Abstract
To support clinical use, a multigram-scale process has been developed to provide 5-MeO-DMT, a psychedelic natural product found in the parotid gland secretions of the toad, Incilius alvarius. Several synthetic routes were initially explored, and the selected process featured an optimized Fischer indole reaction to 5-MeO-DMT freebase in high-yield, from which the 1:1 succinate salt was produced to provide 136 g of crystalline active pharmaceutical ingredient (API) with 99.86% peak area by high-performance liquid chromatography (HPLC) and a net yield of 49%. The report provides in-process monitoring, validated analytical methods, impurity formation and removal, and solid-state characterization of the API essential for subsequent clinical development.
Introduction
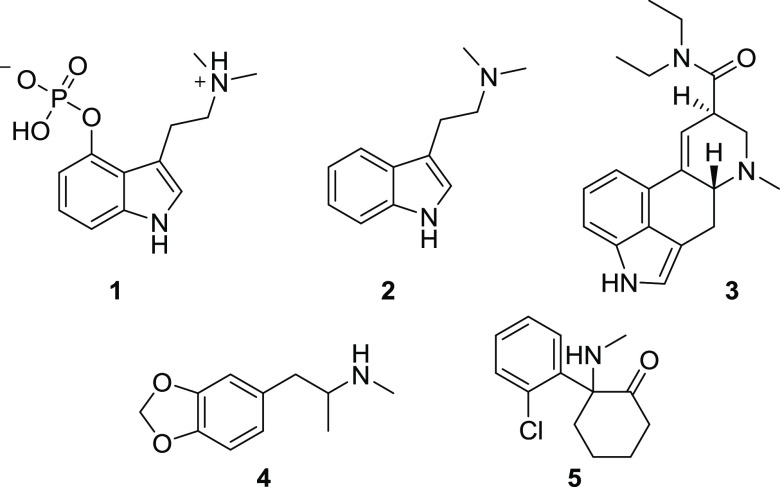
Recently, interest has increased in understanding the clinical applications of psychedelic, entactogenic, and dissociative psychoactive drugs, such as psilocybin (1), DMT (2), LSD (3), MDMA (4), or ketamine (5) in combination with psychotherapeutic support to promote improved mental health conditions (Figure 1).1,2 In particular, research has indicated favorable results in treating post-traumatic stress disorder (PTSD), depression, end of life conditions, and anxiety-related disorders.1,3−6 This research shows that while the therapeutic mechanisms are not fully understood, some factors have been correlated with improvement in mental health. These factors include the intensity of mystical experience occasioned by the psychedelic, the context in which the session was conducted (known as set and setting), the dose at which the drug is administered, psychological flexibility, connectedness, emotional breakthrough, and increased neural entropy.1,7−10
Figure 1.
Structures of clinically explored psychedelic, entactogenic, and dissociative psychoactive drugs.
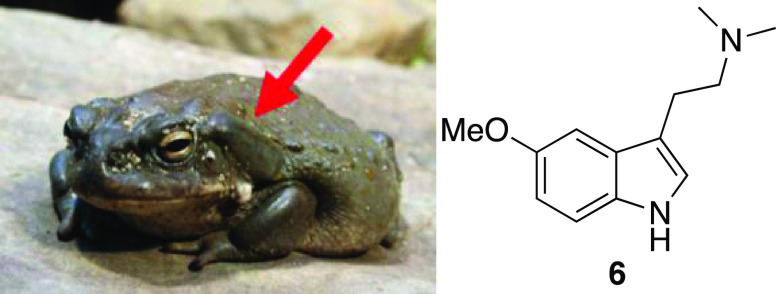
5-MeO-DMT (6) is a tryptamine natural product most commonly identified as the primary psychoactive component of the parotid gland secretions of Incilius alvarius, the Sonoran Desert toad (Figure 2).11 The alkaloid is also known to be present in low concentrations in a variety of plants, shrubs, and seeds. Human consumption of this material for its psychoactive properties has been reported in the scientific literature for at least 100 years.12−15 Although it has been historically suggested that 5-MeO-DMT may have been used by indigenous cultures,11 there is no known documentation to support this assertion. Due to the recent discovery of high concentrations of 5-MeO-DMT in I. alvarius secretions, there has been a reported increase in its recreational and spiritual use.11,16,17 Recent evidence has indicated the presence of 5-MeO-DMT existing in concentrations between 20 and 30% of total dry weight or approximately 200–300 mg of 5-MeO-DMT per dried gram of toad secretion,17 concentrations much higher when compared to plant-derived sources of 5-MeO-DMT.
