Abstract
Background
Inflammasome-induced neuroinflammation is a major pathogenic mechanism underlying the degeneration of nigral dopaminergic neurons in Parkinson’s disease (PD). Baicalein is a flavonoid isolated from the traditional Chinese medicinal herbal Scutellaria baicalensis Georgi with known anti-inflammatory and neuroprotective efficacy in models of neurodegenerative diseases, including PD. However, its effects on inflammasome-induced neuroinflammation during PD remain unclear.
Methods
We used N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to induce PD-like pathology in mice. Behavioral assessments including the pole test, rotarod test, and open field test were conducted to evaluate the effects of baicalein on MPTP-induced motor dysfunction. The efficacies of baicalein against MPTP-induced dopaminergic neuron loss and glial cell activation in the substantia nigra compact were examined by immunohistochemistry, effects on proinflammatory cytokines by quantitative real-time PCR and enzyme-linked immunosorbent assay, and effects on inflammasome pathway activation by immunoblotting and flow cytometry.
Results
Administration of baicalein reversed MPTP-induced motor dysfunction, loss of dopaminergic neurons, and pro-inflammatory cytokine elevation. Baicalein also inhibited NLRP3 and caspase-1 activation and suppressed gasdermin D-dependent pyroptosis. Additionally, baicalein inhibited the activation and proliferation of disease-associated proinflammatory microglia.
Conclusions
These findings suggest that baicalein can reverse MPTP-induced neuroinflammation in mice by suppressing NLRP3/caspase-1/gasdermin D pathway. Our study provides potential insight into the use of baicalein in PD therapy.
Keywords: Parkinson’s disease, neuroinflammation, baicalein, GSDMD, NLRP3
Significance Statement.
Parkinson’s disease (PD) is a progressive and incurable neurodegenerative disorder that markedly impairs motor function and eventually causes many abnormal brain functions, ultimately leading to premature death. Therefore, it is critical to elucidate the pathogenic mechanisms of PD for the development of novel treatment strategies. Baicalein is a flavonoid originally isolated from the traditional medicinal herb Scutellaria baicalensis Georgi with known neuroprotective and anti-inflammatory effects in models of neurodegenerative disorders, including PD models. However, the underlying protective mechanisms remain unclear. In the present study, we demonstrated that baicalein decreases pro-inflammatory cytokine expression and release in the substantial nigra compact (SNc) of MPTP-treated mice by suppressing NLRP3/caspase-1/GSDMD pathway signaling, thereby attenuating neuroinflammation, SNc neuronal loss, and motor dysfunction.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease. It is characterized by the loss of dopaminergic neurons in the substantia nigra compact (SNc), resulting in motor impairments such as resting tremor, rigidity, bradykinesia, and postural instability (Poewe et al., 2017; Krashia et al., 2019). The dopamine precursor levodopa and dopaminergic receptor agonists are widely used clinically for PD therapy; however, these drugs improve clinical symptoms only temporarily and do not stop disease progression (LeWitt et al., 2016). Therefore, it is urgent to identify treatments that prevent loss of SNc neurons by directly targeting the underlying pathogenic processes.
Multiple molecular mechanisms contribute to PD pathogenesis, including neuroinflammation (Zella et al., 2019). The expression levels of proinflammatory cytokines such as tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and IL-6 are substantially elevated in the cerebrospinal fluid and serum of idiopathic PD patients (Mogi et al., 1994, 1996; Dobbs et al., 1999). Inflammasomes are cytosolic multiprotein complexes that induce the production of inflammatory cytokines and promote the development of inflammation (Orning et al., 2019). On recognition of signal from pathogen infection and tissue damage, inflammasome-associated pattern recognition receptors, apoptosis-associated speck-like protein containing CARD (adaptor protein), and pro-inflammatory cysteinyl aspartate specific proteinase (caspases) assemble into oligomers that drive caspase-1 or -11 autoactivation and IL-1β maturation, leading to further cytokine production and proinflammatory programmed cell death known as pyroptosis (Orning et al., 2019). Accumulating evidence suggests that proinflammatory cytokines induced by inflammasomes, especially NLRP3 (NOD-, LRR-, and pyrin domain-containing 3 inflammasome) contribute to the pathogenesis of PD (Heneka et al., 2018; Fan et al., 2020). Gasdermin D (GSDMD), a protein substrate of caspase-1 and -11, has been identified as an executive molecule in pyroptosis; cleavage of GSDMD results in N-domain oligomerization and plasma membrane pore formation (Shi et al., 2015). Recent studies indicate that GSDMD is involved with many diseases, such as disseminated intravascular coagulation (Yang et al., 2019), diabetic heart disease (Wang et al., 2019), and multiple sclerosis (Li et al., 2019). However, whether inflammasome-activated GSDMD contributes to the neuroinflammatory processes involved in PD pathogenesis has not been examined.
Baicalein is a flavonoid originally isolated from the roots of traditional Chinese herbal Scutellaria baicalensis Georgi, which has been widely used for many centuries due to its purported antibacterial, antiviral, and especially anti-inflammatory activities (Lin et al., 1996). Baicalein can reduce the production of several pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6 (Hsieh et al., 2007; Chen et al., 2008), known to increase in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced animal model of PD (Yasuda et al., 2008; Lofrumento et al., 2011). There is also compelling experimental evidence that baicalein is neuroprotective in MPTP-treated mice (Zheng et al., 2019). Baicalein treatment preserved tyrosine hydroxylase (TH)-positive neuron and alleviate muscle tremor in the 6-hydroxydopamine–induced rat model (Yu et al., 2012). Moreover, baicalein attenuated neuroinflammatory astrocyte activation in the MPTP model (Lee et al., 2014). In addition, baicalein inhibited rotenone-induced apoptosis of PC12 cells (Li et al., 2012). These findings strongly suggest that baicalein may have therapeutic value for the prevention of PD-associated neurodegeneration. A recent study demonstrated caspase-1 activation in the MPTP-induced mouse PD model and inhibition of this activation by baicalein (Hung et al., 2016), strongly implicating the inflammasome pathway in experimental PD pathogenesis. However, the specific effects of baicalein on the canonical caspase-1-associated inflammasome pathway in MPTP-induced PD have not been directly examined. The aim of the present study was to evaluate the protective effects of baicalein against MPTP-induced motor dysfunction and SNc dopaminergic neurodegeneration and to elucidate the underlying mechanisms.
