Abstract
Background
Patients with schizophrenia (SCZ) display impaired executive functions compared with healthy controls (HCs). Furthermore, unaffected first-degree relatives (FRs) of patients with SCZ independently perform worse executive functions than do HCs. However, few studies have investigated the differences in executive functions assessed among patients with SCZ, FRs, and HCs, and the findings are inconsistent.
Methods
We investigated diagnostic differences in executive functions, namely (1) numbers of categories achieved (CA), (2) total errors (TE), and (3) percentage of perseverative errors of Nelson types (%PEN), using the Wisconsin card sorting test among patients with SCZ (n = 116), unaffected FRs (n = 62), and HCs (n = 146) at a single institute. Correlations between these executive functions and clinical variables were investigated.
Results
Significant differences existed in all executive functions among diagnostic groups (CA, F2,319 = 15.5, P = 3.71 × 10–7; TE, F2,319 = 16.2, P = 2.06 × 10–7; and %PEN, F2,319 = 21.3, P = 2.15 × 10–9). Patients with SCZ had fewer CA and more TE and %PEN than those of HCs (CA, Cohen’s d = −0.70, P = 5.49 × 10–8; TE, d = 0.70, P = 5.62 × 10–8; and %PEN, d = 0.82, P = 2.85 × 10−10) and FRs (TE, d = 0.46, P = 3.73 × 10–3 and %PEN, d = 0.38, P = .017). Of the 3 executive functions, CA and %PEN of FRs were intermediately impaired between patients with SCZ and HCs (CA, d = −0.41, P = .011 and %PEN, d = 0.41, P = .012). In contrast, no significant difference in TE existed between FRs and HCs (d = 0.22, P = .18). Although CA and TE were affected by the duration of illness (P < .017), %PEN was not affected by any clinical variable in patients with SCZ (P > .017).
Conclusions
Executive function, particularly %PEN, could be a useful intermediate phenotype for understanding the genetic mechanisms implicated in SCZ pathophysiology.
Keywords: Schizophrenia, first-degree relatives, executive function, Wisconsin card sorting test, perseverative errors
Significance Statement.
Differences in 3 executive functions assessed by the WCST were found among patients with SCZ, FRs, and HCs
The difference in %PEN was linearly different among the 3 diagnostic groups
%PEN was not influenced by any clinical variables
%PEN could be a sensitive intermediate phenotype for genetic studies of SCZ
Introduction
Schizophrenia (SCZ) is characterized by widespread cognitive impairments, including executive functions (Palmer et al., 1997; Heinrichs and Zakzanis, 1998; Weickert et al., 2000; Wilk et al., 2005; Dickinson et al., 2008). Cognitive impairment is an important predictor of daily life and social functioning and is essential for the outcome, making it a valuable target for interventions and drug discovery (Lepage et al., 2014). Executive function, which is a higher order and complex cognitive function, encompasses working memory, cognitive flexibility/set-shifting, and inhibition (Miyake et al., 2000). Volumetric alterations in prefrontal–thalamic–cerebellar networks may lead to executive dysfunction in SCZ (Rusch et al., 2007). As the effects of existing pharmacological and behavioral interventions on cognitive impairment in patients with SCZ are weak (Woodward et al., 2005, 2007; Simons et al., 2016), the development of more effective interventions and/or medications is required.
Family, twin, and adoption studies of patients with SCZ have indicated that the risk of occurrence is increased approximately 10-fold in first-degree relatives (FRs) of patients with SCZ (Cardno and Gottesman, 2000; Tsuang, 2000; Okewole et al., 2015). SCZ has a strong genetic component with an estimated heritability of approximately 80% (Sullivan et al., 2003). In addition, most cognitive functions have a genetic basis with an estimated heritability of 33%–85% (Chen et al., 1998; Posthuma et al., 2001; Berrettini, 2005; Husted et al., 2009). To date, genome-wide association studies have identified more than 100 loci linked to general cognitive function (Davies et al., 2018) and 108 loci linked to SCZ (Ripke et al., 2014). These studies revealed that substantial proportions of the heritability of cognitive function and SCZ are explained by a polygenic component consisting of many common genetic variants with small effects (Ohi et al., 2018b). Therefore, cognitive impairments could be useful intermediate phenotypes for understanding the genetic mechanisms implicated in the pathophysiology of SCZ.
