Abstract
Background
Exposure to traffic-related air pollution (TRAP) during development and/or in adulthood has been associated in many human studies with both neurodevelopmental and neurodegenerative diseases, such as autism spectrum disorder (ASD) and Alzheimer’s disease (AD) or Parkinson’s disease (PD).
Methods
In the present study, C57BL/6 J mice were exposed to environmentally relevant levels (250+/−50 μg/m3) of diesel exhaust (DE) or filtered air (FA) during development (E0 to PND21). The expression of several transcription factors relevant for CNS development was assessed on PND3. To address possible mechanistic underpinnings of previously observed behavioral effects of DE exposure, adult neurogenesis in the hippocampus and laminar organization of neurons in the somatosensory cortex were analyzed on PND60. Results were analyzed separately for male and female mice.
Results
Developmental DE exposure caused a male-specific upregulation of Pax6, Tbr1, Tbr2, Sp1, and Creb1 on PND3. In contrast, in both males and females, Tbr2+ intermediate progenitor cells in the PND60 hippocampal dentate gyrus were decreased, as an indication of reduced adult neurogenesis. In the somatosensory region of the cerebral cortex, laminar distribution of Trb1, calbindin, and parvalbumin (but not of Ctip2 or Cux1) was altered by developmental DE exposure.
Conclusions
These results provide additional evidence to previous findings indicating the ability of developmental DE exposure to cause biochemical/molecular and behavioral alterations that may be involved in neurodevelopmental disorders such as ASD.
Keywords: Air pollution, Diesel exhaust, Developmental neurotoxicity, Adult neurogenesis, Cortical lamina organization, Autism spectrum disorder
Background
In utero and early childhood exposure to traffic-related air pollution (TRAP) has been associated with neurodevelopmental diseases such as autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder, and obsessive-compulsive disorder ([4, 23, 30, 50, 59, 62, 67]), and with neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) ([7, 12–14, 24, 25, 43, 63];). We have previously shown that mice exposed developmentally to diesel exhaust (DE) from embryonic day 0 (E0) to postnatal day 3 or 21 (PND3 or PND21) exhibit ASD-like behavioral changes and cortical lamina disorganization [17, 18], as also seen in children with ASD [57]. Other animal studies have also reported behavioral [2, 21, 44, 65] and biochemical [8, 9] effects in rodents following developmental exposure to air pollution. In mice exposed prenatally to DE, differential DNA methylation was reported in promoter regions of genes involving neuronal differentiation and neurogenesis pathways [61].
Air pollution exposure has also been shown to inhibit adult neurogenesis in mice. Our group has shown inhibition of adult neurogenesis in both the sub-ventricular zone (SVZ) and in the hippocampal dentate gyrus (DG) of adult mice exposed acutely to DE [19, 23, 25]. Impaired adult neurogenesis, particularly in the hippocampal sub-granular zone (SGZ), can have important consequences on learning and memory, and has been shown in a number of studies to contribute to the risk of Alzheimer’s disease [1, 32, 42, 46, 66, 70]. Adult neural progenitor cells (NPCs) in the SVZ and SGZ are derived from a slowly dividing subpopulation of embryonic NPCs, whereas fast-dividing embryonic NPCs are responsible for the peak of neurogenesis during CNS development [31, 56]. Environmental toxicants that disrupt the balance between self-renewal and differentiation of NSCs during development could affect the progression of both neurodevelopmental and neurodegenerative disorders.
During CNS development, such processes are controlled in part by the transcription factors paired box 6 (Pax6), T-box brain 2 (Tbr2), and T-box brain 1 (Tbr1), which are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons, respectively [28]. In adult neurogenesis, the SVZ and hippocampal SGZ contain similar progenitor cells and a similar temporal pattern of expression of Pax6, Tbr2, and Tbr1 [11, 39, 52, 53]. Pax6 has been shown to play an essential role in controlling the balance between neural stem cell self-renewal and neurogenesis in both in vivo and in vitro models [33, 45, 54] and regulates the generation of laminae in the cerebral cortex [34]. In Pax77 mice, a transgenic mouse model that overexpresses Pax6, expression of both Tbr2 and Tbr1 is increased in the fetal brain, and overproduction of Tbr2-positive cells was observed at E12.5, followed by microcephaly at E14.5 [54]. Thus, overexpression of Pax6 in a transgenic model leads to increased neurogenesis, compromising the self-renewal potential of NPCs early in development. Additionally, Tbr2 has been reported to play an essential role in regulating laminar fate during cortical genesis [48].
In the present study, we investigated the sex-specific effects of developmental DE exposure on expression of Pax6, Tbr2, and Tbr1, and its long-lasting consequences on both cortical lamina organization in the somatosensory cortex and adult neurogenesis in the hippocampal SGZ. The findings have relevance to hippocampal learning and memory, and also provide further evidence of a possible link between air pollution exposure, altered cortical development, and ASD.
Methods
Animals and exposure
Nine-week-old male and female C57BL/6 J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in the University of Washington Northlake Diesel Exposure Facility under specific-pathogen-free conditions on a 12-h light/dark cycle in an Allenton caging system (Allenton, NJ, USA) connected to filtered air supply with unrestricted access to food and water. The current study was performed simultaneously with our previously published studies [17, 18], with the litter used as the statistical unit, and with mice in the current study representing littermates of the mice that underwent behavioral, biochemical, and histological tests in the previous two studies. Following one week of acclimation, each male was time mated with two females overnight, and evidence of a vaginal plug, confirmation of successful mating, was checked the next morning before 8:30 AM. Female mice with identified vaginal plugs were considered to be at embryonic day 0 (E0). Plug-positive females were assigned randomly to Allentown cage racks designated for either diesel exhaust (DE) or filtered air (FA) exposure groups. Pregnant females were housed individually throughout the entire duration of pregnancy, and after birth the dam and litter continued to be housed throughout the pre-weaning period (E0-PND21), until euthanasia at either PND3 or until weaning. Exposure was for 6 h a day, five days per week (Monday through Friday) at a PM2.5 concentration of 250+/−50 μg/m3, equivalent to a time-weighted hourly average of 44.64-53.51 μg/m3. Mice housed in the FA rack were supplied with HEPA-filtered air. In order to keep the exposure schedule consistent on the same developmental days in different cohorts, mating pairs were always set-up on Sundays to allow the first day of exposure to fall on E0.
