of MMP-2 and the Hamilton Rating Scale for Depression (HAMD) scores in MDD. (2) Between CSF levels of MMP-2 and the Positive and Negative Syndrome Scale (PANSS) scores in SCZ.
Table 2.
Multivariate Linear Regression Analyses Between CSF Levels of MMP-7, -8, and -10, and Diagnosis
| Nonstandardized Coefficient β (pg/mL) | (95% CI) | P value | |
|---|---|---|---|
| (A) MMP-7 levels | |||
| Constant | −0.858 | (−5.069~3.354) | .689 |
| HC (n = 106) (Base) | 0 | ||
| MDD (n = 90) | 2.551 | (0.051~5.051) | .046* |
| SCZ (n = 86) | 1.202 | (−1.331~3.735) | .351 |
| Age | 0.230 | (0.147~0.313) | <.001*** |
| Sex (male) | 3.174 | (1.066~5.282) | .003** |
| R2 = 0.111, ANOVA P < .001***. | |||
| (B) MMP-8 levels | |||
| Constant | 17.196 | (10.945~23.446) | <.001*** |
| HC (n = 106) (Base) | 0 | ||
| MDD (n = 90) | -0.496 | (-4.207~3.215) | .793 |
| SCZ (n = 86) | -0.893 | (-4.653~2.867) | .641 |
| Age | 0.012 | (-0.112~0.135) | .854 |
| Sex (male) | 0.067 | (-3.062~3.196) | .966 |
| R 2 = 0.001, ANOVA P < .992. | |||
| (C) MMP-10 levels | |||
| Constant | 3.788 | (-4.839~12.416) | .388 |
| HC (n = 106) (Base) | 0 | ||
| MDD (n = 90) | 7.334 | (2.212~12.456) | .005** |
| SCZ (n = 86) | 4.507 | (-0.683~9.697) | .088 |
| Age | 0.312 | (0.142~0.482) | <.001*** |
| Sex (male) | 8.062 | (3.744~12.381) | <.001*** |
| R 2 = 0.089, ANOVA P < .001***. |
Abbreviations: CSF, cerebrospinal fluid; HC, healthy controls; MDD, major depressive disorder; MMP, matrix metalloproteinase; SCZ, schizophrenia.
* P < .05, **P < .01, ***P < .001.
Because of potential effects of age and sex on the levels of MMPs in CSF, further analyses of the levels of MMPs in CSF were performed adjusting for age and sex. No significant correlations were observed between other clinical characteristics such as body mass index, IMI equivalent dose, CPZ equivalent doses, glucose, and WCC, and the levels of MMPs in CSF (data not shown).
MMPs Levels in CSF of HC, MDD, and SCZ
The mean levels of MMP-2 in CSF of HC, MDD, and SCZ were 49.8 ± 18.1, 56.4 ± 20.6, 55.9 ± 18.9 ng/mL, respectively (Figure 2A). The MMP-2 levels in the CSF of patients with MDD and SCZ were significantly higher than those of HC (Figure 2B; MDD: nonstandardized coefficient β = 6.543, P = .018; SCZ: nonstandardized coefficient β = 6.082, P = .031).
The levels of MMP-7, -8, and -10 in CSF are presented in Table 2. The MMP-7 levels in the CSF of patients with MDD were also significantly higher than those of HC (MDD: 11.4 ± 10.7 pg/mL vs HC: 8.9 ± 7.2 pg/mL, P = .046). The MMP-10 levels in the CSF of patients with MDD were also significantly higher than those of HC (MDD: 24.3 ± 21.8 pg/mL vs HC: 17.0 ± 17.0 pg/mL, P = .005). In comparison, there were no significant differences in the levels of MMP-7 and -10 in CSF between patients with SCZ and HC. There were also no significant differences in the levels of MMP-8 in CSF among all the groups.
Correlation Between Levels of MMPs in CSF and Psychiatric Symptom Scores
Correlation coefficients were calculated between the levels of MMP-2 in CSF and psychiatric symptom scores (Figure 2C). Within patients with MDD, there was a significant positive correlation between the levels of MMP-2 in CSF and HAMD 17-items (nonstandardized coefficient β = 0.606, P = .045). Furthermore, there were positive correlations between the levels of MMP-2 in CSF and subscale HAMD scores (core symptom, psychic anxiety, and somatic anxiety). However, in patients with SCZ, there were no significant correlations between the levels of MMP-2 in CSF and PANSS scores. There were no significant correlations between the levels of other MMPs in CSF and clinical symptom scores in patients with either MDD or SCZ (data not shown).