To assess executive functions and the related genetic factors, most studies have utilized the Wisconsin card sorting test (WCST) in patients with SCZ and healthy controls (HCs) (Aydin et al., 2017). Patients with SCZ obviously displayed impaired executive functions as assessed by the WCST compared with HCs (Cohen’s d = 1.33). In addition, a meta-analysis using a large sample of 1181 FRs and 992 HCs indicated that unaffected FRs of patients with SCZ perform worse than HCs on the WCST outcome variables of categories achieved (CA) (d = −0.34) and perseverative errors (PE) (d = 0.24) (Jameson et al., 2011). The effect sizes for impaired executive functions in FRs were intermediate between patients with SCZ and HCs. However, there were few studies investigating the differences in executive functions assessed by the WCST among 3 diagnostic groups, that is, patients with SCZ, FRs, and HCs, and the differences in executive functions among the 3 groups were inconsistent among studies (Szöke et al., 2006a, 2006b; Breton et al., 2011; Hu et al., 2011; Sanchez-Torres et al., 2013; Yang et al., 2015) because of the relatively small sample sizes (n = 81–177). We hypothesized that executive functions could be linearly impaired among HCs, FRs, and patients with SCZ. Furthermore, to the best of our knowledge, few studies have examined whether executive functions are affected by clinical variables, including age, years of education, antipsychotics, and psychotic symptoms, among the 3 diagnostic groups.
In this study, we first investigated the differences in executive functions: (1) numbers of CA, (2) total errors (TE), and (3) %PE of Nelson types (%PEN). Functions are well-examined variables in SCZ, assessed by the WCST among patients with SCZ, FRs, and HCs at a single institute using the largest sample sizes (n = 324) in this field. Next, we investigated correlations between executive functions and demographic and clinical variables in each diagnostic group.
Methods
Participants
The participants consisted of 116 patients with SCZ (44 males/72 females, mean age ± SD: 44.0 ± 13.2 years), 62 unaffected FRs (29 mothers/19 fathers/8 sisters/2 brothers/3 offspring, 24 males/38 females, 59.4 ± 14.5 years), and 146 HCs (96 males/50 females, 37.0 ± 15.2 years). The study sample was recruited from the Schizophrenia Non-Affected Relative Project at Kanazawa Medical University (Ohi et al., 2019b, 2020). All participants were of Japanese descent. Patients and their unaffected FRs were recruited from both the outpatient and inpatient populations of Kanazawa Medical University Hospital. Most of unaffected FRs were recruited from the family members of the SCZ patients group. Each patient was diagnosed by at least 2 trained psychiatrists based on unstructured clinical interviews, medical records, and clinical conferences (Ohi et al., 2016, 2017, 2018a, 2019b; Yasuyama et al., 2017). The patients with SCZ were diagnosed according to the criteria in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. Their unaffected FRs were evaluated using the non-patient version of the Structured Clinical Interview for DSM-IV to exclude individuals who had current or past contact with psychiatric services or who had received psychiatric medication. HCs were recruited through local advertisements and from among hospital staff at Kanazawa Medical University and were also evaluated using the non-patient version of the Structured Clinical Interview for DSM-IV to exclude individuals who had current or past contact with psychiatric services, who had received psychiatric medication, or who had a family history of any neuropsychiatric diseases among second-degree relatives. Participants were excluded from the analysis if they had neurological or medical conditions that could affect the central nervous system, including atypical headache, head trauma with loss of consciousness, chronic lung disease, kidney disease, chronic hepatic disease, active cancer, cerebrovascular disease, thyroid disease, epilepsy, seizures, substance-related disorders, current steroid use, or intellectual disability. To measure the premorbid IQ, we administered the Japanese version of the National Adult Reading Test. Current clinical symptoms in patients with SCZ were evaluated using the Positive and Negative Syndrome Scale (Kay et al., 1987). Chlorpromazine equivalents of total antipsychotics (CPZ-eq.) and the Biperiden equivalent of antiparkinsonisms were calculated based on the report by Inagaki (Inada and Inagaki, 2015). The demographic information of the 3 diagnostic groups is summarized in Table 1. The mean age, gender ratio, years of education, and estimated premorbid IQ differed significantly among the groups (P < .05). Written informed consent was obtained from all participants after the procedures had been thoroughly explained. This study was performed in accordance with the Declaration of Helsinki from the World Medical Association and was approved by the Research Ethical Committee of Kanazawa Medical University.
Table 1.