DE was generated on site from a Yanmar YDG5500 diesel generator fueled with standard highway-grade No. 2 diesel fuel obtained from local fuel distributors and operated under load. Generated DE then passed through a two-step dilution system with dynamic control of fine particulate matter (PM2.5), maintaining constant exposure levels at 250+/−50 μg/m3. Chemical composition and particle size characterization of the DE have been previously described in detail [29, 68]. During the exposure period, mice in both exposure groups (DE or FA) were housed in the same room under identical conditions, subjected to the same noise level and light cycle. All animal experiments were approved by the University of Washington Institutional Animal Care and Use Committee.
Pregnancy outcomes, body weights, and postnatal behavioral testing
The current study was part of a larger study involving 13 FA- and 14 DE-exposed litters that were generated over 12 overlapping exposure cohorts. Mice for the current study were littermates of the mice used for behavioral, biochemical, and histological measures in our previous studies [17, 18]. Both DE- and FA-exposed pups were born in similar litter size and sex ratio [17]. There were no significant differences in pup weights, and the appearance of the righting reflex was the same for both DE- and FA-exposed pups [17]. There were no significant differences in maternal behavior between the DE- and FA-exposed dams during the early postnatal period, including time spent on pup-care activities such as grooming, nursing, and nest building [17]. Postnatal behavioral testing of littermates of the mice used in the current study has been reported previously and includes significant effects on social behaviors, communication, and repetitive behaviors [17].
Tissue collection
On PND3, male and female pups (one per litter) born to DE- and FA-exposed dams were euthanized by rapid decapitation with scissors, and cortex samples were rapidly dissected and snap-frozen in liquid nitrogen, then stored at −80 °C for later quantitative real-time PCR (qRT–PCR) analysis. On PND60, additional mice (one per sex per litter) were euthanized with CO2 followed by cervical dislocation, and perfused transcardially with 10 ml of phosphate-buffered saline (PBS) followed by 10 ml of 4% paraformaldehyde at the rate of 2 ml/min. Brains were then carefully removed from the skull, placed into 4% paraformaldehyde at 4 °C overnight for additional fixing then cryoprotected in 30% sucrose at 4 °C until the brains sank. After cryoprotection, brains were hemisected at midline and embedded in Tissue-Tek* CRYO-OCT cutting matrix (Fischer Scientific, Pittsburgh, PA) with midline facing the bottom of a standard-size Cryomold® (25 × 20 × 5 mm, Sakura Finetek, Netherlands) to ensure a consistent sectioning angle. The embedded brains were stored in −80 °C in moisture-trapping resealable zip bags for later immunohistochemistry analysis.
Quantitative real time-PCR
Levels of mRNA of Pax6, Tbr1, Tbr2, Sp1, and Creb1 were measured in the cerebral cortex of DE- and FA-exposed PND3 mice. In brief, RNA was extracted by homogenizing frozen brain samples in TRIzol reagent (Thermo Fisher Scientific, Rockford, IL) with a tissue homogenizer followed by standard chloroform extraction and ethanol precipitation procedures. RNA was further purified with the GeneJET RNA purification kit (Thermo Fisher Scientific Inc., Rockford, IL) according to the protocol provided by the manufacturer. Quality and concentration of RNA samples were confirmed by NanoDrop (Thermo Fisher Scientific Inc., Rockford, IL) measurements. For qRT-PCR analysis, only samples with 260/280 ratio > 1.8 and 260/230 ratio between 2.0 and 2.2 were used. Reverse transcription was performed using the iScript cDNA Synthesis kit (Biorad; Hercules, CA) with 1 μg of RNA per 20 μl reaction. The iTaq™ Universal SYBR® Green One-Step Kit (Biorad; Hercules, CA) was used for signal detection during qRT-PCR on a Bio-Rad CFX384 Real-Time PCR Detection System (Biorad; Hercules, CA), using the primers indicated in Supplemental Table 1. Expression of target genes was normalized to the housekeeping gene GAPDH, and relative expression levels (ddCq) were calculated according to Haimes and Kelley [36]. Expression levels in DE-exposed animals were compared with same-sex animals exposed to FA.
Immunohistochemical analysis of cortical lamina organization and of hippocampal neurogenesis
OCT-embedded PND60 mouse brains were cut sagittally at 10-12 μm starting 2000 μm away from the midline, and the somatosensory cortex region was sampled 200 μm apart for five serial sets. The sections were direct-mounted on glass slides and air dried, then stored at −80 °C. Immunohistochemistry was performed as previously described [28] with the following primary antibodies at the indicated dilutions: rabbit anti-Tbr1 (1:2000; obtained from Dr. Robert Hevner), rat anti-Tbr2 (1:200; Ebioscience, 14-4875-82), rabbit anti-CUX1 (1:200; Santa Cruz, sc-13024), rat anti-Ctip2 (1:1,000; Abcam, 18465), mouse anti-Calb (1:3,000; Sigma, c9848), and rabbit anti-Parvalbumin (PV) (1:1,000; Swant, PV27). Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit (1:600, Thermo Fisher Scientific), 568-conjugated goat anti-mouse (1:600, Thermo Fisher Scientific), and 568-conjugated goat anti-mouse (1:600, Thermo Fisher Scientific). The chromosome counterstain DAPI (4′,6-diamidino-2-phenylindole) (Sigma, St. Louis, MO) was used to label DNA after incubation with primary and secondary antibodies, following the manufacturer’s protocol. Brains from five animals in each experimental group were processed and analyzed, with 3-5 sections collected per brain. Digital immunofluorescence images were obtained on a Zeiss Axio Imager Z1. In each image, cortical depth (i.e., distance between the ventricle and pia mater), was divided into 10 evenly spaced bins, with bin 1 nearest the pia mater. Fluorescent-labeled cells were counted in each bin and the area of each bin was measured using Adobe Photoshop, with the researcher blinded to experimental groups. Cell density in each bin was calculated by dividing cell count by bin area.