Figure 2.
(Left) I. alvarius (image courtesy of Holger Krisp, Ulm, Germany, 2011 under CC BY 3.0) with the parotid gland highlighted. (Right) Structure of 5-MeO-DMT (6).
Anecdotally, and suggested by research over the last 5 years, 5-MeO-DMT has been reported to be helpful in treating clinical mental health conditions.8,9,16−18 These data suggest that 5-MeO-DMT produces mystical experiences with comparative intensity as seen with psilocybin,8 has a significantly shorter duration of effect—between 10 and 45 min depending on the route of administration used,19 and produces increased desired effects when the context of the experience is carefully curated.20,21
An extensively supported hypothesis is that commonly encountered psychedelic effects in humans (e.g., visual hallucinations, altered sense of self, time, and space, and atypical thought patterns) are mediated primarily via activation of the serotonergic 5-HT2A receptor in the central nervous system (CNS).22,23 Notably, all currently known psychedelics are also nonselective, simultaneously interacting with numerous other monoaminergic receptors and transporters in the CNS, and hence exhibit variable degrees of synergistic polypharmacology in addition to agonist activity at the 5-HT2A receptor.24 5-MeO-DMT has demonstrated sub-micromolar binding affinity across most serotonin receptor subtypes expressed in the CNS, with about 300-fold selectivity for the human 5-HT1A (3 ± 0.2 nM) versus 5-HT2A (907 ± 170 nM) receptor subtypes.25 Data has suggested that activation of the 5-HT1A receptor may also play a significant role in contributing to the subjective and behavioral effects elicited by psychedelics in a synergistic way with 5-HT2A activation.26−28 In contrast to 5-MeO-DMT, psilocin (the active metabolite of psilocybin) is about 5-fold more selective for human 5-HT2A receptors (107 nM) versus 5-HT1A (567 nM).29 In a controlled study in healthy human volunteers, coadministration of psilocin with the antianxiety medication buspirone, a selective 5-HT1A agonist, altered the subjective effects produced by psilocin, notably reducing the intensity of certain visual hallucinations.30 Interestingly, anecdotal reports on 5-MeO-DMT consumption have described a general lack of colorful geometric visual hallucinations typically associated with other psychedelics.31
To date, a comprehensive understanding of the correlation between psychedelics’ polypharmacology and the corresponding influence on their subjective effects is not well established. While a number of potential mechanisms have been hypothesized to rationalize the therapeutic mode of action of psychedelics, such as increased structural plasticity in the prefrontal cortex,32 still no direct connection has been made between specific psychedelic pharmacodynamics and positive therapeutic outcomes.33 Nevertheless, randomized clinical trials with the psychedelic psilocybin (1) in the treatment of serious mental health conditions such as major depressive disorder (MDD) continue to show promise.34 To this end, 5-MeO-DMT appears to be pharmacodynamically unique compared to previous clinically studied psychedelics and could provide a useful comparator in contemporary controlled clinical studies with psychedelics to better understand their mode of action.
Unlike psilocybin, psychedelic tryptamines such as DMT (2) and 5-MeO-DMT (6) are subject to rapid first-pass metabolism by monoamine oxidase and are therefore not orally active. When consumed parenterally, they produce a significantly shorter duration of action, typically less than 1 h, compared to the 5–8 h duration of effects produced by psilocybin. The shorter duration of action may help in reducing the amount of time a patient would spend in the clinic. Additionally, compared to DMT, 5-MeO-DMT is known to be approximately 10–20 times more potent in humans.13 With a short duration of action and possibly significant 5-HT1A receptor selectivity, 5-MeO-DMT possesses unique pharmacodynamic and pharmacokinetic properties compared to other clinically studied psychedelics. These features may correlate with more positive therapeutic outcomes in controlled human clinical trials. To test this hypothesis and to better understand the psychotherapeutic utility of 5-MeO-DMT and enable such clinical trials, the preparation of active pharmaceutical ingredient (API) is required with adequate controls to ensure its identity, potency, purity, and strength. The development of this process is the topic of this report.