Demographic Variables of the Included Diagnostic Groups
| Schizophrenia | First-degree relatives | Controls | |||
|---|---|---|---|---|---|
| Variables | (n = 116) | (n = 62) | (n = 146) | P values (F2,321) | Post hoc |
| Age (y) | 44.0 ± 13.2 | 59.4 ± 14.5 | 37.0 ± 15.2 | 1.40 × 10 –20 (52.9) | FR > SCZ > HC |
| Gender (male/female) | 44/72 | 24/38 | 96/50 | 5.31 × 10 –6 (24.3) a | – |
| Education (y) | 12.6 ± 2.1 | 12.8 ± 2.2 | 16.0 ± 2.4 | 1.76 × 10 –30 (85.5) | HC > FR, SCZ |
| Estimated premorbid IQ | 98.7 ± 10.6 | 99.3 ± 9.2 | 108.9 ± 7.6 | 3.71 × 10 –17 (43.1) | HC > FR, SCZ |
| CPZ-eq. (mg/d) | 550.4 ± 519.0 | – | – | – | – |
| Biperiden-eq. (mg/d) | 1.0 ± 2.7 | – | – | – | – |
| Age at onset (y) | 27.5 ± 10.6 | – | – | – | – |
| Duration of illness (y) | 16.4 ± 11.8 | – | – | – | – |
| PANSS positive symptoms | 16.3 ± 6.4 | – | – | – | – |
| PANSS negative symptoms | 17.5 ± 6.7 | – | – | – | – |
| CAb | 2.7 ± 2.0 | 2.5 ± 2.1 | 4.4 ± 2.0 | 3.36 × 10 –13 (31.5) | HC > FR, SCZ |
| TEb | 24.3 ± 9.9 | 23.8 ± 10.4 | 16.0 ± 9.1 | 4.02 × 10 –12 (28.5) | SCZ, FR > HC |
| %PENb | 31.6 ± 18.9 | 30.4 ± 20.9 | 13.9 ± 16.0 | 4.85 × 10 –15 (36.6) | SCZ, FR > HC |
Abbreviations: Biperiden-eq., Biperiden equivalent; CA, category achieved; CPZ-eq., chlorpromazine equivalents of total antipsychotics; FR, first-degree relatives; HC, controls; IQ, intelligence quotient; PANSS, Positive and Negative Syndrome Scale; %PEN, percentage of perseverative errors of Nelson types; SCZ, schizophrenia; TE, total errors.
Means ± SD are shown. Complete demographic information was not obtained for all participants (estimated premorbid IQ in HC, n = 122; in FR, n = 61). P < .017 (α = 0.05/3) are shown in boldface and post hoc analysis was performed.
a χ 2 test.
bRaw scores of executive functions were indicated.
Executive Function
Executive function, including cognitive flexibility in response to feedback, was assessed using the WCST. We used a modified and computerized Keio version of the WCST (Igarashi et al., 2002; Tomida et al., 2010). According to the manual for the Keio version of the WCST, the participants classified a single card shown at the bottom of a computer screen in terms of color (red, green, yellow, blue), shape (triangle, star, cross, circle), and number (1–4) and selected 1 type of card from 4 basic types of cards shown at the top. Without letting the participants know the correct category, the computer gave feedback regarding whether the selection was correct or incorrect. If the participants made 6 continuous correct selections, the categories in the computer were changed, and the participants had to select another category to make a correct selection. This test was carried out for up to 48 selections.
The main outcomes commonly used in the WCST were (1) CA, (2) TE, and (3) %PEN. CA is the number of categories for which 6 consecutive correct responses are achieved (8 is the maximum number of categories that can be achieved) and is frequently used as a measure of overall WCST performance. TE is the total number of incorrect responses (maximum TE is 48). PEN is the number of incorrect responses in the same category as the immediately preceding incorrect response (maximum of PE is 47), and %PEN is the percentage of PEN in TE (maximum %PEN is 98). Values for %PEN imply impairment in problem solving, set shifting, mental flexibility, and abstract thinking (Baran et al., 2009). Three executive functions (CA, TE, and %PEN) were correlated; however, the degrees of correlations among the variables were not strong (Lee et al., 2009; Banno et al., 2012), suggesting that these variables would be independent indicators of executive functions.
Statistical Analyses
All statistical analyses were performed using the IBM SPSS Statistics 25.0 software (IBM Japan, Tokyo, Japan). Differences in continuous variables, such as age and years of education, among the 3 diagnostic groups were analyzed using the ANOVA. Differences in categorical variables, such as gender, were analyzed using Pearson’s χ2 test. To control for confounding factors, the effects of the diagnostic status on executive functions were analyzed using the ANCOVA with the executive functions (CA, PEN, and %PEN) as the dependent variables, diagnostic status (SCZ, FR, or HC) as the independent variable, and age and gender as the covariates. Post hoc tests with Fisher’s least significant difference test were used to evaluate the significant differences among groups. Correlations between the executive function and clinical variables were evaluated using Pearson’s correlation coefficient in each of the 3 diagnostic groups. Standardized effects were calculated using Cohen d method (https://lbecker.uccs.edu/). The significance level was set at a 2-tailed P < .017 (α = 0.05/3 executive functions) to control for type I errors.
Results
Differences in Executive Functions Among Patients with SCZ, FRs, and HCs
First, we examined correlations among 3 executive functions (CA, TE, and %PEN) in each diagnostic group. The CA, TE, and %PEN were highly correlated with each other (Table 2, all P < 1.00 × 10–3), and the degrees of these correlations were similar among diagnostic groups. Consistent with previous studies (Lee et al., 2009; Banno et al., 2012), these 3 indicators were not identical, particularly CA and %PEN.