Statistical analysis
Statistical analyses for cortical lamina quantifications were performed as previously described [18]. The two-tailed t test was used to assess differences in cell density between FA and DE brains of the same sex in each bin. The F test was also used to assess equality of variances between FA and DE brains of the same sex in each bin. In the hippocampus, Tbr2+ cells in the DG were counted in 3-5 tissue sections from each of the five mice sampled per experiment group. Averaged Tbr2+ cell counts for each animal were normalized to same-sex FA control. The unpaired T test with Welch’s correction was used to compare differences in Tbr2+ cell counts in the hippocampus between FA- and DE-exposed brains of mice of the same sex.
Results
Developmental DE exposure is associated with increased expression of the transcription factors Pax6, Tbr2, Tbr1, Sp1, and Creb1 on PND3
Levels of mRNA of transcription factors known to modulate neurogenesis and neuronal differentiation were measured by qRT-PCR in whole brains from PND3 male and female mice exposed to DE or FA from E0 to PND3. The relative expression of each of the target genes Pax6, Tbr2, Tbr1, Sp1, and Creb1 was normalized to the housekeeping gene GAPDH. As shown in Fig. 1, expression of all transcription factors increased significantly in brains of DE-exposed male mice compared to FA controls. In female mice, there was a slight trend toward increased expression, particularly for Tbr1 and Creb1, but it was not statistically significant (Fig. 1). There were no differences in GAPDH expression between DE- and FA-exposed animals (not shown).
Fig. 1.
mRNA levels of neurogenic transcription factors at PND3. mRNA levels of transcription factors Pax6 (a), Tbr2 (b), Tbr1 (c), Sp1 (d), and Creb1 (e) were measured in cortex samples from PND3 pups exposed to either DE or FA from E0 to PND3. mRNA levels were normalized to the house-keeping gene GAPDH. Results represent the mean (± SE) of 5 mice from different litters for each experimental group. For all five transcription factors measured, mRNA levels were significantly increased in DE exposed males when compared to FA males (*p < 0.05; **p < 0.01; ***p < 0.001; two-way ANOVA with Bonferroni post-test)
Developmental DE exposure is associated with decreased adult neurogenesis
The presence of T-box brain 2 (Tbr2) has been used as a marker for neurogenesis during CNS development and in the adult brain, as it is expressed by intermediate neuronal progenitors [28, 39]. In the adult brain, neurogenesis occurs in two discrete areas, the sub-ventricular zone (SVZ), and the hippocampal sub-granular zone (SGZ) of the dentate gyrus (DG) [1, 66]. At PND60, mice exposed developmentally to DE exhibited a significant decrease in the number of Tbr2+ cells in the hippocampal SGZ region, with the decrease seen in both males and females (Fig. 2). Thus, exposure of mice to DE during development was associated with a decrease in hippocampal adult neurogenesis later in life at PND60.
Fig. 2.
Adult neurogenesis in the hippocampal dentate gyrus. Adult neurogenesis in the hippocampal dentate gyrus, as measured by Tbr2 immunohistochemistry. Tbr2 is expressed in intermediate progenitor cells [28, 39], and in the adult brain is a marker of newly-divided neurons in the subventricular zone (SVZ) and hippocampal subgranular zone (SGZ) [11, 39, 52, 53]. Representative images from a FA-exposed male (a) and DE-exposed male (b) are shown, with Tbr2+ cells (in green), counterstained with the nuclear stain DAPI (blue). DE exposure was associated with a significant decrease in the number of Tbr2+ cells in the SGZ in both males and females (c). Results represent the mean (± SE) of 5 mice from different litters for each experimental group (**p < 0.01; ***p < 0.001; unpaired t test with Welch’s correction)
Disorganization of cortical laminae in PND60 mice exposed developmentally to DE
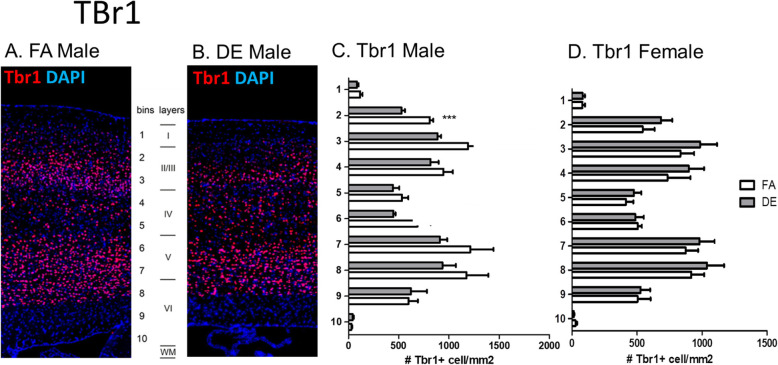
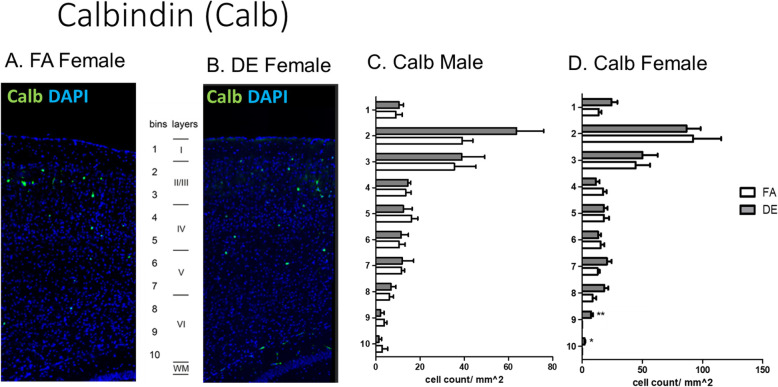
Disruptions of cerebral cortex development have been demonstrated in mice genetically modified to overexpress Pax6 [40], as well as in mice exposed prenatally to PM2.5 [69] or DE [17]. To assess potential effects on lamina organization due to developmental DE exposure, an immunohistochemical analysis was carried out at PND60 in the somatosensory cortex, using the lamina-specific markers T-box brain 1 (Tbr1), COUP-TF-interacting protein 2 (Ctip2), and CUT-like homeobox 1 (Cux1), as well as Calbindin (Calb) and Parvalbumin (Pv). Statistically significant differences were found in the cortical distribution of Tbr1-, Calb-, and Pv-positive cells. Tbr1 is a transcription factor known for its role in glutamatergic neuron differentiation [28, 49]. Developmental DE exposure affected the localization pattern of Tbr1-positive cells at PND60, with a less distinct bi-laminar pattern than that seen in control mice and a statistically significant decrease in Tbr1-positive cells in cortical layer II/III in the DE-exposed males, but not females (Fig. 3). In contrast, FA-exposed control mice of both sexes and DE-exposed female mice exhibited a distinct bi-laminar localization pattern, with Tbr1-positive cells localizing in layers II/III and V (Fig. 3c, d). Calbindin (Calb) is a calcium-binding protein, and Calb+ interneurons have been shown to localize primarily but not exclusively in cortical layer II/III [5]. In DE-exposed female mice a statistically significant increase in Calb+ cell density was found in cortical layer VI when compared to FA-exposed females, whereas no significant difference in Calb+ cell distribution was found between FA- and DE-exposed male mice (Fig. 4c, d). Parvalbumin (Pv) is a calcium-binding albumin protein expressed by interneurons localizing primarily in layer II/III [5, 35]. In DE-exposed male mice, a statistically significant increase in Pv + cell density was found in cortical layers IV and VI when compared to FA-exposed males, whereas no significant differences in Pv + cell distribution were found between FA- and DE-exposed female mice (Fig. 5c, d). No significant differences were found in the cortical distribution of Ctip2- and Cux1-positive cells following DE exposure (Supplemental Figs. 1 and 2).