Materials and Methods
Animals and Reagents
Male C57BL/6J mice (2 months old, 18–22 g) were purchased from Xipuer-bikai Company (Shanghai, China). Mice were housed in conventional cages at 22°C ± 2°C and 55% ± 5% humidity under a 12-hour-light/-dark cycle with ad libitum access to food and water for at least 1 week before the experiment. All experiments were conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and all animal procedures were approved by the Ethical Review Committee for Laboratory Animal Welfare of Nanjing Medical University. Mice were randomly divided into 5 groups (n = 25/group): control group, MPTP alone group, and 3 MPTP plus baicalein treatment groups. Control group mice were administered phosphate buffered saline (PBS) (vehicle) for 9 consecutive days by i.p. injection, while mice of the MPTP alone and MPTP + baicalein groups were injected i.p. with 30 mg/kg/d MPTP for 5 consecutive days to induce PD (Zhang et al., 2017). Mice in the 3 MPTP + baicalein groups also received intragastric baicalein at 140, 280, or 560 mg/kg/d 1 hour before MPTP treatment for 5 days and continued administration for an additional 4 days (total of 9 days), as previously described (Mu et al., 2011). All mice were killed after behavioral testing conducted the day following final administration of baicalein or PBS for immunohistochemistry, flow cytometry, western blotting, and quantitative real-time PCR, as described below.
Baicalein (98% in purity) was purchased from Cheng Desite Bio-technology. MPTP-HCL (M0896), anti-glial fibrillary acidic protein (GFAP) (G3893), and anti-β-Actin (A1978) were purchased from Sigma-Aldrich. Anti-NLRP3 (AG-20B-0014) and anti-caspase-1 (AG-20B-0042) were purchased from AdipoGen. Anti-IL-1β (AF-401-NA) was purchased from R&D Systems. Anti-GSDMD (ab209845), anti-Ki67 (ab16667), and anti-APOE (ab1906) were purchased from Abcam. Anti-TH (AB152) was purchased from Merck-Millipore. FAM fluorescent-labeled inhibitors of caspases probe assay (FLICA) Caspase-1 kit (ICT098) and anti-CD11b (MCA275G) were purchased from Bio-Rad. Anti-ionized calcium binding adapter molecule 1 (IBA1) (019-19741) was purchased from Wako.
Behavioral Assessments
Open Field Test
On the day after final administration of baicalein or equivalent (i.e., 10 days after the first saline or MPTP injection and before all other behavioral tests), open field tests were conducted as described (Zhang et al., 2017). The apparatus was made of white plastic (50 × 50 cm), and the area was divided into a central region and a peripheral region, each comprising 50% of the total open field area. Mice were transferred to the experimental room 1 hour before testing for adaptation and then placed into the open field chamber under evenly distributed low light for 5 minutes. The total distance travelled, center area duration time, and rearing times were recorded automatically during test.
Pole Test
A pole test was performed as previously described (Zhang et al., 2017). Briefly, a 0.5-m long pole was placed vertically into the home cage, and mice were placed at the top. The time required to descend back into the cage was recorded. On the day before the test, each mouse received 5 consecutive training trials, each separated by 30-minute rest periods. On the test day, 3 test trials were conducted with 3-minute intervals, and the average time to descend back into the cage was recorded.
Accelerating Rotarod Test
The accelerating rotarod test was conducted as previously described (Zhang et al., 2017). Briefly, mice were placed on the accelerating rotarod, which accelerates from 5 to 30 rpm in 300 seconds and the latency to fall off was recorded. Mice were pre-trained over 3 consecutive days with 5 trials per day at a 30-minute intervals. On the test day, mice were evaluated on 5 trials conducted with 3-minute intervals.
Immunohistochemistry
Mice were sacrificed after behavioral tests and perfused with 4% paraformaldehyde. Brains were extracted, dehydrated in 30% sucrose solution, embedded in optimal cutting temperature compound, and continuously sliced at 25-µm thickness. Slices were blocked, incubated with primary antibodies, and then treated with fluorescent- or horseradish peroxidase-labeled secondary antibodies. The number of TH-positive cells in the SNc was determined by stereological methods, as described (Hu et al., 2019).
Western-Blot Analysis
Mouse brain substantia nigra (SN) specimens were quickly collected on ice and homogenized 1:10 (w/v) in homogenization buffer (containing 20 mM Tris-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM b-glycerol phosphate, 1 mM sodium vanadate, 1% Triton X-100, and 1 mg/mL leupeptin; pH 7.5) supplemented with protease inhibitor cocktail. After centrifugation at 12 000 g for 10 minutes, protein lysates were quantified by bicinchoninic acid method (Bio-Rad). Then the protein specimens were subjected to western blot with different antibodies.
Real-Time PCR Quantification
RNA extraction, cDNA synthesis, and the PCR reactions were performed as previously described (Rui et al., 2016). Briefly, total RNA was extracted from the SN tissues by using TRIzol regent (Invitrogen, CA) and subjected to cDNA synthesis. Then quantitative real-time PCR was performed by SYBR Premix Ex Taq (Takara). Primers were used as follows: for Il-1b, 5´-TCA TTG TGG CTG TGG AGA AG-3´ and 5´-AGG CCA CAG GTA TTT TGT CG-3´; for Il-18, 5´-CCG CCT CAA ACC TTC CAA AT-3´ and 5´-TGT GTT TCT TTT CTG GGT GCC-3´; for Tnf-a, 5´-CAT CTT CTC AAA ATT CGA GTG ACAA-3´ and 5´-TGG GAG TAG ACA AGG TAC AAC CC-3´; for Il-6, 5´-ATC CAG TTG CCT TCT TGG GAC TGA-3´and 5´-TAA GCC TCC GAC TTG TGA AGT GGT-3´; for Inos, 5´-GGG AAT CTT GGA GCG AGT TG-3´ and 5´-TGT CCA GGA AGT AGG TGA GGG-3´; for Hprt, 5´-GTC CCA GCG TCG TGA TTA GC-3´ and 5´-TGG CCT CCC ATC TCC TTCA-3´.
Enzyme-Linked Immunosorbent Assay
Brain SN tissue samples were collected from mice to determinate the protein level of inflammatory factors. The levels of IL-1β, IL-6, IL-18, Inos, and TNF-α were measured by enzyme-linked immunosorbent assay kits (purchased from Shanghai Excell Co.) according to the manufacturer’s protocol. Microplate reader (Antobio, Zhengzhou, China) was used to test the absorbance of all inflammatory cytokines.
Cell Culture and Baicalein Treatment
Mixed glial cells were prepared from brain of postnatal day (P)1–3 mouse pups (n = 25). Briefly, whole brains were isolated and meninges removed. Tissue was minced and digested in 0.25% trypsin for 5 minutes at 37°C under a 5% CO2 atmosphere. The cell suspension was briefly centrifuged, washed, and filtered through a cell strainer (100 μM). Cells were transferred to T-75 flasks and cultured for 15 days in Dulbecco’s modified Eagles medium/F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C under a 5% CO2 atmosphere with the medium replacement every 3 days. To evaluate the effect of baicalein on glial reactivity in vitro, cells were treated with MPTP (40 μM, 15 hours) or MPP+ (40 μM, 15 hours) followed by ATP (2.5 mM, 1 hour) as previously reported (Lee et al., 2019) in the presence of vehicle (control) or baicalein (10, 20, 40 μM), as described in a previous report (Zhang et al., 2010).