Correlation Among MMP Levels in CSF
Correlation coefficients were calculated for the levels of MMPs in CSF. There were significant positive correlations between the levels of MMP-2 and MMP-7 in CSF among all groups (supplementary Table 1A). There were significant positive correlations between the levels of MMP-2 and MMP-10 in CSF among all groups (supplementary Table 1B). There were also significant positive correlations between the levels of MMP-7 and MMP-10 in CSF among all groups (supplementary Table 1C). However, there were no correlations observed between the levels of MMP-2 and MMP-8 in CSF among all groups. There were also no correlations obseved between the levels of MMP-7 and MMP-8 and between MMP-8 and MMP-10 in CSF among all groups (data not shown).
Total Protein Levels in CSF
TP in CSF is widely used as a marker of CNS inflammation (Orlovska-Waast et al., 2019). The levels of TP in HC, MDD, and SCZ were 33.4 ± 8.2, 39.4 ± 17.1, 37.8 ± 13.8 mg/dl, respectively (supplementary Fig. 1A). The levels of TP in patients with MDD and SCZ were significantly higher than those of HC (MDD: nonstandardized coefficient β = 6.028, P = .002; SCZ: nonstandardized coefficient β = 4.319, P = .028). There were significant positive correlations between the levels of MMP-2 and TP in CSF among HC (nonstandardized coefficient β = .767, P < .001), patients with MDD (nonstandardized coefficient β = .767, P < .001), and patients with SCZ (nonstandardized coefficient β = .595, P < .001), respectively. Because of potential effects of age and sex on the levels of TP in CSF as well as the levels of MMPs in CSF, further analyses of the levels of TP in CSF were performed adjusting for age and sex. In patients with MDD, there was a significant positive correlation between the levels of TP in CSF and HAMD scores (supplementary Fig. 1B1: HAMD 17-items, somatic anxiety). In patients of SCZ, no significant correlations were observed between the levels of TP in CSF and PANSS scores (supplementary Fig. 1B2).
Discussion
Here we showed that the levels of MMP-2 in CSF were significantly elevated in patients with MDD and SCZ compared with HC. In addition, an association between MMP-2 levels and symptom severity was observed in patients with MDD, while no such association was observed in patients with SCZ. Although the levels of MMP-2, -7, and -10 were positively correlated with each other in all MDD, SCZ, and HC, the levels of MMP-7 and -10 were elevated only in patients with MDD. MMP-2 is constitutively expressed in organs throughout the body. Within the CNS, MMP-2 is distributed in the soma of neurons (Planas et al., 2001), endothelium (Planas et al., 2001), astrocytes (Planas et al., 2001; del Zoppo et al., 2007; Candelario-Jalil et al., 2011), and microglia (Planas et al., 2001; Lorenzl et al., 2002; Konnecke and Bechmann, 2013). MMP-2 is expressed under physiological conditions and is involved in homeostatic functioning (Sbardella et al., 2012). MMP-2 activates glial cells, especially microglia, the immune cells of CNS, which enhances neuro-immune modulation (Nakaji et al., 2006). MMP-2 is also crucial in opening the blood-brain barrier (BBB) by disrupting tight junction proteins (Feng et al., 2011) and degrading collagen-IV and laminin, which constitute the basal lamina of the BBB (Suofu et al., 2012). Therefore, impaired BBB due to increased MMP-2 can allow harmful substances such as cytokines to enter the systemic circulation, which may lead to persistent brain inflammation.
As mentioned above, activated microglia can release MMP-2 (Lorenzl et al., 2002). In addition, MMP-2, produced from other cells such as neurons and astrocytes (Planas et al., 2001), activates microglia (Nakaji et al., 2006). Activated microglia reflects neuroinflammation, which can be quantitated in vivo by positron emission tomography (PET) with a translocator protein (TSPO) ligand, a marker of microglial activation (Bloomfield et al., 2016b; Holmes et al., 2016; Marques et al., 2019). PET studies for patients with MDD (Setiawan et al., 2018; Enache et al., 2019) and SCZ (Bloomfield et al., 2016b; Marques et al., 2019) showed microglial activation and a correlation of TSPO level with total score of depressive symptoms in patients with MDD (Li et al., 2018) and specific symptoms in patients with SCZ (Holmes et al., 2016). Therefore, elevated MMP-2 levels in CSF of patients with MDD and SCZ may suggest the existence of a common inflammation process-induced activation of microglia in MDD and SCZ.