Fig. 3.
Tbr1 in PND60 cortex. Tbr1 immunohistochemistry in cortex of DE- and FA-exposed mice. Tbr1 is normally localized to neurons in layers II/II and V, as demonstrated in the Allen Brain Atlas [60]. (a, b) Representative images of Tbr1+ cells (red), counterstained with the nuclear stain DAPI (blue), from FA- and DE-exposed males. DE-exposed males showed a statistically significant decrease in Tbr1-positive cell density in bin 2 (cortical layer II/III) compared to FA-exposed males (c). No differences in Tbr1-positive cell distribution were observed with DE exposure in female mice (d). Results represent the mean (± SE) of 5 mice from different litters for each experimental group; 3-5 sections/mouse were examined (***p < 0.001; unpaired t test with Welch’s correction)
Fig. 4.
Calbindin in PND60 cortex. Calbindin (Calb) immunohistochemistry in cortex of DE- and FA-exposed mice. Calb is a calcium-binding protein; Calb-expressing interneurons have been shown to localize in cortical layer II/III [5]. (a, b) Representative images of Calb-positive cells (green), counterstained with the nuclear stain DAPI (blue), from FA- and DE-exposed females. In DE-exposed females, a statistically significant increase in Calb-positive cell density was found in bins 9-10 (cortical layer VI) when compared to FA-exposed females (c, d). No significant differences in Calb-positive cell distribution were found between FA- and DE-exposed male mice (c, d). Results represent the mean (± SE) of 5 mice from different litters for each experimental group; 3-5 sections/mouse were examined (*p < 0.05, **p < 0.01; unpaired T test with Welch’s correction)
Fig. 5.
Parvalbumin in PND60 cortex. Parvalbumin (PV) immunohistochemistry in cortex of DE- and FA-exposed mice. PV is a calcium-binding albumin protein expressed by interneurons localized to cortical layer II/III [5, 35]. (a, b) Representative images of PV-positive cells (red), counterstained with the nuclear stain DAPI (blue), from FA- and DE-exposed males. In DE-exposed males a statistically significant increase in PV-positive cell density was found in bins 5 and 9 (cortical layers IV and VI) when compared to FA-exposed males (c, d). No significant differences in PV-positive cell distribution were found between FA- and DE-exposed female mice (c, d). Results represent the mean (± SE) of 5 mice from different litters for each experimental group; 3-5 sections/mouse were examined (*p < 0.05; unpaired T test with Welch’s correction)
Discussion
The expression of five different transcription factors (Pax6, Tbr2, Tbr1, Sp1, and Creb1) known to modulate neurogenic pathways [27, 28, 34, 54, 58] were found to be increased in DE-exposed males on PND3 (Fig. 1). PND3 was selected for the analyses because cortical neurogenesis is complete and markers of different neuron types are strongly expressed, enabling analysis of cortical neuron types quantitatively by PCR. Temporal expression of Pax6, Tbr2, and Tbr1 has been shown to control the process of neurogenesis and neurodifferentiation, as these transcription factors are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons, respectively, during neurogenic events in both the fetal and adult brain [11, 28, 39, 52, 53]. In addition, Pax6 has been shown to play an essential role in controlling the balance between neural stem cell self-renewal and neurogenesis in both in vivo and in vitro models [33, 54], and in transgenic mice that overexpress Pax6, the expression of both Tbr2 and Tbr1 has been shown to be positively regulated [54]. Our findings of increased Pax6, Tbr2, and Tbr1 mRNA levels in the cortex of PND3 male mice exposed developmentally to DE (Fig. 1) suggest that DE exposure caused a delay in the differentiation of cortical progenitors and neurons in male mice, since transcription-factor levels normally decline postnatally, but remained elevated after DE exposure. These findings would be consistent with the reported upregulation of Tbr2 and Tbr1 expression by Pax6 [54], leading to promotion of neurogenesis at the expense of neural stem cell self-renewal. In the DE-exposed males, expression of Pax6, Tbr2, and Tbr1 were all increased, despite their different developmental roles. One possibility to explain this observation is that there could be an overall increased and prolonged abundance of developmental cell types (both progenitor cells and new neurons) in the DE-exposed male mice. If the population of radial glial cells (Pax6+ cells) is increased, they may generate more intermediate progenitors (Tbr2+) and neurons (Tbr1+). The predicted consequence would be that overall neurogenesis, and therefore brain size, is increased. Interestingly, this fits with clinical observations of increased brain volume in a significant proportion of autism cases, marked by brain overgrowth during the late gestational and early postnatal developmental periods [37].