LDH Analysis
Lactate dehydrogenase (LDH) level in supernatant of glial cells was tested using LDH cytotoxicity detection kit (Jiancheng Bio, Nanjing, China) according to the manufacturer’s instructions. The absorbance was detected at 440 nm using a microplate reader (Antobio, Zhengzhou, China).
Flow Cytometry
Single-cell suspensions were prepared from brain tissues, as described previously (Li et al., 2019). In brief, brain tissues were excised from mice, and digested with collagenase IV (0.5 mg/mL; Sigma-Aldrich) and DNase I (10 U/mL; Roche) in RPMI 1640 under agitation (200 rpm) at 37°C for 60 minutes. Cell were then filtered through a 100-μm sieve, centrifuged over a Percoll density gradient (GE Healthcare), separated by collecting the interface fractions between 37% and 70% Percoll. The cells were then suspended in PBS containing 2% (wt/vol) FBS. After intensive washing, the FLICA was conducted to estimate caspase-dependent cell death. Briefly, the cell suspension was incubated with caspase-1-FLICA probe for 1 hour at 37°C under 5% CO2 in the dark then washed 3 times with PBS containing 2% FBS. Cells were stained with propidium iodide (PI) for 30 minutes in the dark and subjected to flow cytometry. For cell surface CD11b staining, the cells were blocked with Fc blocking antibodies and incubated with CD11b antibody for 30 minutes on ice. Staining for nuclear factor Ki67 was performed using the eBioscience Foxp3/Transcription Factor Staining Buffer Set. Flow cytometry was performed using the FACSCalibur (BD Biosciences), and data were analyzed by FlowJo 7.6.1 software.
Statistical Analysis
Results are expressed as the means ± SEM. Accelerating rotarod test results were compared among treatment groups by 2-way ANOVA followed by post hoc Dunnett’s t tests for pair-wise comparisons. Results of other experiments were compared among groups by 1-way ANOVA followed by post-hoc Dunnett’s t tests. All statistical analyses in this study were performed using the GraphPad Prism 6.0 software (Graph Pad Software Inc., San Diego, CA). A P < .05 (2 tailed) was considered significant for all tests. Significance levels in figures are indicated as *P < .05, **P < .01, and ***P < .001.
Results
Baicalein Ameliorated MPTP-Induced Motor Dysfunction
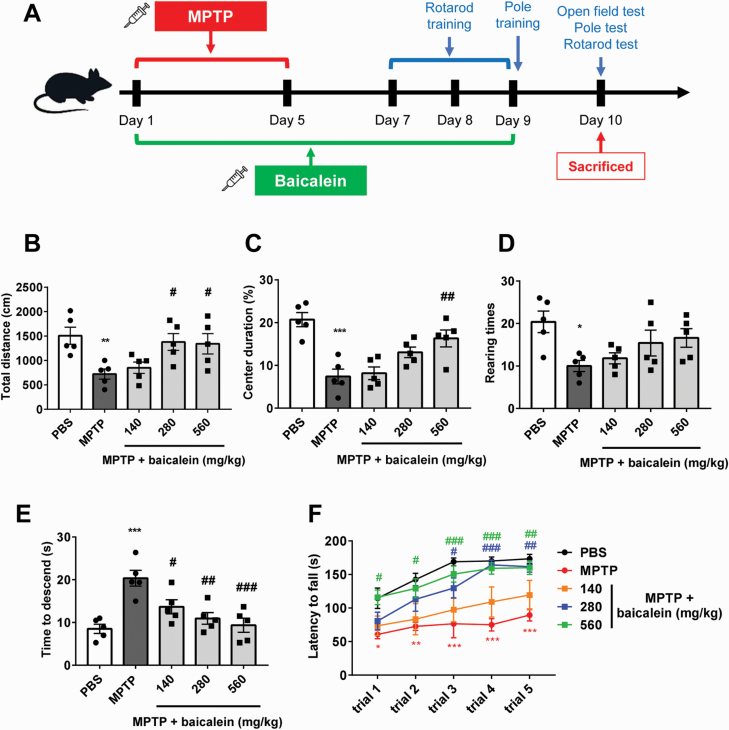
Motor dysfunction is the main pathological character of PD patients and MPTP-induced model PD in mice (Blanchet et al., 1997). To examine the protective effects of baicalein in MPTP-treated mice, we first measured motor function in the open field, accelerating rotarod, and pole tests on the day after the final baicalein or PBS injection (Figure 1A). Open field test was performed firstly before other behavioral tests. Total travelled distance and rearing times during 5 minutes of mice treated with MPTP was significantly reduced compared with control mice, which indicated the defect in autonomous activity (P < .01, Figure 1B; P < .05, Figure 1D). Meanwhile, mice with stress and anxiety conditions, including MPTP treatment, tend to spend less time exploring the center area of the open field (Miyata et al., 2019; Su et al., 2019), as we also observed in the present study (P < .01; Figure 1C). After baicalein administration for 9 days, the activities and the center area duration of mice in open field increased, especially the baicalein 280-mg/kg and 560-mg/kg groups (F4, 20 = 4.763, 280 mg/kg and 560 mg/kg, P < .05, Figure 1B; F4, 20 = 11.68, 560 mg/kg, P < .01, Figure 1C; F4, 20 = 3.516, P > .05, Figure 1D). In accord with pervasive motor dysfunction, MPTP-treated mice required more time to descend the pole and fell off the rotarod in less time (at lower speeds) than controls (P < .01, Figure 1E; trial 1, P < .05, trials 2–5, P < .01, Figure 1F). Baicalein treatment dose-dependently reduced pole descent time (F4, 20 = 9.608, 140 mg/kg, P < .05, 280 mg/kg and 560 mg/kg, P < .01; Figure 1E). Further, 280 mg/kg baicalein significantly increased rotarod time starting on trial 3, while 560 mg/kg increased rotarod time starting on trial 1 compared with the MPTP alone group (F4, 20 = 8.426, 280 mg/kg, trial 3, P < .05, trial 4–5, P < .01, 560 mg/kg, trial 1–2, P < .05, trial 3–5, P < .01; Figure 1F). Collectively, these findings indicated that baicalein can effectively reverse the deleterious effects of MPTP on motor function.
Figure 1.
Baicalein improved N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced behavior disorder. (A) The scheme of experimental procedure. (B-D) Open field analysis of indicated mice. Total travelled distance (B), percentages of time spend in the center area (C), and rearing times (D) in 5 minutes during test were recorded and analyzed. (E) Times required for mice to descend from the pole were recorded and analyzed. (F) Latency to fall during test on accelerated rotarod for 5 consecutive trials was recorded and analyzed. *P < .05, **P < .01, ***P < .001 vs control group. #P < .05, ##P < .01 vs MPTP group. Data are expressed as means ± SEM (n = 5/group).