Results and Discussion
5-MeO-DMT Dosage and Salt Form Selection
The most commonly reported route of administration is by vaporization of the freebase drug, which is generally not a pharmaceutically acceptable approach compared to other dosage forms. While other intraperitoneal routes of administration with 5-MeO-DMT such as dry powder inhalation, transdermal, or intravenous administration are possible, an intramuscular injection has been identified as a preferable compromise for administering this material. In addition to allowing precise metering of dose, the intramuscular injection of 5-MeO-DMT in a naturalistic setting has been previously reported and was claimed to possess an advantageous duration of action compared to the intense rapid-onset produced by other intraperitoneal routes.19 The injectable drug formulated as a 20 mg/mL solution of API in sterile water with excipients is capable of delivering a precise dose of API in the range of 2–15 mg, consistent with the dose range described in previous anecdotal reports with this material. 5-MeO-DMT freebase has low water solubility (<10 mg/mL) and the unionized amine may degrade on exposure to atmospheric oxygen to give the corresponding N-oxide degradant (vide infra). A water-soluble, pharmaceutically acceptable salt form of 5-MeO-DMT was therefore required.
In parallel to the exploration of viable synthetic routes to 5-MeO-DMT freebase, a range of pharmaceutically acceptable salt forms were considered from acids with sufficient pKa difference to fully protonate 6, including the counterions chloride, sulfate, fumarate, succinate, maleate, lysate, oxalate, benzoate, tartrate, mesylate, or acetate.35 Using analytically pure 5-MeO-DMT freebase, the hydrochloride, sulfate, fumarate, and succinate salts were initially evaluated. Attempts at formation of the sulfate salt yielded an intractable gum and the approach was abandoned. The hydrochloride salt was readily prepared as an apparent crystalline solid, but the material was found to be hygroscopic and was deliquescent under high-humidity conditions. Both the fumarate and succinate salts were readily prepared and provided stable, free-flowing, crystalline materials. The fumarate salts of structurally analogous tryptamines are commonly reported, possibly due to their ease of synthesis.36 DMT (2) fumarate, for example, has been previously used in clinical studies as an intravenous injection.37 Fumaric acid is, however, a known Michael acceptor and has been shown to form covalent products with amine-containing APIs under mild conditions.38,39 Given that terminal sterilization by an autoclave may be required in the future preparation of sterile solutions of the 5-MeO-DMT drug product, the potential for this known reactivity with fumaric acid eliminated it as an acceptable salt form. Succinic acid is a structurally similar dicarboxylic acid but lacks the conjugated double bond present in fumaric acid and would not exhibit similar chemical reactivity.
The succinate salt was therefore explored further as a potential pharmaceutically acceptable salt form. The material was prepared and subjected to thorough solid-state characterization, including equilibrium water solubility, X-ray powder diffraction (XRPD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), hyper-DSC, dynamic vapor sorption (DVS), 1H nuclear magnetic resonance (NMR), and optical microscopy (see the Supporting Information). Briefly, 5-MeO-DMT succinate (1:1) was not hygroscopic and XRPD indicated that multiple crystallization conditions resulted in a common stable crystalline anhydrate (form A) with only a few conditions that formed unique solvated forms (see the Supporting Information). The data supported the use of 5-MeO-DMT succinate (1:1) as a stable and pharmaceutically acceptable salt form. Given its ease of synthesis and favorable solid-state properties, this salt form was selected for further development.
5-MeO-DMT Route Scouting
For clinical development, the ideal synthetic route to 5-MeO-DMT would utilize commercially available starting materials, would be scalable to readily provide the product in the range of 0.1–1 kg, would not rely on flash silica gel chromatography or fractionation, and would provide a high-purity final product with no unidentified individual impurity >0.15% peak area by a validated high-performance liquid chromatography (HPLC) method. The literature survey revealed three potentially viable synthetic routes, and each was explored and evaluated for the ability to meet the above criteria.