Immunohistochemistry analysis in the hippocampal DG of PND60 mice exposed developmentally to DE (E0-PND21) showed a significant decrease in Tbr2-positive cells in the SGZ of both male and female mice (Fig. 2). Since Tbr2 is only expressed by newly divided intermediate neural progenitor cells [28, 39], this finding suggests that developmental DE exposure decreases adult neurogenesis. Given the importance of the hippocampus for learning and memory, this effect of DE exposure on adult neurogenesis in the SGZ could account for neurodevelopmental problems and even deleterious effects in adults. Other studies have shown decreased adult neurogenesis due to acute and subacute exposure to traffic-related air pollution in adult mice [19, 20], but this long-lasting effect of developmental exposure is novel. In Pax77 mice, a line of transgenic mice that overexpress Pax6, an increased number of Tbr-positive cells was observed in the neocortex at E12.5, followed by microcephaly at E14.5 with no increase in apoptosis [54]. Our findings suggest that over-promotion of neurogenesis early in CNS development depletes the self-renewal potential of neural progenitor cells, which leads to decreased neurogenesis during the later stages of corticogenesis, as well as to decreased adult neurogenesis in the SGZ. Alternatively, DE exposure may impair the postnatal migration and integration of neural stem and progenitor cells in the hippocampal SGZ. Consistent with the latter possibility, developmental DE exposure has been shown to affect the expression and distribution of reelin [18], which plays important roles in migration and differentiation of neurons in the cortex and hippocampus [10]. Furthermore, mRNA levels of the transcription factor Creb1 were also increased in brains of PND3 male mice exposed developmentally to DE (Fig. 1e). As Creb1 is reported to play a role in neuronal survival during adult neurogenesis [38, 71], further assessment of whether Creb1 upregulation due to DE exposure persists in adult mice would be of interest.
We also found increased mRNA levels of the transcription factor Sp1 in the brains of PND3 male mice exposed developmentally to DE (Fig. 1d). Elevated expression of Sp1 has been observed in the brains of autism patients [64], along with altered expression of a number of ASD candidate genes that have Sp1 binding sites, including reelin (RELN). Increases in Sp1 expression and downregulation of RELN expression were observed in both ASD patients [64] and in the brains of mice exposed developmentally to DE using the same experimental paradigm as the current study [18]. Further, in the gene encoding, the MET receptor tyrosine kinase, a functional promoter variant has been identified that was associated with ASD in two separate studies and that alters Sp1 binding [15, 16]. The diverse downstream pathways mediated by the Sp1-regulated genes, along with the environmental and intracellular signal-related regulation of Sp1, could explain the complex phenotypes associated with ASD. Increased Sp1 expression is also of interest to neurodegenerative disease, as Sp1 has been found to upregulate the β-amyloid precursor protein (APP) as a result of environmental influences during brain development (e.g., exposure to the neurotoxic metal lead), leading to amyloidogenesis and cognitive decline at a later age [3, 6].
In the somatosensory cortex, we found a significantly decreased number of Tbr1-positive cells in cortical layers II/III from PND60 male mice exposed developmentally to DE (Fig. 3c), similarly to what was found in the Pax77 mouse model, where cortical layers are generated with an “inside-out” pattern, with neurons in deeper layers born during early stages of corticogenesis [41, 51, 55]. This sex-specific finding from the Tbr1 immunohistochemistry analysis is also in agreement with the developmental effects of DE exposure on Pax6, Tbr2, and Tbr1 expression levels, which were also male specific (Fig. 1). The male specificity of these effects is of particular interest given the higher incidence of ASD in males and is consistent with our previous studies showing that males were often more sensitive to the behavioral and biochemical effects of developmental DE exposure [17, 18]. It is intriguing that the inhibition of hippocampal adult neurogenesis following developmental DE exposure occurred in both males and females, whereas the cortical effects and effects on ASD-related behaviors were more male-specific. The underlying factors affecting the different sensitivities of males and females are unknown. Adult mice exposed to DE have also shown increased sensitivity of males to DE-associated oxidative stress and neuroinflammation [22].
We have previously reported downregulation of reelin (RELN) expression in brains of mice at PND3 and PND60 following developmental DE exposure using the same experimental paradigm as the current study [18]. Since reelin has been known to play a critical role in guiding the process of neuronal migration [26, 47], we carried out in the current study an immunohistochemical analysis with four additional cortical lamina markers (Calb, Pv, Ctip2, and Cux1) to further evaluate the extent of potential cortical organizational effects due to developmental DE exposure. We found subtle changes in the distribution of Calb+ and Pv + cells in deeper layers of the cortex (Figs. 4 and 5), while no significant changes in cell distribution were found with the markers Ctip2 and Cux1 (Supplemental Figs. 1 and 2). Although the level of cortical disorganization found in DE-exposed mice is mild compared to that observed in the reelin knockout mouse [10], our results parallel reports of small patches of disorganization found in prefrontal cortex of adolescent ASD patients [57]. The consequences of such subtle effects on cortical layering is not known, but even minor alterations in cortical structure may be of importance, particularly when they occur in a region such as the somatosensory cortex that can be altered in ASD. Our previous studies have demonstrated ASD-related behavioral consequences of developmental DE exposure, including effects on communication, social behaviors, and repetitive behaviors [17]. This would suggest that the subtle effects of DE exposure on cortical layering in the current study have direct phenotypic relevance.