Baicalein Reduced Dopaminergic Neuron Loss and Glial Cell Activation in MPTP Mice
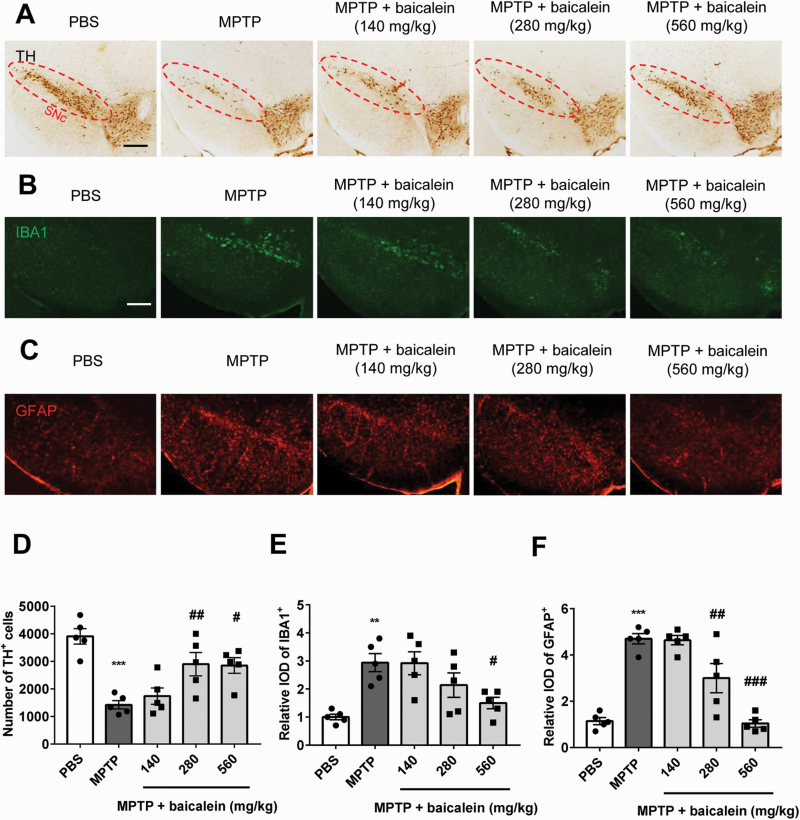
We next examined the neuroprotective efficacy of baicalein by comparing nigral dopaminergic neuron numbers among groups by TH immunostaining. MPTP-treated mice exhibited severe loss of TH-positive neurons (nearly 80%‒90%) compared with controls, and baicalein treatment partially rescued this population (F4, 20 = 11.11, 280 mg/kg, P < .01 and 560 mg/kg, P < .05; Figure 2A, D). This finding suggested that the motor improvements demonstrated in Figure 1 resulted from protection of basal ganglion circuits. Previous studies have shown that activation of microglia and astrocytes is a central process in neuroinflammation, which is in turn a major contributor to the pathogenesis of neurodegenerative disease (Yasuda et al., 2008). Consistent with this notion, baicalein reduced microglial and astrocyte activation in response to MPTP, especially at the highest dose of 560 mg/kg (F4, 20 = 7.207, 560 mg/kg, P < .05, Figure 2B, E; F4, 20 = 29.78, 280 mg/kg and 560mg/kg, P < .01, Figure 2C, F).
Figure 2.
The protective effect of baicalein on dopaminergic neurons and glial cells. (A) Immunohistochemical staining of tyrosine hydroxylase (TH) neurons in brain substantia nigra compact (SNc) from indicated mice. Scale bar = 100 μm. (B) Immunofluorescence analysis of microglia (ionized calcium binding adapter molecule 1 [IBA1], green) and astrocytes (glial fibrillary acidic protein (GFAP), red) in brain SNc from indicated mice. Scale bar = 100 μm. (C) Quantified cell numbers of TH+ neurons and relative IOD values of IBA1 and GFAP. **P < .01, ***P < .001 vs control group. #P < .05, ##P < .01, ###P < .001 vs MPTP group. Data are expressed as means ± SEM (n = 5/group).
Baicalein Attenuated Neuroinflammation by Suppressing Inflammasome-Dependent Pyroptosis in MPTP Mice
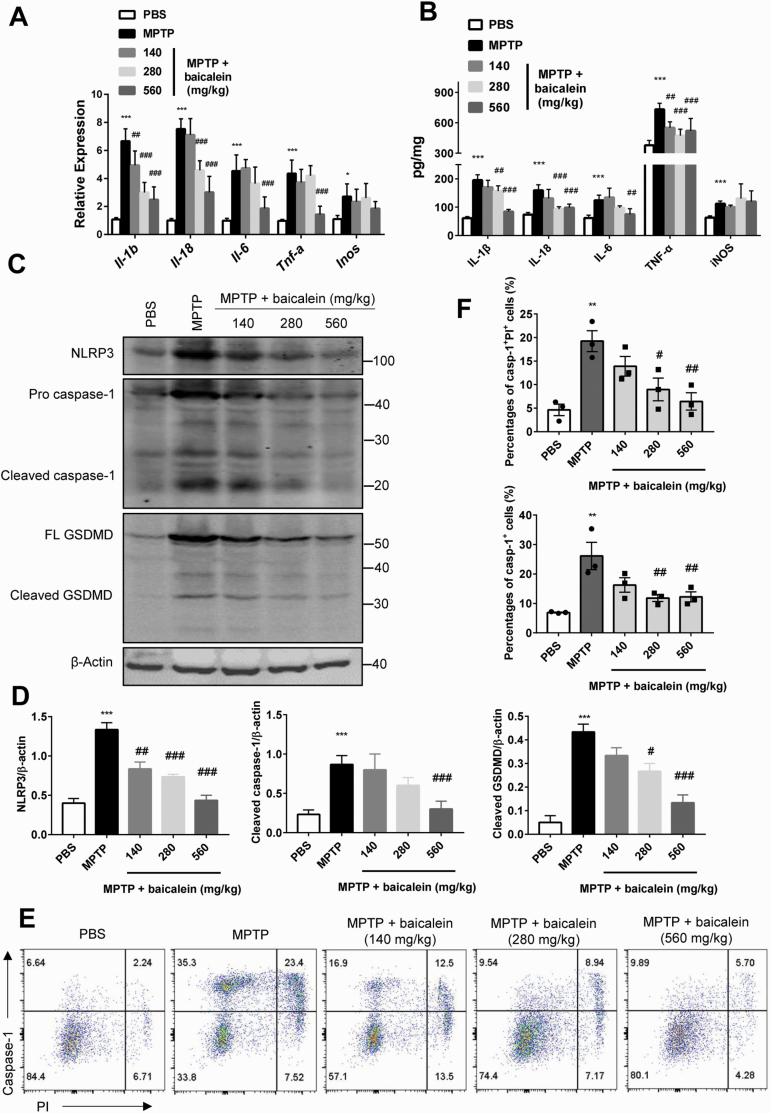
To examine the mechanisms underlying the suppressive effect of baicalein on neuroinflammation, we first compared SN expression levels of proinflammatory cytokines among groups at the mRNA and protein levels. Administration of MPTP enhanced expression levels of IL-1β, IL-18, IL-6, TNF-α, and iNOS, and these increases were reversed dose-dependently by baicalein (protein levels: IL-1β, F4, 20 = 37.74, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P < .01; IL-18, F4, 20 =17.61, 280 mg/kg and 560 mg/kg, P < .01; IL-6, F4, 20 = 12.65, 560 mg/kg, P < .01; TNF-α,F4, 20 = 15.19, 560 mg/kg, P < .01; iNOS, F4, 20 = 4.016, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P > .05; Figure 3A; mRNA levels: IL-1β, F4, 20 = 37.74, 280 mg/kg and 560 mg/kg, P < .01; IL-18, F4, 20 = 51.71, 280 mg/kg and 560 mg/kg, P < .01; IL-6, F4, 20 = 18.35, 560 mg/kg, P < .01; TNF-α,αF4, 20 = 24.2, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P < .01; iNOS, F4, 20 = 3.529, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P > .05; Figure 3B). Activation of the NLRP3 inflammasome by upstream pattern recognition receptors led to increased caspase-1 cleavage and release of proinflammatory cytokines, including IL-1β, processes that drive neuroinflammation in PD (Heneka et al., 2018). We then investigated whether baicalein-mediated attenuation of neuroinflammation is associated with the NLRP3 inflammasome pathway by western blotting and FACS. Western blotting demonstrated significantly increased expression of NLRP3, pro-caspase-1, and cleaved-caspase-1 in brain SN tissue from MPTP-treated mice compared with