Microglial activation in the frontal cortex (Hannestad et al., 2013; Bloomfield et al., 2016a, 2016b; Li et al., 2018), anterior cingulate cortex (Holmes et al., 2016, 2018; Su et al., 2016; Richards et al., 2018; Setiawan et al., 2018), temporal cortex (Hannestad et al., 2013; Bloomfield et al., 2016a, 2016b; Li et al., 2018; Setiawan et al., 2018), and hippocampus (Doorduin et al., 2009; Li et al., 2018; Setiawan et al., 2018) were observed in both MDD and SCZ. However, the level of microglial activation differed between MDD and SCZ in some regions of the brain, such as the prefrontal cortex (Holmes et al., 2018; Setiawan et al., 2018), insula (Holmes et al., 2016; Setiawan et al., 2018), total grey matter (Bloomfield et al., 2016a, 2016b), and dorsolateral prefrontal cortex (Holmes et al., 2016). Additionally, another PET study revealed that TSPO binding in the middle frontal gyrus of SCZ patients tended to be lower compared with those of matched control participants (Notter et al., 2018). Therefore, such regional differences in microglial activation might be crucial in defining each psychiatric disorder.
In this study, the levels of MMP-7 and -10 in CSF were detectable at a low level. In past studies, MMP-7 and -10 were normally undetectable or present at a low level in CNS (Yong et al., 2001; Yong, 2005), which corresponds to this study. In addition, the levels of MMP-7 and -10 in CSF were elevated in MDD but not SCZ. In comparison, they were positively correlated with MMP-2 levels in both MDD and SCZ. MMP-7 is secreted from microglia (Burke, 2004) in response to TNF-α (Wang et al., 2018), which suggests that the elevated levels of MMP-7 may reflect neuro-immune activation (Conant et al., 1999). Although the role of MMP-10 in CNS remains unclear, some past studies showed that MMP-10 activates pro-MMPs (Sbardella et al., 2012). In addition, MMP-10 is also secreted from microglia (Molin et al., 2017) and activates MMP-7 (Murphy et al., 1991), which in turn activates MMP-2 (Barille et al., 1999). These findings and past studies suggest a potential inflammatory cascade, including an interaction among MMP-2, -7, and -10, which might be activated in patients with MDD. Therefore, further investigation of this inflammatory cascade among MMP-2, -7, and -10 is expected to contribute to elucidating the pathophysiology of MDD.
Activated microglia secretes numerous pro-inflammatory cytokines (Gottschall et al., 1995; Lee et al., 2014; Mondel li et al., 2017; Pan et al., 2018), which are differentially expressed depending on each disorder, such as an elevated expression of TNF-α in MDD (Enache et al., 2019) and that of IL-8 in SCZ (Orlovska-Waast et al., 2019). In addition, TNF-α induces the expression of MMP-7 (Li et al., 2017) and MMP-10 (Pedersen et al., 2009), which may explain why elevated levels of MMP-7 and MDD-10 were observed in patients with MDD but not SCZ.
This study also showed that the levels of TP, an indirect marker of brain inflammation (Orlovska-Waast et al., 2019), were higher in the CSF of both patients with MDD and SCZ compared with HC, which is consistent with a previous meta-analysis of inflammatory markers levels in CSF of patients with MDD and SCZ (Orlovska-Waast et al., 2019). This study also found a correlation between the levels of TP in CSF and depressive symptoms as well as between the levels of MMP-2 in CSF and depressive symptoms. Meanwhile, there was no correlation between TP levels in CSF and schizophrenic symptoms. Therefore, the increased levels of MMP-2 and TP may reflect the occurrence of neuroinflammation in MDD and SCZ. MMP-2 and TP may be inflammatory markers of both MDD and SCZ and simultaneously be state markers of MDD.
This study has several limitations. First, because of the limited CSF volume, it was not possible to measure CSF cytokine levels such as IL-6, IL-8, and TNF-α, and tissue inhibitors of MMPs, which is useful to confirm associations with the levels of MMPs. Next, since peripheral blood and CSF could not be compared in the same participant, this study did not examine the relationship between BBB impairment and MMPs with CSF/serum albumin ratio. Finally, most patients were medicated at the time of lumbar puncture. The correlations between CSF levels of MMPs and IMI and CPZ equivalent doses were nonsignificant. However, it is impossible to completely rule out the effects of psychotropic drugs on the levels of MMPs in CSF because previous studies suggest that norepinephrine closely related to psychotropic drugs, especially antidepressants, could enhance MMP-2 expression in cultured cells (Yang et al., 2006) and in the mouse hypothalamus (Maolood et al., 2008). Therefore, it is necessary to investigate drug-free patients in a future study to exclude the effects of medications on MMPs.