Conclusions
Our findings show that developmental DE exposure is associated with upregulation of the Pax6, Tbr2, and Tbr1 neurogenic pathway, likely, disrupting the temporal balance between neurogenesis and progenitor cell self-renewal and leading to decreased neurogenesis during late cortical development. Related to this finding, we found that developmental DE exposure from E0-PND21 was associated with decreased adult neurogenesis in the hippocampus at PND60, detected by a decrease in Tbr2-positive cells in the SGZ. This important finding suggests that developmental DE exposure could have long-lasting consequences on learning and memory that extend into adulthood. In addition to this effect on hippocampal adult neurogenesis, we found perturbations in cortical layering of the somatosensory cortex at PND60 that were associated with developmental DE exposure, specifically with respect to neurons expressing Tbr1, Calb, and Pv. These findings are of relevance to neurodevelopmental disorders, and particularly ASD, as our previous findings showed ASD-related behavioral alterations arising after developmental DE exposure. The effects on cortical development are likely due to perturbations of reelin, an important mediator of cortical development that is affected by developmental DE exposure [18]. Taken together, these findings suggest that DE exposure affects postnatal migration and integration of neural stem and progenitor cells in both the SGZ and cortex. Much more still remains unknown about the risk from developmental air pollution in respect to both neurodegenerative diseases and neurodevelopmental disorders. Given the pervasive nature of traffic-related air pollution and the size of populations exposed globally, future studies are warranted to carefully evaluate both the potential hazard and the exact mode of toxicity of TRAP.
Supplementary Information
Additional file 1: Supplemental Table 1. Sequences of primers for qRT-PCR. Supplemental Figure 2. Ctip2 in PND60 Cortex. Supplemental Figure 3. CUX1 in PND60 Cortex.
Acknowledgements
The authors thank Mr. Jim Stewart and Dr. Joel Kaufman for providing and characterizing DE exposure, and Elise Hickman and Natalya Matlashchuk for assistance with the qPCR assays and cortical-layering analyses.
Abbreviations
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- ASD
Autism spectrum disorder
- Calb
Calbindin
- CNS
Central nervous system
- Creb1
cAMP-responsive element binding protein 1
- Ctip2
Coup-TF interacting protein 2
- Cux1
Cut-like homeobox 1
- DE
Diesel exhaust
- DG
Dentate gyrus
- E0
Embryonic day 0
- FA
Filtered air
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- NPCs
Neural progenitor cells
- Pax6
Paired box 6
- PD
Parkinson’s disease
- PV
Parvalbumin
- PND
Postnatal day
- SGZ
Subgranular zone of the hippocampal dentate gyrus
- Sp1
Specificity protein 1
- SVZ
Subventricular zone
- Tbr1
T-box brain 1
- Tbr2
T-box brain 2
- TRAP
Traffic-related air pollution
Authors’ contributions
YCC, RD, RH, LGC, and TBC designed the experiments, which were carried out by YCC, TBC, KD, and RD. LGC obtained funding for the study. TBC, LGC, and YCC wrote the paper. All authors read and approved the final manuscript.
Funding
Research by the authors was supported by grants to L.G.C. and T.B.C from NIEHS (R01ES028273, R01ES022949, P30ES07033, P42ES04696), NICHD (U54HD083091), and by funds from the Department of Environmental and Occupational Health Sciences, University of Washington, and by R01 NS092339 to R.F.H.
Availability of data and materials
The original data are available at the University of Washington.
Ethics approval and consent to participate
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Washington and were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health. The UW is an AAALAC accredited institution.
Consent for publication
All listed authors have given consent for publication of this paper.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Toby B. Cole and Yu-Chi Chang contributed equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11689-020-09340-3.