Table 2.
Correlations Among 3 Executive Functions (CA, TE, and %PEN) in Each Diagnostic Group
| CA | TE | %PEN | |
|---|---|---|---|
| CA | – | −0.86 a/−0.81a/−0.86a | −0.59 a/−0.61a/−0.59a |
| TE | – | 0.73 a/0.80a/0.73a | |
| %PEN | – |
Abbreviations: CA, category achieved; %PEN, percentage of perseverative errors of Nelson types; TE, total errors.
Pearson’s correlation coefficient in each diagnostic group (schizophrenia/first-degree relatives/controls) is represented. P < .017 are shown in boldface.
a P < .001.
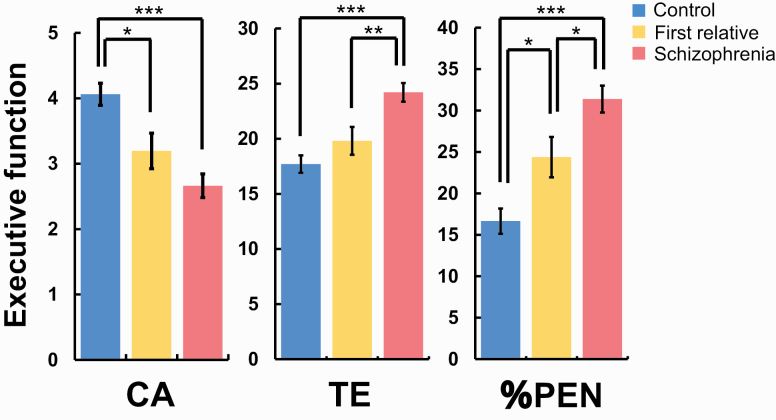
We next investigated diagnostic differences in 3 executive functions among patients with SCZ, FRs, and HCs. We found significant differences in all executive functions among patients with SCZ, FRs, and HCs (Figure 1; CA, F2,319 = 15.5, P = 3.71 × 10–7; TE, F2,319 = 16.2, P = 2.06 × 10–7; and %PEN, F2,319 = 21.3, P = 2.15 × 10–9). Post hoc analyses showed that patients with SCZ had lower CA and higher TE and %PEN than those of HCs (CA, Cohen’s d = −0.70, P = 5.49 × 10–8; TE, d = 0.70, P = 5.62 × 10–8; and %PEN, d = 0.82, P = 2.85 × 10−10). There were also significant differences in TE and %PEN between patients with SCZ and FRs (TE, d = 0.46, P = 3.73 × 10–3 and %PEN, d = 0.38, P = .017), while there was no significant difference in CA between these groups (d = −0.26, P = .10). Of the 3 executive functions, CA and %PEN in FRs were lower and higher than those in HCs, respectively (CA, d = −0.41, P = .011 and %PEN, d = 0.41, P = .012). In contrast, there was no significant difference in TE between FRs and HCs (d = 0.22, P = .18).
Figure 1.
Differences in the executive functions among patients with schizophrenia, their unaffected first-degree relatives, and healthy controls. Abbreviations: %PEN; percentage of perseverative errors of Nelson types; CA; categories achieved; TE; total errors. Age- and gender-corrected scores of executive functions were indicated. Error bars indicate the standard error of the mean scores.*Post hoc P < .05, **post hoc P < .01, ***post hoc P < .001.
Correlations Between Executive Functions and Demographic and Clinical Variables in Each Diagnostic Group
To identify potential impacts of demographic variables on executive functions, we further investigated the correlations of demographic variables, such as age and years of education, in each diagnostic group (Table 3). Age was negatively correlated with CA and positively correlated with TE and %PEN in most diagnostic groups. In contrast, the estimated premorbid IQ was positively correlated with CA and negatively correlated with TE and %PEN only in patients with SCZ, except for a marginal negative correlation with TE in FRs. Years of education were positively correlated with CA and negatively correlated with TE and %PEN in patients with SCZ and HCs but not in FRs.
Table 3.