In conclusion, significant increases of CSF levels of MMP-2 suggested chronic brain inflammation in MDD and SCZ. In addition, both the increase of MMP-2 in a state-dependent manner for MDD and the activation of the MMP-2 cascade interacting with MMP-7 and MMP-10 might be involved in the pathophysiology of MDD.
Supplementary Material
Acknowledgments
The collection, storage, and provision of sample and associated data were supported by the National Center of Neurology and Psychiatry (NCNP) biobank. We wish to thank Professor Junko Tanaka and Tomoyuki Akita for advice on statistical analysis and Dr Aldric Hama for editorial assistance. We also thank Ms. Hiromi Abe and Dr Kei Itagaki for helpful comments.
This work was supported by JSPS KAKENHI (grant nos. 18K15534 to N.K., 18H02756 to M.T., and 18K07620 to M.O.-T.), Japan Agency for Medical Research and Development grants (18dk0307081h0001, 18dm0107100h0003, and 18dk0307062h0003 to H.K.), grants from the Takeda Science Foundation to N.K., and grants from the SENSHIN Medical Research Foundation to M.T.
Statement of Interest
Hiroshi Kunugi MD, PhD, has received donation for research from Takeda Pharmaceutical Co. and Daiichi-Sankyo Co. No financial support for this manuscript was received from any pharmaceutical companies.
References
- American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Barillé S, Bataille R, Rapp MJ, Harousseau JL, Amiot M (1999) Production of metalloproteinase-7 (matrilysin) by human myeloma cells and its potential involvement in metalloproteinase-2 activation. J Immunol 163:5723–5728. [PubMed] [Google Scholar]
- Bloomfield PS, Howes OD, Turkheimer F, Selvaraj S, Veronese M (2016a) Response to Narendran and Frankle: the interpretation of PET microglial imaging in schizophrenia. Am J Psychiatry 173:537–538. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD (2016b) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B. (2004) The role of matrix metalloproteinase 7 in innate immunity. Immunobiology 209:51–56. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Thompson J, Taheri S, Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J, Rosenberg GA (2011) Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke 42:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN (1999) Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol 46:391–398. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA (2007) Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38:646–651. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- Enache D, Pariante CM, Mondelli V (2019) Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 81:24–40. [DOI] [PubMed] [Google Scholar]
- Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z (2011) Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One 6:e20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall PE, Yu X, Bing B (1995) Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res 42:335–342. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee JY, O’Connor KC, Pelletier D, Carson RE (2013) The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [¹¹C]PBR28 PET study. Brain Behav Immun 33:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN (2000) Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain 123(Pt 4):698–709. [DOI] [PubMed] [Google Scholar]
- Hattori K, Ota M, Sasayama D, Yoshida S, Matsumura R, Miyakawa T, Yokota Y, Yamaguchi S, Noda T, Teraishi T, Hori H, Higuchi T, Kohsaka S, Goto Y, Kunugi H (2015) Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci Rep 5:11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS (2016) In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C]®-PK11195 positron emission tomography study. Mol Psychiatry 21:1672–1679. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS (2018) Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry 83:61–69. [DOI] [PubMed] [Google Scholar]
- Inada T, Inagaki A, (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447. [DOI] [PubMed] [Google Scholar]
- Kim YS, Joh TH (2012) Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol Ther (Seoul) 20:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könnecke H, Bechmann I (2013) The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol 2013:914104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A (1999) Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood 94:2080–2089. [PubMed] [Google Scholar]
- Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT, Lee HJ (2014) Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int 2014:297241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sagar AP, Kéri S (2018) Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry 83:1–7. [DOI] [PubMed] [Google Scholar]
- Li W, Cui N, Mazzuca MQ, Mata KM, Khalil RA (2017) Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen type I deposition in hypertension in pregnancy: role of TNF-α. Am J Physiol Heart Circ Physiol 313:H491–H507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi S, Albers DS, Narr S, Chirichigno J, Beal MF (2002) Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson’s disease. Exp Neurol 178:13–20. [DOI] [PubMed] [Google Scholar]
- Mano Y, Shibata K, Sumigama S, Hayakawa H, Ino K, Yamamoto E, Kajiyama H, Nawa A, Kikkawa F (2009) Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9 secretions from human amnion cells in preterm premature rupture of membranes. Gynecol Obstet Invest 68:145–153. [DOI] [PubMed] [Google Scholar]