control mice, suggesting activation of the NLRP3 inflammasome pathway (NLRP3, P < .01, cleaved caspase-1, P < .01; Figure 3C, D), and baicalein dose-dependently reduced NLRP3 activation (NLRP3, F4, 10 = 29.16, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P < .01; cleaved caspase-1, F4, 10 = 16.02, 560 mg/kg, P < .01; Figure 3C, D). Notably, the downstream pyroptosis effector molecule GSDMD was also activated by MPTP (P < .01; Figure 3C, D), a response reversed by baicalein (F4, 10 = 22.32, 280 mg/kg, P < .05 and 560 mg/kg, P < .01; Figure 3C, D). These results were also confirmed by FACS analysis. The increasing number of caspase-1+ PI+ cells, even caspase-1+ cells, from brain tissue induced by MPTP was lessened after the administration of baicalein in different doses (caspase-1+ PI+ cells, F4, 10 = 8.808, 280 mg/kg, P < .05 and 560 mg/kg, P < .01, Figure 3D; caspase-1+ cells, F4, 10 = 8.164, 280 mg/kg and 560 mg/kg, P < .01, Figure 3E, F). Collectively, we demonstrated that baicalein can reverse MPTP-induced increases in the expression and release of pro-inflammatory cytokines and as well as the activation of the NLRP3 inflammasome.
Figure 3.
Baicalein inhibited inflammasome dependent pyroptosis induced by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). (A) Quantitative PCR analysis of mRNA relative expression of proinflammatory cytokines in brain substantia nigra (SN) tissue from indicated mice (n = 5/group). Data were normalized to a reference gene, Hprt. (B) ELISA analysis of protein expression of proinflammatory cytokines in brain SN tissue from indicated mice (n = 5/group). (C) Immune blotting analysis of NLRP3, caspase-1, and GSDMD (full-length and cleaved form) in the brain SN tissue from indicated mice (n = 3/group). Data are shown as representative plots (C) and quantified immunoblotting bands (D). (E–F) Flow-cytometric analysis of caspase-1+ PI+ cells from brain tissue (n = 3/group). Data are shown as representative plots (E) and quantified percentages (F). *P < .05, **P < .01, ***P < .001 vs control group. #P < .05, ##P < .01, ###P < .001 vs MPTP group. Data are expressed as means ± SEM.
Baicalein Prevented MPTP-Induced Pyroptosis in Mixed Glial Cells
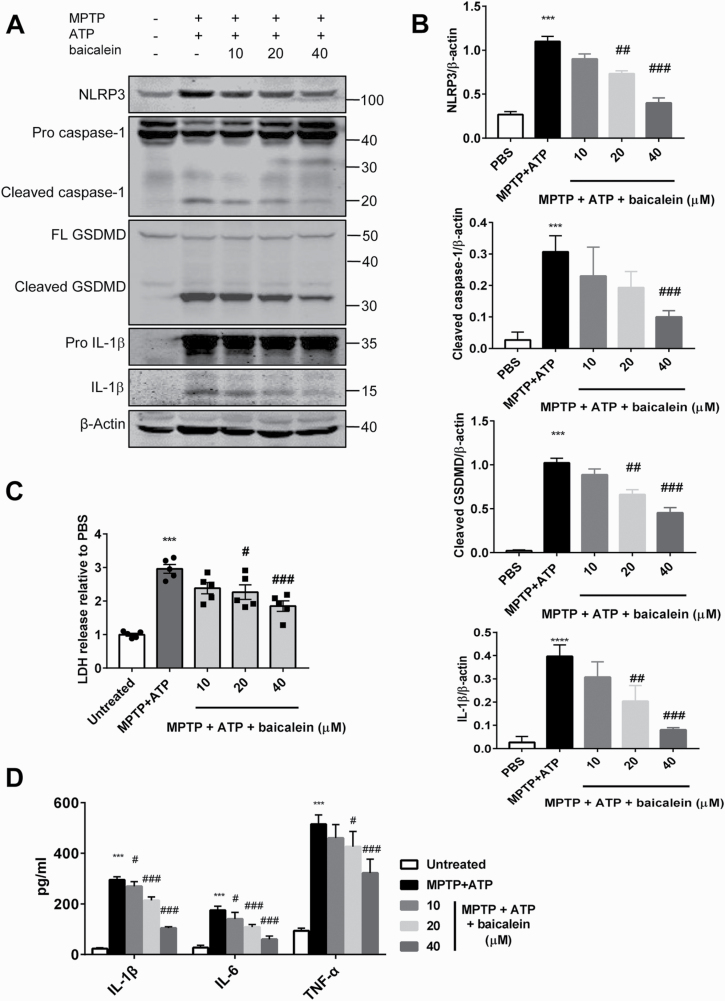
To further confirm the suppressive effect of baicalein on canonical inflammasome pathway observed in vivo, additional analysis in vitro was performed. Primary mixed glial cells were first primed with MPTP in the presence or absence of baicalein, followed by exposure to ATP. Western blotting revealed that priming with MPTP and ATP treatment markedly increased NLRP3 and pro-IL-1β expression levels and promoted the cleavage (activation) of caspase-1, IL-1β,and GSDMD(NLRP3, P < .01; cleaved caspase-1, P < .01; IL-1β, P < .01; GSDMD, P < .01; Figure 4A, B). Further, MPTP/ATP treatment increased LDH activity in the culture supernatant, indicating plasma membrane leakage consistent with pyroptosis (P < .01; Figure 4C). Consistent with the findings in vivo, treatment with baicalein reduced the protein level of NLRP3, inhibited the activation of caspase-1 and IL-1β, and further declined GSDMD cleavage in mixed glial cells, leading to less LDH leakage (NLRP3, F4, 10 = 48.77, 20 μM and 40 μM, P < .01; cleaved caspase-1, F4, 10 = 12.31, 40 μM, P < .01; cleaved GSDMD, F4, 10 = 55.85, 20 μM and 40 μM, P < .01; IL-1β, F4, 10 = 28.96, 20 μM and 40 μM, P < .01, Figure 4A, B; LDH, F4, 20 = 22.05, 20 μM, P < .05 and 40 μM, P < .01, Figure 4C). To exclude the impact of baicalein on the