Correlations Between Executive Functions and Clinical Variables in the 3 Diagnostic Groups
| Schizophrenia | First-degree relatives | Controls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CA | TE | %PEN | CA | TE | %PEN | CA | TE | %PEN | |
| Age (y) | −0.23b | 0.27 b | 0.13 | −0.23 | 0.26a | 0.29a | −0.42b | 0.52 b | 0.46 b |
| Education (y) | 0.32 b | −0.29 b | −0.14 | 0.05 | −0.13 | −0.16 | 0.24 b | −0.30 b | −0.30 b |
| Estimated premorbid IQ | 0.44 b | −0.42b | −0.38b | 0.17 | −0.25a | −0.20 | −0.05 | −0.01 | 0.03 |
| CPZ-eq. (mg/d) | −0.25b | 0.23 b | 0.15 | – | – | – | – | – | – |
| Biperiden-eq. (mg/d) | −0.15 | 0.13 | 0.06 | – | – | – | – | – | – |
| Age at onset (y) | < 0.01 | −0.02 | < 0.01 | – | – | – | – | – | – |
| Duration of illness (y) | −0.27b | 0.32 b | 0.14 | – | – | – | – | – | – |
| PANSS positive symptoms | 0.02 | −0.07 | −0.12 | – | – | – | – | – | – |
| PANSS negative symptoms | −0.17 | 0.22a | 0.18a | – | – | – | – | – | – |
Abbreviations: Biperiden-eq., Biperiden equivalent; CA, category achieved; CPZ-eq., chlorpromazine equivalents of total antipsychotics; PANSS, Positive and Negative Syndrome Scale; PEN, percentage of perseverative errors of Nelson types; TE, total errors.
Pearson’s correlation coefficient is represented. P < .017 are shown in boldface.
a P < .05.
b P < .017.
We next investigated the correlations of clinical variables, such as medications and psychiatric symptoms, in patients with SCZ (Table 3). CPZ-eq. and duration of illness were negatively correlated with CA and positively correlated with TE. There were no significant correlations between %PEN and any clinical variables or correlations of Biperiden equivalent, age at onset, and psychiatric symptoms with CA, TE, and %PEN (P > .017).
Discussion
We investigated the differences in executive functions assessed by WCST among patients with SCZ, FRs, and HCs at a single institute using the largest sample sizes. Compared with previous studies (Szöke et al., 2006a, 2006b; Breton et al., 2011; Hu et al., 2011; Sanchez-Torres et al., 2013; Yang et al., 2015), the sample sizes in the current study were the largest in this field. We found significant differences in all executive functions, CA, TE, and %PEN among the 3 diagnostic groups. Consistent with previous studies (Ma et al., 2007; Birkett et al., 2008; Breton et al., 2011; Hu et al., 2011; Sanchez-Torres et al., 2013; Yang et al., 2015), patients with SCZ showed lower CA and higher TE and %PEN than did HCs. Patients with SCZ displayed higher TE and %PEN than did FRs. Furthermore, FRs had lower CA and higher %PEN than did HCs. Of the 3 measures, %PEN was significantly different among each diagnostic group. Considering that %PEN was not affected by clinical variables in patients with SCZ, our findings suggest that executive function, especially in %PEN, could be a useful intermediate phenotype for understanding the genetic mechanisms implicated in the pathophysiology of SCZ.
We revealed that the difference in %PEN among the 3 diagnostic groups was the most significant and linearly different among the 3 diagnostic groups. PEN is the number of incorrect responses in the same category as that of the immediately preceding incorrect response, and %PEN is defined as the percentage of PEN in TE. To avoid conflating the tendency to perseverate with the tendency to make errors of all kinds, such as TE and unique errors, the tendency to perseverate is expressed as the percentage of PE in TE (Birkett et al., 2008). PE is regarded as the main indicator of frontal dysfunction, and it provides a robust and valid measure of executive attention processes related to the inhibition of prepotent responses, shifting set, cognitive control, and performance monitoring (Koren et al., 1998). PE was strongly and negatively correlated with the gray matter volume of the total left prefrontal cortex in healthy participants, while there was a lack of correlation between PE and left prefrontal volumes in patients with SCZ because of floor effects in both impaired phenotypes (Nestor et al., 2019). Compared with HCs, reduced right prefrontal volumes were found in FRs with cognitive impairments assessed by several cognitive tests, including the WCST (Bhojraj et al., 2011). Considering that patients with SCZ failed to increase right prefrontal blood flow during WCST performance compared with HCs (Ortuno et al., 2006), impaired right prefrontal cortex in FRs as well as patients with SCZ might contribute to differences in %PEN among the 3 diagnostic groups.
We examined correlations between the executive functions and demographic variables in each diagnostic group. Years of education was positively correlated with CA and negatively correlated with TE and %PEN, while age was negatively correlated with CA and positively correlated with TE and %PEN in patients with SCZ and HCs but not in FRs. The reason why we did not find a correlation between executive functions and these demographic variables in FRs would be the relatively small sample sizes, as the correlation coefficient was similar among the 3 groups. On the other hand, the premorbid IQ was positively correlated with CA and negatively correlated with TE and %PEN only in patients with SCZ and not in FRs or HCs. Considering that premorbid IQ and years of education were highly correlated in each group (SCZ, r = 0.37; FRs, r = 0.44; HCs, r = 0.33), low variances (SD) of the premorbid IQ in FRs and HCs (FRs, 9.2; HCs, 7.6) would contribute to the lack of significant correlations between premorbid IQ and executive functions in the 2 groups. In patients with SCZ, CA and TE were negatively and positively correlated with CPZ-eq. and duration of illness, respectively. In contrast, %PEN was not significantly correlated with any clinical variable. These findings suggest that %PEN might be a trait marker but not a state marker in the pathophysiology of SCZ.