- Maolood N, Hardin-Pouzet H, Grange-Messent V (2008) Matrix metalloproteinases MMP2 and MMP9 are upregulated by noradrenaline in the mouse neuroendocrine hypothalamus. Eur J Neurosci 27:1143–1152. [DOI] [PubMed] [Google Scholar]
- Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, Sommer IEC, Howes OD (2019) Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med 49:2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM (2000) Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 289:1202–1206. [DOI] [PubMed] [Google Scholar]
- Molin CJ, Westerberg E, Punga AR (2017) Profile of upregulated inflammatory proteins in sera of Myasthenia Gravis patients. Sci Rep 7:39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM (2017) Brain microglia in psychiatric disorders. Lancet Psychiatry 4:563–572. [DOI] [PubMed] [Google Scholar]
- Murphy G, Cockett MI, Ward RV, Docherty AJ (1991) Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J 277(Pt 1):277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaji K, Ihara M, Takahashi C, Itohara S, Noda M, Takahashi R, Tomimoto H (2006) Matrix metalloproteinase-2 plays a critical role in the pathogenesis of white matter lesions after chronic cerebral hypoperfusion in rodents. Stroke 37:2816–2823. [DOI] [PubMed] [Google Scholar]
- Nissinen L, Kähäri VM (2014) Matrix metalloproteinases in inflammation. Biochim Biophys Acta 1840:2571–2580. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, Meyer U (2018) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry 23:323–334. [DOI] [PubMed] [Google Scholar]
- Orlovska-Waast S, Köhler-Forsberg O, Brix SW, Nordentoft M, Kondziella D, Krogh J, Benros ME (2019) Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry 24:869–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo T, Tanaka K, Koda R, Shinoda J, Sano N, Tanaka S, Aoyama H, Mimura M, Kamijima K (2005) Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci 59:517–526. [DOI] [PubMed] [Google Scholar]
- Pan C, Wang C, Zhang L, Song L, Chen Y, Liu B, Liu WT, Hu L, Pan Y (2018) Procyanidins attenuate neuropathic pain by suppressing matrix metalloproteinase-9/2. J Neuroinflammation 15:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G, Saermark T, Kirkegaard T, Brynskov J (2009) Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol 155:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Solé S, Justicia C (2001) Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis 8:834–846. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Kähäri VM (2000) Matrix metalloproteinases in wound repair (review). Int J Mol Med 6:391–407. [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, Machado-Vieira R, Yuan P, Niciu MJ, Lyoo CH, Henter ID, Salvadore G, Drevets WC, Kolb H, Innis RB, Zarate CA Jr (2018) PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella D, Fasciglione GF, Gioia M, Ciaccio C, Tundo GR, Marini S, Coletta M (2012) Human matrix metalloproteinases: an ubiquitarian class of enzymes involved in several pathological processes. Mol Aspects Med 33:119–208. [DOI] [PubMed] [Google Scholar]
- Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E (1999) Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry 4:280–283. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Xu C, Sharma S, Kish S, Houle S, Meyer JH (2018) Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 5:339–347. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC(1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shibasaki C, Takebayashi M, Itagaki K, Abe H, Kajitani N, Okada-Tsuchioka M, Yamawaki S (2016) Altered serum levels of matrix metalloproteinase-2, -9 in response to electroconvulsive therapy for mood disorders. Int J Neuropsychopharmacol 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silacci P, Dayer JM, Desgeorges A, Peter R, Manueddu C, Guerne PA (1998) Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J Biol Chem 273:13625–13629. [DOI] [PubMed] [Google Scholar]
- Su L, Faluyi YO, Hong YT, Fryer TD, Mak E, Gabel S, Hayes L, Soteriades S, Williams GB, Arnold R, Passamonti L, Rodríguez PV, Surendranathan A, Bevan-Jones RW, Coles J, Aigbirhio F, Rowe JB, O’Brien JT (2016) Neuroinflammatory and morphological changes in late-life depression: the NIMROD study. Br J Psychiatry 209:525–526.27758838 [Google Scholar]
- Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A (2012) Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience 212:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint P, Libert C (2007) Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 82:1375–1381. [DOI] [PubMed] [Google Scholar]
- Wang P, Gorter RP, de Jonge JC, Nazmuddin M, Zhao C, Amor S, Hoekstra D, Baron W (2018) MMP7 cleaves remyelination-impairing fibronectin aggregates and its expression is reduced in chronic multiple sclerosis lesions. Glia 66:1625–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, Lemeshow S, Glaser R (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66:10357–10364. [DOI] [PubMed] [Google Scholar]
- Yong VW. (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 6:931–944. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR (2001) Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.