conversion of MPTP metabolites due to the anti-oxidation effects of baicalein, we also repeated the in vitro study with MPP+ instead of MPTP and obtained consistent results (NLRP3, F4, 10 = 39.1, 20 μM and 40 μM, P < .01; cleaved caspase-1, F4, 10 = 9.937, 20 μM, P < .05 and 40 μM, P < .01; cleaved GSDMD, F4, 10 = 74.75, 20 μM, P < .05 and 40 μM, P < .01; IL-1β, F4, 10 = 21.63, 40 μM, P < .05; supplementary Figure 1A, B). Furthermore, baicalein also inhibited the marked elevation of IL-1β, IL-6, and TNF-α protein induced by MPTP (IL-1β, F4, 20 = 444.1, 10 μM, P < .05, 20 μM and 40 μM, P < .01; IL-6, F4, 20 = 68.2, 10 μM, P < .05, 20 μM and 40 μM, P < .01; TNF-α, F4, 20 = 63.23, 20 μM, P < .05 and 40 μM, P < .01; Figure 4D). These results suggest that baicalein can prevent MPTP-induced inflammasome activation and pyroptosis.
Figure 4.
Baicalein suppressed N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced pyroptosis in vitro. (A–B) Immune blotting analysis of NLRP3, caspase-1, and GSDMD (full-length and cleaved forms), pro-IL-1β, and matured IL-1β from the mixed glial cells primed with PBS, or MPTP (40 μM, 15 hours) followed by ATP (2.5 mM, 1 hour), or MPTP (40 μM, 5 hours) + baicalein (10 μM, 20 μM, 40 μM; 15 hours) followed by ATP (2.5 mM, 1 hour) (n = 3/group). Data are shown as representative plots (A) and quantified immunoblotting bands (B). Culture supernatants and cellular lysates mixture were immunoblotted with the indicated antibodies. (C) Quantification of LDH in the culture supernatants of mixed glial cells (n = 5/group). (D) ELISA analysis of protein level of proinflammatory cytokines in the culture supernatants of mixed glial cells (n = 5/group). ***P < .001 vs control group. #P < .05, ##P < .01, ###P < .001 vs MPTP group. Data are expressed as means ± SEM.
Baicalein Inhibited Activation and Proliferation of Proinflammatory Microglia
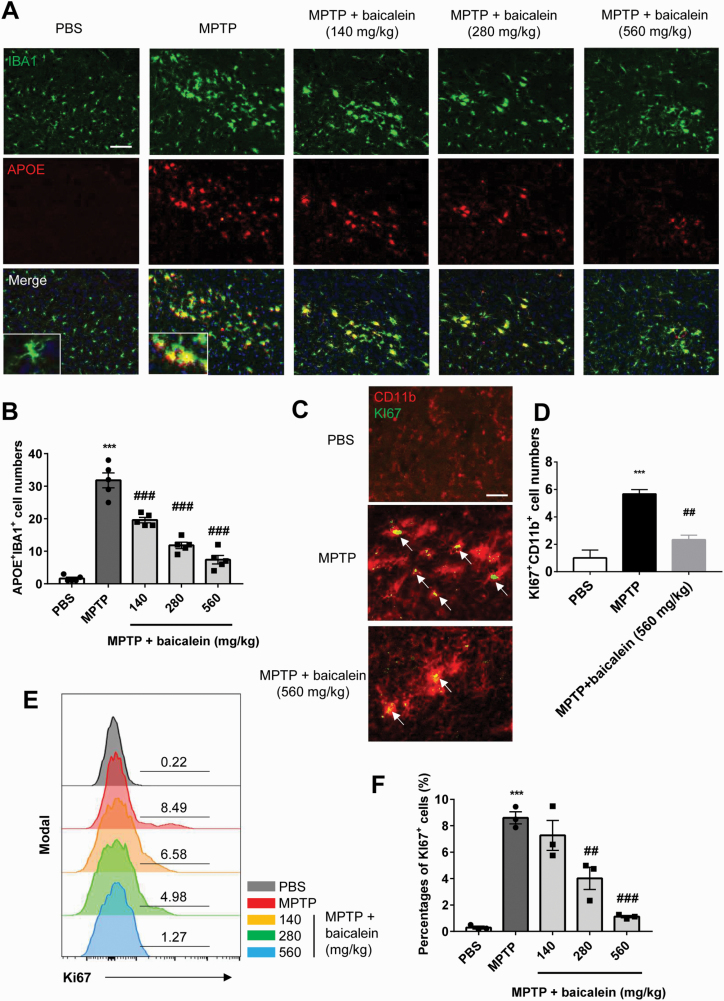
Recent studies have identified several functionally distinct microglia. Disease-associated microglia (MGnD) is characterized by its proinflammatory and pathogenesis effect, while homeostatic-associated microglia (M0) could maintain homeostasis and immune monitoring (Krasemann et al., 2017). Based on our previous results, we speculated that microglia activated by MPTP are proinflammatory. To verify our hypothesis, we performed immunofluorescence colocalization analysis on brain SNc of mice from all groups. As expected, the MGnD phenotype of activated microglia was confirmed by the colocalization of IBA1 and APOE (P < .01; Figure 5A, B). Baicalein administration reduced these activated proinflammatory microglia (F4, 20 = 79.3, 140 mg/kg, 280 mg/kg, and 560 mg/kg, P < .01; Figure 5A, B). We next examined the proliferation of microglia by Ki67 immunofluorescence and FACS analysis. Compared with control mice, the SNc of MPTP-treatment mice exhibited more numerous cells with an activated morphology coexpressing Ki67 and the microglial marker CD11b (P < .01; Figure 5C, D), implying that proinflammatory microglia proliferate in response to MPTP treatment. Moreover, the percentage of Ki67+ cells gated with CD11b+ cells was increased in MPTP-treated mouse brain (P < .01; Figure 5E, F), while baicalein dose-dependently reversed this proliferative response, especially at 560 mg/kg (F2, 6 = 31.2, 560 mg/kg, P < .01, Figure 5C, D; F4, 10 = 30.22, 280 mg/kg and 560 mg/kg, P < .01, Figure 5E, F). These observations revealed the inhibitive effect of baicalein on activation and proliferation of proinflammatory microglia.