As age was a serious confounding factor (Rhodes 2004; Miranda et al., 2019) and our findings might be driven by the significant age differences observed in the groups, we performed subgroup analysis using age-matched samples (n = 58 in each group; supplementary Table 1) selected from our participants. Because the sample sizes were decreased, some differences in executive functions between diagnostic groups became insignificant (supplementary Figure 1; P > .017). However, effect sizes (d) for the differences between groups were not changed (supplementary Table 2). Furthermore, we checked differences in 3 executive functions between genders in each group because gender would be a potential confounding variable for the 3 executive functions. There were no differences in 3 executive functions between genders in any diagnostic groups (P > .05). Therefore, our findings might not be driven by age and gender.
Because SCZ is a polygenic disorder caused by multiple susceptibility genes (Ripke et al., 2014), the severity of impairments of intermediate phenotypes in patients with SCZ and their FRs may increase with the increase in familial genetic loading for SCZ. Supporting this notion is the familial aggregation on executive function in patients with SCZ and FRs (Lin et al., 2013). Patients in multiplex families exhibited more prominent impairments of executive function than those in simplex families, while the impairments were limited to multiplex families and not simplex families in FRs (Lin et al., 2013). In contrast, another study reported no familial aggregations on the executive function in patients with SCZ or FRs (Birkett et al., 2008). In this study, we did not focus on familial aggregation on the executive function in FRs in simplex or multiplex families because of the relatively small sample sizes in the FR group (simplex, n = 60; multiplex, n = 2). Further replication studies are needed to confirm the familial aggregation on the executive function in FRs.
There are some limitations to the interpretations of our findings. We could not exclude the possibility of some methodological selection bias in our samples. Since 77% of the FRs consisted of the parents of patients with SCZ, the mean age in the FRs was increased. In contrast, the HC group had a lower mean age and higher premorbid IQ, as the group included some medical students. Compared with the sample sizes of patients with SCZ and HCs, that of the FRs was relatively small. Thus, false positive findings are possible. Future studies attempting to replicate our findings using larger FR sample sizes are needed.
In this study, we investigated executive functions assessed by the WCST among patients with SCZ, their unaffected FRs, and HCs using the largest sample sizes at a single institute. The difference in %PEN but not in CA or TE was the most significantly and linearly revealed among the 3 diagnostic groups. %PEN was not significantly influenced by any clinical variable. Our findings suggest that %PEN could be the most sensitive intermediate phenotype of the WCST for understanding the genetic mechanisms implicated in the pathophysiology of SCZ as well as be useful for the prevention for onset of SCZ and the early intervention of individuals at risk for SCZ.
Supplementary Material
Acknowledgments
We thank all individuals who participated in this study.
This work was supported by Grants-in-Aid for Scientific Research (C) (19K08081) and Young Scientists (B) (16K19784) from the Japan Society for the Promotion of Science (JSPS), a grant from the Uehara Memorial Foundation, a grant from the Takeda Science Foundation, a grant from the YOKOYAMA Foundation for Clinical Pharmacology (YRY-1807), a grant from the Smoking Research Foundation, a grant from the Matsubara Saburo Memorial Research Foundation for Psychiatry, and a grant for Assist KAKEN (K2017-8, K2018-16, and K2018-17) and Promoted Research (S2017-3, S2018-5, and S2019-8) from Kanazawa Medical University.
Statement of Interest
None.