References
- 1.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 2.Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. NeuroToxicology. 2016;59:140–154. doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge Y-W, Lahiri S, Debomoy K, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J. Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles County. California. Environ. Health Perspect. 2013;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beguin S, Crépel V, Aniksztejn L, Becq H, Pelosi B, Pallesi-Pocachard E, Bouamrane L, Pasqualetti M, Kitamura K, Cardoso C, Represa A. An epilepsy-related ARX polyalanine expansion modifies glutamatergic neurons excitability and morphology without affecting gabaergic neurons development. Cerebral Cortex. 2013;23:1484–1494. doi: 10.1093/cercor/bhs138. [DOI] [PubMed] [Google Scholar]
- 6.Bihaqi SW, Bahmani A, Subaiea GM, Zawia NH. Infantile exposure to lead and late-age cognitive decline: relevance to AD. Alzheimers Dement. 2014;10:187–195. doi: 10.1016/j.jalz.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD. Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front. Synaptic Neurosci. 2017;9:10. doi: 10.3389/fnsyn.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- 10.Boyle MP, Bernard A, Thompson CL, Ng L, Boe A, Mortrud M, Hawrylycz MJ, Jones AR, Hevner RF, Lein ES. Cell-type-specific consequences of reelin deficiency in the mouse neocortex, hippocampus, and amygdala. J. Comp. Neurol. 2011;519:206–289. doi: 10.1002/cne.22655. [DOI] [PubMed] [Google Scholar]
- 11.Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascón S, Erdelyi F, Szabo G, Parras C, Guillemot F, Frotscher M, Berninger B, Hevner RF, Raineteau O, Götz M. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderón-Garcidueñas L, Franco-Lira M, Mora-Tiscareño A, Medina-Cortina H, Torres-Jardón R, Kavanaugh M. Early Alzheimer’s and Parkinson’s disease pathology in urban children: friend versus foe responses--it is time to face the evidence. Biomed. Res. Int. 2013;2013:161687. doi: 10.1155/2013/161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderón-Garcidueñas L, Kulesza RJ, Doty RL, D’Angiulli A, Torres-Jardón R. Megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ. Res. 2015;137:157–169. doi: 10.1016/j.envres.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Calderón-Garcidueñas L, Reynoso-Robles R, Vargas-Martínez J, Gómez-Maqueo-Chew A, Pérez-Guillé B, Mukherjee PS, Torres-Jardón R, Perry G, Gónzalez-Maciel A. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ. Res. 2016;146:404–417. doi: 10.1016/j.envres.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1(3):159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc. Natl. Acad. Sci. U S A. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YC, Cole TB, Costa LG. Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Part. Fibre Toxicol. 2018;15(1):18. doi: 10.1186/s12989-018-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang YC, Daza R, Hevner R, Costa LG, Cole TB. Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: evidence for a reelin-related pathogenetic pathway induced by interleukin-6. Brain Behav. Immun. 2019;78:105–115. doi: 10.1016/j.bbi.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn JL, Cole TB, Khoi D, Costa LG. Acute exposure to diesel exhaust impairs adult neurogenesis in mice: prominence in males and protective effect of pioglitazone. Arch. Toxicol. 2018;92:1815–1829. doi: 10.1007/s00204-018-2180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L, Lau WKW, Fung TKH, Lau BWM, Chau BKH, Liang Y, Wang Z, So KF, Wang T, Chan C, Lee TMC. PM2.5 exposure suppresses dendritic maturation in subgranular zone in aged rats. Neurotox. Res. 2017;32:50–57. doi: 10.1007/s12640-017-9710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Church JS, Tijerina PB, Emerson FJ, Coburn MA, Blum JL, Zelikoff JT, Schwartzer JJ. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. Neurotoxicology. 2018;65:231–240. doi: 10.1016/j.neuro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole TB, Coburn J, Dao K, Roqué P, Chang Y, Kalia V, Guilarte TR, Dziedzic J, Costa LG. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. doi: 10.1016/j.tox.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa LG, Chang YC, Cole TB. Developmental neurotoxicity of traffic-related air pollution: focus on autism. Curr. Environ. Health Rep. 2017;4:156–165. doi: 10.1007/s40572-017-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res. Int. 2014;2014:736385. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa LG, Cole TB, Coburn J, Chang Y, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–139. doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Arcangelo G. Reelin in the years: controlling neuronal migration and maturation in the mammalian brain. Adv. Neurosci. 2014;2014:597395. [Google Scholar]
- 27.Dworkin S, Mantamadiotis T. Targeting CREB signalling in neurogenesis. Expert Opin. Ther. Targets. 2010;14:869–879. doi: 10.1517/14728222.2010.501332. [DOI] [PubMed] [Google Scholar]
- 28.Englund C, Fink A, Charmaine L, Pham D, Ray DAM, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox JR, Cox DP, Drury BE, Gould TR, Kavanagh TJ, Paulsen MH, Sheppard L, Simpson CD, Stewart JA, Larson TV, Kaufman JD. Chemical characterization and in vitro toxicity of diesel exhaust particulate matter generated under varying conditions. Air Qual. Atmos. Health. 2014;8:507–519. doi: 10.1007/s11869-014-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuertes E, Standl M, Forns J, Berdel D, Garcia-Aymerich J, Markevych I, Schulte-Koerne G, Sugiri D, Schikowski T, Tiesler CM, Heinrich J. Traffic-related air pollution and hyperactivity/inattention, dyslexia and dyscalculia in adolescents of the German GINIplus and LISAplus birth cohorts. Environ. Int. 2016;97:85–92. doi: 10.1016/j.envint.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Furutachi S, Miya H, Watanabe T, Kawai H, Yamasaki N, Harada Y, Imayoshi I, Nelson M, Nakayama KI, Hirabayashi Y, Gotoh Y. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015;18:657–665. doi: 10.1038/nn.3989. [DOI] [PubMed] [Google Scholar]
- 32.Fuster-Matanzo A, Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Tau protein and adult hippocampal neurogenesis. Front. Neurosci. 2012;6:104. doi: 10.3389/fnins.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan Q, Lee A, Suzuki R, Yamagami T, Stokes A, Nguyen BC, Pleasure D, Wang J, Chen HW, Zhou CJ. Pax6 mediates β-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells. Stem Cells. 2014;32:45–58. doi: 10.1002/stem.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgala PA, Manuel M, Price DJ. The generation of superficial cortical layers is regulated by levels of the transcription factor Pax6. Cereb. Cortex. 2011;21:81–94. doi: 10.1093/cercor/bhq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2008;2008:1–3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haimes J, Kelley M. Demonstration of a ΔΔ C q calculation method to compute relative gene expression from qPCR data. GE Healthcare, Tech Note, 1–4. 2010. http://dharmacon.gelifesciences.com/uploadedfiles/resources/delta-cq-solaris-technote.pdf. Accessed 15 May 2018.