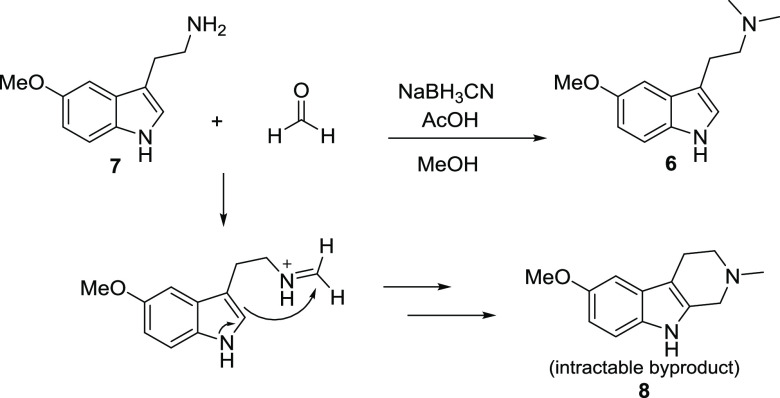
Route 1
A seemingly attractive single-step process employed a modified Eschweiler–Clarke reaction via reductive amination between formaldehyde and commercially available 5-methoxy tryptamine (7) with sodium cyanoborohydride as the reducing agent (Scheme 1).40 Several small-scale attempts were initially evaluated with reaction monitoring by liquid chromatography-mass spectrometry (LCMS). Though product formation was evident, the reaction was plagued by challenges that would likely multiply at larger scales. The Pictet–Spengler reaction to the corresponding tryptoline (8) was difficult to suppress and removal of this structurally similar and possibly biologically active byproduct was challenging. Further optimization to Route 1 may be possible, but ultimately, the reaction was not recommended for further development. A related reaction involving N-methylation of tryptamine 7 by methyl iodide has also been suggested; however, this approach would inevitably lead to difficult-to-control quaternization at the amine and was therefore also not considered for large-scale synthesis.
Scheme 1. Eschweiler–Clarke Reaction to 6 and Mechanism of Pictet–Spengler Byproduct Formation.
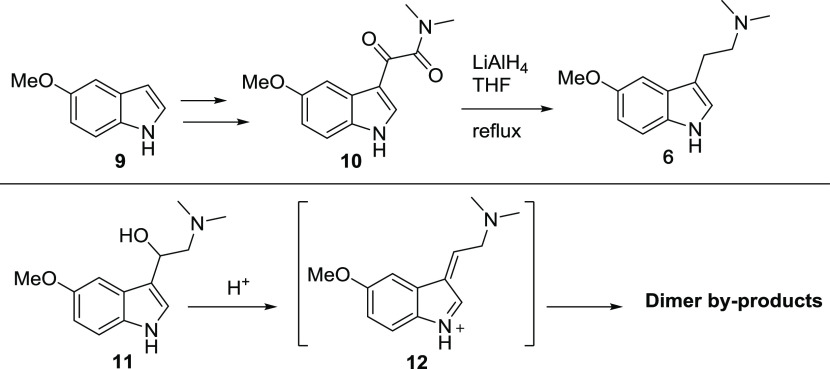
Route 2
The Speeter–Anthony tryptamine synthesis (Scheme 2) is the most cited general method for preparing substituted psychedelic tryptamines and has also been used to prepare 5-MeO-DMT previously.31,41 Given recent learnings and optimizations from the large-scale synthesis of psilocin and psilocybin produced by an analogous process, the route was considered for the large-scale synthesis of 6 from 5-methoxyindole 9.42−44 A key consideration in this approach is performing the final reduction on the ketoamide 10 with pyrophoric lithium aluminum hydride (LAH) with the subsequent quench and tedious extraction from solid aluminum waste salts; the difficulty of this process tends to increase with scale. Our data has indicated that in most cases when synthesizing tryptamines, the reduction step will stall at approximately 90% conversion with 5–10% of an expected β-hydroxy intermediate, such as 11, remaining (Scheme 2). On workup, further manipulations of the crude freebase, especially acidic conditions, can initiate conversion of the β-hydroxy impurity to a reactive electrophile, such as 12 (Scheme 2), and give mixtures of isomeric dimerized impurities. Crookes et al. provided a thorough investigation into the formation of analogous dimeric byproducts in the LAH reduction to produce DMT (2) by the mechanism analogous to the depiction in Scheme 2.45 Though Route 2 was a viable process, given the known challenges with scale-up, this route would require additional process development to ensure that the final product could reliably meet high-purity specifications without relying on column chromatography. Therefore, a single-step procedure based on the Fischer indole reaction was next explored.