References
- Aydın E, Cansu Ülgen M, Tabo A, Devrim Balaban Ö, Yeşilyurt S, Yumrukçal H (2017) Executive function and genetic loading in nonpsychotic relatives of schizophrenia patients. Psychiatry Res 248:105–110. [DOI] [PubMed] [Google Scholar]
- Banno M, Koide T, Aleksic B, Okada T, Kikuchi T, Kohmura K, Adachi Y, Kawano N, Iidaka T, Ozaki N (2012). Wisconsin Card Sorting Test scores and clinical and sociodemographic correlates in schizophrenia: multiple logistic regression analysis. BMJ Open 2:e001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Tekcan AI, Gürvit H, Boduroglu A (2009) Episodic memory and metamemory in Parkinson’s disease patients. Neuropsychology 23:736–745. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. (2005) Genetic bases for endophenotypes in psychiatric disorders. Dialogues Clin Neurosci 7:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Montrose DM, Keshavan MS (2011) Grey matter and cognitive deficits in young relatives of schizophrenia patients. Neuroimage 54Suppl 1:S287–S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett P, Sigmundsson T, Sharma T, Toulopoulou T, Griffiths TD, Reveley A, Murray R (2008) Executive function and genetic predisposition to schizophrenia–the Maudsley family study. Am J Med Genet B Neuropsychiatr Genet 147:285–293. [DOI] [PubMed] [Google Scholar]
- Breton F, Planté A, Legauffre C, Morel N, Adès J, Gorwood P, Ramoz N, Dubertret C (2011) The executive control of attention differentiates patients with schizophrenia, their first-degree relatives and healthy controls. Neuropsychologia 49:203–208. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II (2000) Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 97:12–17. [PubMed] [Google Scholar]
- Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG (1998) Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry 155:1214–1220. [DOI] [PubMed] [Google Scholar]
- Davies G, et al. (2018). Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun 9:2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC (2008) General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry 64:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12:426–445. [DOI] [PubMed] [Google Scholar]
- Hu M, Chen J, Li L, Zheng Y, Wang J, Guo X, Wu R, Zhao J (2011) Semantic fluency and executive functions as candidate endophenotypes for the early diagnosis of schizophrenia in Han Chinese. Neurosci Lett 502:173–177. [DOI] [PubMed] [Google Scholar]
- Husted JA, Lim S, Chow EW, Greenwood C, Bassett AS (2009) Heritability of neurocognitive traits in familial schizophrenia. Am J Med Genet B Neuropsychiatr Genet 150B:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K, Oguni H, Osawa M, Awaya Y, Kato M, Mimura M, Kashima H (2002) Wisconsin Card Sorting Test in children with temporal lobe epilepsy. Brain Dev 24:174–178. [DOI] [PubMed] [Google Scholar]
- Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440– 447. [DOI] [PubMed] [Google Scholar]
- Jameson KG, Nasrallah HA, Northern TG, Welge JA (2011) Executive function impairment in first-degree relatives of persons with schizophrenia: a meta-analysis of controlled studies. Asian J Psychiatr 4:96–99. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Koren D, Seidman LJ, Harrison RH, Lyons MJ, Kremen WS, Caplan B, Goldstein JM, Faraone SV, Tsuang MT (1998) Factor structure of the Wisconsin Card Sorting Test: dimensions of deficit in schizophrenia. Neuropsychology 12:289–302. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee HK, Kweon YS, Lee CT, Lee KU (2009) The impact of executive function on emotion recognition and emotion experience in patients with schizophrenia. Psychiatry Investig 6:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Bodnar M, Bowie CR (2014) Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry 59:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Liu CM, Hwang TJ, Hsieh MH, Hsiao PC, Faraone SV, Tsuang MT, Hwu HG, Chen WJ (2013) Performance on the Wisconsin Card Sorting Test in families of schizophrenia patients with different familial loadings. Schizophr Bull 39:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang Q, Sham PC, Liu X, Rabe-Hesketh S, Sun X, Hu J, Meng H, Chen W, Chen EY, Deng W, Chan RC, Murray RM, Collier DA, Li T (2007) Neurocognitive deficits in first-episode schizophrenic patients and their first-degree relatives. Am J Med Genet B Neuropsychiatr Genet 144B:407–416. [DOI] [PubMed] [Google Scholar]
- Miranda AR, Franchetto Sierra J, Martinez Roulet A, Rivadero L, Serra SV, Soria EA (2019) Age, education and gender effects on Wisconsin Card Sorting Test: standardization, reliability and validity in healthy Argentinian adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 191–19. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol 41:49–100. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Forte M, Ohtani T, Levitt JJ, Newell DT, Shenton ME, Niznikiewicz M, McCarley RW (2019) Faulty executive attention and memory interactions in schizophrenia: prefrontal gray matter volume and neuropsychological impairment. Clin EEG Neurosci 51:267–274. 1550059419881529 [DOI] [PubMed] [Google Scholar]