Figure 5.
The inhibitory effect of baicalein on proinflammatory microglia. (A–B) Immunofluorescence analysis of ionized calcium binding adapter molecule 1 (green) and APOE (red) cells in brain substantia nigra compact (SNc) from indicated mice. Data are shown as representative images (A) and quantified cell numbers (B) (n = 5/group). Scale bar, 50 μm. (C-D) Immunofluorescence staining of CD11b+ (red) and Ki67+ (green) cells in brain SNc from indicated mice. Data are shown as representative images (C) and quantified cell numbers (D) (n = 3/group). Scale bar = 20 μm. (E–F) Flow-cytometric analysis of Ki67+ cells gated from CD11b+ cells in brain tissue. Data are shown as representative plots (E) and quantified percentages (F) (n = 3/group). ***P < .001 vs control group. ##P < .01, ###P < .001 vs N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine group. Data are expressed as means ± SEM.
Discussion
Recent studies have demonstrated that baicalein protects neurons in animal models of Alzheimer’s disease (AD) and PD by inhibiting neuroinflammation (Li et al., 2017). The current work suggested the baicalein suppresses MPTP-induced nigral dopaminergic neuron death, glial activation, and motor dysfunction by inhibiting the canonical inflammasome pathway NLRP3/caspase-1/GSDMD. Specifically, daily administration of baicalein prior to MPTP significantly improved motor function in pole and rotarod tests, increased autonomous motor activity in the open field, and prevented MPTP-induced dopaminergic neuron loss and glial cell activation compared with MPTP alone. Notably, baicalein also inhibited NLRP3 activation and suppressed the cleavage of caspase-1 and GSDMD in the SNc as well as in mixed cultured glial cells. Finally, baicalein also inhibited activation and proliferation of proinflammatory microglia. Collectively, these findings indicated that baicalein can attenuate NLRP3-induced neuroinflammation in a MPTP-induced PD model.
Neuroinflammation driven by inflammasomes is an important component of neurodegenerative disease etiopathogenesis. The NLRP3 complex in particular is the most studied inflammasome in AD and PD. Knockout of NLRP3 ameliorates amyloid plaque pathology and neuroinflammatory process during the development of AD (Venegas et al., 2017; Ising and Heneka, 2018). In PD patients, the NLRP3 inflammasome pathway is activated by oxidative stress and insoluble α-synuclein aggregates (Codolo et al., 2013; Walsh et al., 2014; Zhou et al., 2016). Moreover, baicalein reduces MPTP-induced caspase-1 activation and IL-1β elevation in the mouse SN following MPTP treatment (Hung et al., 2016). These latter findings were confirmed here as well, as we demonstrated that baicalein has direct inhibitory effects on the NLRP3 inflammasome. In 2015, 2 research groups independently identified GSDMD as a key substrate of the inflammasome proteolytic pathway and the primary driver of NLRP3-dependent pyroptosis (Shi et al., 2015; Kayagaki et al., 2015). A recent study also suggested that GSDMD-induced pyroptosis promotes neuroinflammation in multiple sclerosis by enhancing the release of IL-1β and IL-18 (Li et al., 2019). We detected MPTP-induced cleavage and activation of GSDMD concomitant with NLRP3/caspase-1 activity both in vivo and in vitro and demonstrated that this response can be suppressed by baicalein.
In 1988, McGeer and co-workers described microglial activation in the SNc of the postmortem PD brain, thus providing the first evidence for neuroinflammatory processes in PD pathogenesis. These cells are identified as immune cells by immunoreactivity antigen for antigens such as HLA-DP, HLA-DQ, HLA-DR (CR3/43), and ferritin (McGeer et al., 1988; Mirza et al., 1999). As brain-resident innate-immune cells, microglia of various phenotypes regulate CNS homeostasis and disease progression according to the local microenvironment (Tang and Le, 2016; Butovsky and Weiner, 2018). A recent study identified 2 distinct cell types according to differences in function and gene expression profile: the homeostatic M0 and disease-associated MGnD. These MGnD microglia are activated by TREM2, leading to the activation of APOE and subsequently suppression of homeostatic genes (Krasemann et al., 2017). APOE is a major mediator of neuroinflammation as knockout alleviated neurodegeneration in experimental autoimmune encephalomyelitis model mice (Shin et al., 2014). Moreover, microglia isolated from ALS model mice at the peak stage of disease showed the highest expression of APOE (Butovsky et al., 2012). Neurotoxic Aβ-plaque–associated microglia also exhibits a highly APOE-enriched MGnD phenotype in AD patients and AD model mice, suggesting a major role in AD progression (Huang et al., 2017). In our study, we observed more numerous activated microglia with high APOE expression in MPTP-treated mice, implying expansion of the MGnD phonotype induced. In addition, we found elevated proliferation of activated microglia in PD mice and observed that baicalein treatment suppressed expression of the proliferation marker Ki67.
In conclusion, our results provide evidence that the PD-like pathology induced by MPTP is mediated in part by the neuroinflammatory NLRP3/caspase-1/GSDMD pathway and that suppression of this pathway by baicalein is neuroprotective. Targeting this pathway may be an effective strategy for PD treatment. However, the precise cellular and molecular details of this anti-inflammatory effect in PD model mice require further exploration.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Nanjing Medical University Foundation (no. NMUB2018203).
Statement of Interest
None.