- 37.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell. Neurosci. 2011;46:79–88. doi: 10.1016/j.mcn.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imamura F, Greer CA. Pax6 regulates Tbr1 and Tbr2 expressions in olfactory bulb mitral cells. Mol. Cell. Neurosci. 2013;54:58–70. doi: 10.1016/j.mcn.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent BA, Mistlberger RE. Sleep and hippocampal neurogenesis: implications for Alzheimer’s disease. Front. Neuroendocrinol. 2017;45:35–52. doi: 10.1016/j.yfrne.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Lee PC, Raaschou-Nielsen O, Lill CM, Bertram L, Sinsheimer JS, Hansen J, Ritz B. Gene-environment interactions linking air pollution and inflammation in Parkinson’s disease. Environ. Res. 2016;151:713–720. doi: 10.1016/j.envres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Li K, Li L, Cui B, Gai Z, Li Q, Wang S, Yan J, Lin B, Tian L, Liu H, Liu X, Xi Z. Early postnatal exposure to airborne fine particulate matter induces autism-like phenotypes in male rats. Toxicol. Sci. 2018;162:188–199. doi: 10.1093/toxsci/kfx240. [DOI] [PubMed] [Google Scholar]
- 45.Manuel M, Georgala PA, Carr CB, Chanas S, Kleinjan DA, Martynoga B, Mason JO, Molinek M, Pinson J, Pratt T, Quinn JC, Simpson TI, Tyas DA, van Heyningen V, West JD, Price DJ. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development. 2006;134:545–555. doi: 10.1242/dev.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maruszak A, Pilarski A, Murphy T, Branch N, Thuret S. Hippocampal neurogenesis in Alzheimer’s disease: is there a role for dietary modulation? J. Alzheimers Dis. 2014;38:11–38. doi: 10.3233/JAD-131004. [DOI] [PubMed] [Google Scholar]
- 47.Michetti C, Altabella L, Caruso A, Castelluccio P, Bedse G, Gaetani S, Canese R, Laviola G, Scattoni ML. Mapping pathological phenotypes in reelin mutant mice. Front. Pediatr. 2014;2:95. doi: 10.3389/fped.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mihalas AB, Elsen GE, Bedogni F, Daza RAM, Ramos-laguna KA, Arnold SJ, Hevner RF. Intermediate progenitor cohorts differentially generate cortical layers and require Tbr2 for timely acquisition of neuronal subtype identity article intermediate progenitor cohorts differentially generate cortical layers and require Tbr2 for timely acquis. Cell Rep. 2016;16:92–105. doi: 10.1016/j.celrep.2016.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihalas AB, Hevner RF. Control of neuronal development by T-box genes in the brain. Curr. Top. Dev. Biol. 2017;122:279–312. doi: 10.1016/bs.ctdb.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Min JY, Min KB. Exposure to ambient PM10and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ Int. 2017;99:221–7. [DOI] [PubMed]
- 51.Rakic P. Neurons in Rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 52.Roybon L, Deierborg T, Brundin P, Li JY. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur. J. Neurosci. 2009;29:232–243. doi: 10.1111/j.1460-9568.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 53.Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, Brundin P. Neurogenin2 directs granule neuroblast production and amplification while neuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4:e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 2009;5:20–23. doi: 10.1371/journal.pgen.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat. Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 56.Singh S, Solecki DJ. Polarity transitions during neurogenesis and germinal zone exit in the developing central nervous system. Front. Cell. Neurosci. 2015;9:62. doi: 10.3389/fncel.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ström AC, Forsberg M, Lillhager P, Westin G. The transcription factors Sp1 and Oct-1 interact physically to regulate human U2 snRNA gene expression. Nucleic Acids Res. 1996;24:1981–1986. doi: 10.1093/nar/24.11.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suades-González E, Gascon M, Guxens M, Sunyer J. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013;41:D996–D1001. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tachibana K, Takayanagi K, Akimoto A, Ueda K, Shinkai Y. Prenatal diesel exhaust exposure disrupts the DNA methylation pro file in the brain of mouse offspring. J. Toxicol. Sci. 2015;40:1–11. doi: 10.2131/jts.40.1. [DOI] [PubMed] [Google Scholar]
- 62.Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, Stacy SL. Fine particulate matter and the risk of autism spectrum disorder. Environ. Res. 2015;140:414–420. doi: 10.1016/j.envres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimers Dement. 2014;10(Suppl):S213–S225. doi: 10.1016/j.jalz.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Thanseem I, Anitha A, Nakamura K, Suda S, Iwata K, Matsuzaki H, Ohtsubo M, Ueki T, Katayama T, Iwata Y, Suzuki K, Minoshima S, Mori N. Elevated transcription factor specificity protein 1 in autistic brains alters the expression of autism candidate genes. Biol Psychiatry. 2012;71(5):410–418. doi: 10.1016/j.biopsych.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 65.Thirtamara RK, Doherty-Lyons S, Bolden C, Willis D, Hoffman C, Zelikoff J, Chen LC, Gu H. Prenatal and early-life exposure to high-level diesel exhaust particles leads to increased locomotor activity and repetitive behaviors in mice. Autism Res. 2013;6:248–257. doi: 10.1002/aur.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toda T, Gage FH. Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2017;373:693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 67.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weldy CS, Liu Y, Chang YC, Medvedev IO, Fox JR, Larson TV, Chien WM, Chin MT. In utero and early life exposure to diesel exhaust air pollution increases adult susceptibility to heart failure in mice. Part. Fibre Toxicol. 2013;10:59. doi: 10.1186/1743-8977-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang T, Zheng X, Wang X, Zhao H, Wang T, Zhang H, Li W, Shen H, Yu L. Maternal exposure to PM2.5 during pregnancy induces impaired development of cerebral cortex in mice offspring. Int. J. Mol. Sci. 2018;19:E257. doi: 10.3390/ijms19010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng JY, Liang KS, Wang XJ, Zhou XY, Sun J, Zhou SN. Chronic estradiol administration during the early stage of Alzheimer’s disease pathology rescues adult hippocampal neurogenesis and ameliorates cognitive deficits in Aβ1-42 mice. Mol. Neurobiol. 2016;54:7656–7669. doi: 10.1007/s12035-016-0181-z. [DOI] [PubMed] [Google Scholar]
- 71.Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Sequences of primers for qRT-PCR. Supplemental Figure 2. Ctip2 in PND60 Cortex. Supplemental Figure 3. CUX1 in PND60 Cortex.
Data Availability Statement
The original data are available at the University of Washington.