Scheme 2. Speeter–Anthony Tryptamine Synthesis and Byproduct Formation via Reactive Impurity 11.
Route 3
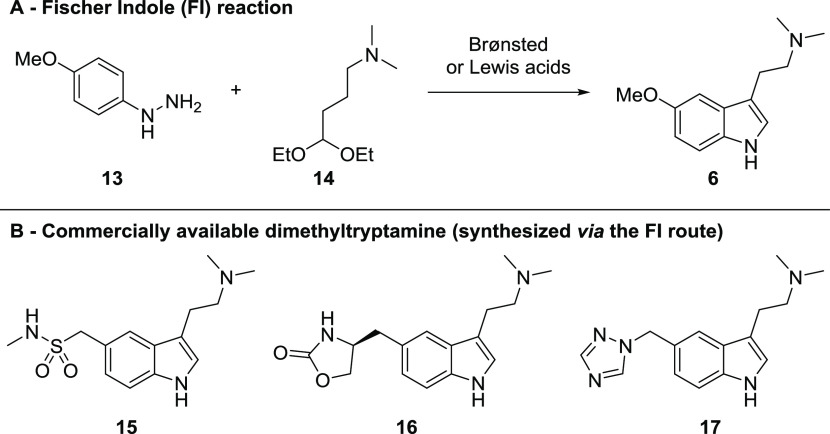
Several attributes inherent to the Fischer indole reaction approach to 6 from 4-methoxyphenylhydrazine (13) and 4,4-diethoxy-N,N-dimethylbutan-1-amine (14), a masked aldehyde protected as the diethyl acetal derivative (Scheme 3A), were attractive for the development of a scalable process: the transformation occurs in a single step, it does not rely on high temperatures, occurs in aqueous solvent, and does not rely on air-sensitive or pyrophoric reactants such as lithium aluminum hydride. Additionally, literature precedent exists for its use specifically in the synthesis of 5-MeO-DMT in addition to related substituted N,N-dimethyltryptamines,46 with reported examples for the use of an analogous process in the commercial manufacture of structurally similar 5-substituted dimethyltryptamine antimigraine medicines, such as sumatriptan (15), zolmitriptan (16), and rizatriptan (17) (Scheme 3B).47 Importantly, the pharmaceutical relevance of tryptamines 15–17 provided some assurance that the key butanamine starting material 14 common to all three processes was well-characterized and would remain commercially available and inexpensive.
Scheme 3. (A) Fischer Indole Reaction in the preparation of 6 and (B) Approved Antimigraine Medications Prepared by the Analogous Process.
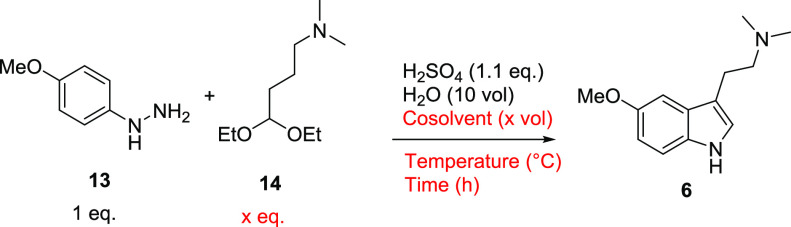
As per the previously published protocol, the reaction was first conducted in refluxing dilute aqueous sulfuric acid solution (Table 1, entry 1).46 Reaction monitoring by LCMS indicated that the phenylhydrazine limiting reagent 13 was consumed within 2 h with a crude reaction purity of about 63% peak area, including several high-molecular-weight impurities representing the remaining 37% peak area. With the significant impurity profile, the reaction would have likely required chromatography to isolate the product of sufficient purity. Serendipitously, we observed that an aliquoted LCMS sample removed prior to reflux prepared in acetonitrile instead of water proceeded to near completion at or below room temperature and contained almost exclusively 6 with few byproducts. Following this observation, an experiment was repeated using 1:1 water/acetonitrile as the solvent system at room temperature overnight to confirm 88% conversion to the product by LCMS (Table 1, entry 2). Based on the encouraging results, the process was repeated, and additional conditions were explored.