References
- Blanchet PJ, Papa SM, Metman LV, Mouradian MM, Chase TN (1997) Modulation of levodopa-induced motor response complications by NMDA antagonists in Parkinson’s disease. Neurosci Biobehav Rev 21:447–453. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL (2012) Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122:3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19:622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SF, Hsu CW, Huang WH, Wang JY (2008) Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. Br J Pharmacol 155:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M (2013) Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. Plos One 8:e55375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs RJ, Charlett A, Purkiss AG, Dobbs SM, Weller C, Peterson DW (1999) Association of circulating TNF-alpha and IL-6 with ageing and parkinsonism. Acta Neurol Scand 100:34–41. [DOI] [PubMed] [Google Scholar]
- Fan Z, Pan YT, Zhang ZY, Yang H, Yu SY, Zheng Y, Ma JH, Wang XM (2020) Systemic activation of NLRP3 inflammasome and plasma α-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J Neuroinflammation 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. [DOI] [PubMed] [Google Scholar]
- Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS (2007) Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, Südhof TC (2017) ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168:427–441.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZL, Sun T, Lu M, Ding JH, Du RH, Hu G (2019) Kir6.1/K-ATP channel on astrocytes protects against dopaminergic neurodegeneration in the MPTP mouse model of Parkinson’s disease via promoting mitophagy. Brain Behav Immun 81:509–522. [DOI] [PubMed] [Google Scholar]
- Hung KC, Huang HJ, Wang YT, Lin AM (2016) Baicalein attenuates α-synuclein aggregation, inflammasome activation and autophagy in the MPP+-treated nigrostriatal dopaminergic system in vivo. J Ethnopharmacol 194:522–529. [DOI] [PubMed] [Google Scholar]
- Ising C, Heneka MT (2018) Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis 9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. [DOI] [PubMed] [Google Scholar]
- Krasemann S, et al. (2017) The TREM2-APOE Pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47:566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashia P, et al. (2019) Author correction: blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun 10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Park HR, Ji ST, Lee Y, Lee J (2014) Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-κB, ERK, and JNK. J Neurosci Res 92:130–139. [DOI] [PubMed] [Google Scholar]
- Lee E, Hwang I, Park S, Hong S, Hwang B, Cho Y, Son J, Yu JW (2019) MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ 26:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWitt PA, Fahn S (2016) Levodopa therapy for Parkinson disease: a look backward and forward. Neurology 86:S3–12. [DOI] [PubMed] [Google Scholar]
- Li S, Wu Y, Yang D, Wu C, Ma C, Liu X, Moynagh PN, Wang B, Hu G, Yang S (2019) Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J Exp Med 216:2562–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XX, He GR, Mu X, Xu B, Tian S, Yu X, Meng FR, Xuan ZH, Du GH (2012) Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur J Pharmacol 674:227–233. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao J, Hölscher C (2017) Therapeutic potential of baicalein in Alzheimer’s disease and Parkinson’s disease. CNS Drugs 31:639–652. [DOI] [PubMed] [Google Scholar]
- Lin CC, Shieh DE (1996) The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med 24:31–36. [DOI] [PubMed] [Google Scholar]
- Lofrumento DD, Saponaro C, Cianciulli A, De Nuccio F, Mitolo V, Nicolardi G, Panaro MA (2011) MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation 18:79–88. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291. [DOI] [PubMed] [Google Scholar]
- Mirza B, Hadberg H, Thomsen P, Moos T (1999) The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 95:425–432. [DOI] [PubMed] [Google Scholar]
- Miyata S, Kumagaya R, Kakizaki T, Fujihara K, Wakamatsu K, Yanagawa Y (2019) Loss of glutamate decarboxylase 67 in somatostatin-expressing neurons leads to anxiety-like behavior and alteration in the Akt/GSK3β signaling pathway. Front Behav Neurosci 13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180:147–150. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211:13–16. [DOI] [PubMed] [Google Scholar]
- Mu X, He GR, Yuan X, Li XX, Du GH (2011) Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol Biochem Behav 98:286–291. [DOI] [PubMed] [Google Scholar]
- Orning P, Lien E, Fitzgerald KA (2019) Gasdermins and their role in immunity and inflammation. J Exp Med 216:2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:1–21. [DOI] [PubMed] [Google Scholar]
- Rui W, Zou Y, Lee J, Nambiar SM, Lin J, Zhang L, Yang Y, Dai G (2016) Nuclear factor Erythroid 2-related factor 2 deficiency results in amplification of the liver fat-lowering effect of estrogen. J Pharmacol Exp Ther 358:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. [DOI] [PubMed] [Google Scholar]
- Shin S, Walz KA, Archambault AS, Sim J, Bollman BP, Koenigsknecht-Talboo J, Cross AH, Holtzman DM, Wu GF (2014) Apolipoprotein E mediation of neuro-inflammation in a murine model of multiple sclerosis. J Neuroimmunol 271:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Deng MF, Xiong W, Xie AJ, Guo J, Liang ZH, Hu B, Chen JG, Zhu X, Man HY, Lu Y, Liu D, Tang B, Zhu LQ (2019) MicroRNA-26a/Death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson’s disease. Biol Psychiatry 85:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194. [DOI] [PubMed] [Google Scholar]
- Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D, Vieira-Saecker A, Schwartz S, Santarelli F, Kummer MP, Griep A, Gelpi E, Beilharz M, Riedel D, Golenbock DT, Geyer M, Walter J, Latz E, Heneka MT (2017) Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552:355–361. [DOI] [PubMed] [Google Scholar]
- Walsh JG, Muruve DA, Power C (2014) Inflammasomes in the CNS. Nat Rev Neurosci 15:84–97. [DOI] [PubMed] [Google Scholar]
- Wang X, Pan J, Liu H, Zhang M, Liu D, Lu L, Tian J, Liu M, Jin T, An F (2019) AIM2 gene silencing attenuates diabetic cardiomyopathy in type 2 diabetic rat model. Life Sci 221:249–258. [DOI] [PubMed] [Google Scholar]
- Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, Wu J, Wang Z, Liu Y, Chen F, Xiao X, Mackman N, Billiar TR, Han J, Lu B (2019) Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity 51:983–996.e6. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Yagi K, Suzuki K, Fujita S (2008) The effects of MPTP on the activation of microglia/astrocytes and cytokine/chemokine levels in different mice strains. J Neuroimmunol 204:43–51. [DOI] [PubMed] [Google Scholar]
- Yu X, He GR, Sun L, Lan X, Shi LL, Xuan ZH, Du GH (2012) Assessment of the treatment effect of baicalein on a model of Parkinsonian tremor and elucidation of the mechanism. Life Sci 91:5–13. [DOI] [PubMed] [Google Scholar]
- Zella MAS, Metzdorf J, Ostendorf F, Maass F, Muhlack S, Gold R, Haghikia A, Tönges L (2019) Novel immunotherapeutic approaches to target alpha-synuclein and related neuroinflammation in Parkinson’s disease. Cells 8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH, Chen NH (2017) Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol Sin 38:1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ye J, Dong G (2010) Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. J Mol Neurosci 40:311–320. [DOI] [PubMed] [Google Scholar]
- Zheng ZV, Cheung CY, Lyu H, Chan HY, Li Y, Bian ZX, Wang KKW, Poon WS (2019) Baicalein enhances the effect of low dose Levodopa on the gait deficits and protects dopaminergic neurons in experimental Parkinsonism. J Clin Neurosci 64:242–251. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu M, Du RH, Qiao C, Jiang CY, Zhang KZ, Ding JH, Hu G (2016) MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener 11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.