- Ohi K, Matsuda Y, Shimada T, Yasuyama T, Oshima K, Sawai K, Kihara H, Nitta Y, Okubo H, Uehara T, Kawasaki Y (2016) Structural alterations of the superior temporal gyrus in schizophrenia: detailed subregional differences. Eur Psychiatry 35:25–31. [DOI] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kihara H, Yasuyama T, Sawai K, Matsuda Y, Oshima K, Okubo H, Nitta Y, Uehara T, Kawasaki Y (2017) Impact of familial loading on prefrontal activation in major psychiatric disorders: a Near-Infrared Spectroscopy (NIRS) Study. Sci Rep 7:44268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kuwata A, Kataoka Y, Okubo H, Kimura K, Yasuyama T, Uehara T, Kawasaki Y (2018a) Smoking rates and number of cigarettes smoked per day in schizophrenia: a large cohort meta-analysis in a Japanese Population. Int J Neuropsychopharmacol 22:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Sumiyoshi C, Fujino H, Yasuda Y, Yamamori H, Fujimoto M, Shiino T, Sumiyoshi T, Hashimoto R (2018b) Genetic overlap between general cognitive function and schizophrenia: a review of cognitive GWASs. Int J Mol Sci 19:3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Kataoka Y, Shimada T, Kuwata A, Okubo H, Kimura K, Yasuyama T, Uehara T, Kawasaki Y (2019a) Meta-analysis of physical activity and effects of social function and quality of life on the physical activity in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 269:517–527. [DOI] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kataoka Y, Koide Y, Yasuyama T, Uehara T, Okubo H, Kawasaki Y (2019b) Intelligence decline between present and premorbid IQ in schizophrenia: Schizophrenia Non-Affected Relative Project (SNARP). Eur Neuropsychopharmacol 29:653–661. [DOI] [PubMed] [Google Scholar]
- Ohi K, Nishizawa D, Shimada T, Kataoka Y, Hasegawa J, Shioiri T, Kawasaki Y, Hashimoto R, Ikeda K (2020) Polygenetic risk scores for major psychiatric disorders among schizophrenia patients, their first-degree relatives, and healthy participants. Int J Neuropsychopharmacol 23:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okewole AO, Adewuya AO, Makanjuola RO, Owoeye OA (2015) Morbidity profile of first-degree relatives of probands with schizophrenia: a comparison with mood disorder and healthy control. Soc Psychiatry Psychiatr Epidemiol 50:389–395. [DOI] [PubMed] [Google Scholar]
- Ortuño F, Moreno-Iñiguez M, Millán M, Soutullo CA, Bonelli RM (2006) Cortical blood flow during rest and Wisconsin Card Sorting Test performance in schizophrenia. Wien Med Wochenschr 156:179–184. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV (1997) Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology 11:437–446. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Boomsma DI (2001) Perceptual speed and IQ are associated through common genetic factors. Behav Genet 31:593–602. [DOI] [PubMed] [Google Scholar]
- Rhodes MG. (2004) Age-related differences in performance on the Wisconsin Card Sorting Test: a meta-analytic review. Psychol Aging 19:482–494. [DOI] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, Holmans PA, Lee P, Bulik-Sullivan B, Collier DA, Huang H (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G (2007) Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res 93:79–89. [DOI] [PubMed] [Google Scholar]
- Sánchez-Torres AM, Basterra V, Moreno-Izco L, Rosa A, Fañanás L, Zarzuela A, Peralta V, Cuesta MJ (2013) Executive functioning in schizophrenia spectrum disorder patients and their unaffected siblings: a ten-year follow-up study. Schizophr Res 143:291–296. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, Stine-Morrow EA (2016) Do “brain-training” programs work? Psychol Sci Public Interest 17:103–186. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC (2003) Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60:1187–1192. [DOI] [PubMed] [Google Scholar]
- Szöke A, Schürhoff F, Golmard JL, Alter C, Roy I, Méary A, Etain B, Bellivier F, Leboyer M (2006a) Familial resemblance for executive functions in families of schizophrenic and bipolar patients. Psychiatry Res 144:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöke A, Schürhoff F, Méary A, Mathieu F, Chevalier F, Trandafir A, Alter C, Roy I, Bellivier F, Leboyer M (2006b) Lack of influence of COMT and NET genes variants on executive functions in schizophrenic and bipolar patients, their first-degree relatives and controls. Am J Med Genet B Neuropsychiatr Genet 141B:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida K, Takahashi N, Saito S, Maeno N, Iwamoto K, Yoshida K, Kimura H, Iidaka T, Ozaki N (2010) Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin Neurosci 64:62–69. [DOI] [PubMed] [Google Scholar]
- Tsuang M. (2000) Schizophrenia: genes and environment. Biol Psychiatry 47:210–220. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR (2000) Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry 57:907–913. [DOI] [PubMed] [Google Scholar]
- Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW (2005) No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology 19:778–786. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH (2005) A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol 8:457–472. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH (2007) A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophr Res 89:211–224. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang T, Li Z, Heeramun-Aubeeluck A, Liu N, Huang N, Zhang J, He L, Li H, Tang Y, Chen F, Liu F, Wang J, Lu Z (2015) The relationship between facial emotion recognition and executive functions in first-episode patients with schizophrenia and their siblings. BMC Psychiatry 15:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama T, Ohi K, Shimada T, Uehara T, Kawasaki Y (2017) Differences in social functioning among patients with major psychiatric disorders: interpersonal communication is impaired in patients with schizophrenia and correlates with an increase in schizotypal traits. Psychiatry Res 249:30–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.