Table 1. Reaction Optimization Conditions.
| entry | equiv 14 (x) | cosolvent, (vol) | time (h) | temp. (°C) | conversion (area %)a |
|---|---|---|---|---|---|
| 1 | 1.2 | (0) | 2 | 100 | 63 |
| 2 | 1.2 | MeCN, (10) | 19 | 22 | 88 |
| 3 | 1.2 | MeCN, (10) | 3 | 40 | 90 |
| 4 | 1.2 | MeOH, (10) | 3 | 40 | 84 |
| 5 | 1.2 | DMSO, (10) | 3 | 40 | 87 |
| 6 | 1.2 | MeTHF, (10) | 3 | 40 | 79 |
| 7 | 1.2 | DCM, (10) | 3 | 40 | 77 |
| 8 | 1.2 | H2O, (10)b | 3 | 40 | 66 |
| 9 | 1.05 | MeCN, (5) | 3 | 35 | 90 |
| 9b | 28 | 35 | 89 | ||
| 10 | 1.05 | MeCN, (5) | 3 | 35 | 90 (80)c |
UPLC-UV percent area at 269 nm.
Total water was 20 vol.
Isolated yield.
Raising the temperature to 40 °C, the reaction was found to reach completion within 3 h with acetonitrile cosolvent (Table 1, entry 3). To better understand the role of the cosolvent, several additional reactions were trialed with different cosolvents, including methanol, dimethyl sulfoxide (DMSO), 2-methyltetrahydrofuran (2-MeTHF), and dichloromethane (DCM) (Table 1, entries 4–7) compared to the same volume of only water under otherwise identical conditions (Table 1, entry 8). The results indicated that all cosolvents tested were advantageous in increasing reaction conversion, with water-miscible polar aprotic DMSO providing results comparable to that of acetonitrile. Methanol also exhibited a significant enhancing effect on the reaction. The water-immiscible solvents 2-MeTHF and DCM also moderately improved reaction conversion. These data indicated that most cosolvents improved the conversion and purity profile of the reaction, and the water-miscible polar aprotic cosolvents demonstrated a significant rate-enhancing effect and minimized side reactions in the formation of 6. Though both reactants 13 and 14 appeared to be water soluble in the absence of cosolvent, we hypothesized that the addition of cosolvent possibly assisted in solubilizing either reactant or prevented the formation of hydrophobic clusters. The hypothesis is supported by the observation that in the absence of cosolvent, the major side reaction impurities formed were indicative of high-molecular-weight oligomers, which could potentially form from localized high-concentration clusters of starting reactants. Though DMSO and acetonitrile performed comparably, acetonitrile was selected for further development as the high-boiling point and low volatility of DMSO may have introduced additional complexity by its eventual removal in the workup.
Further optimization revealed that diethyl acetal 14 could be reduced to 1.05 equiv relative to limiting reagent 13. Acetonitrile cosolvent was reduced from 10 to 5 vol, and the temperature was reduced to 35 °C without measurable impact on the crude reaction profile or reaction rate (Table 1, entry 9). Further, stressing the same reaction with an extended 28-h hold time had only a slight impact on the reaction profile with an overall 1% reduction in a HPLC purity of the crude reaction mixture (Table 1, entry 9b). The reaction’s indifference to extended hold times was advantageous and suggested that reaction time was not a critical process parameter and could allow for some flexibility with timing when running the process at scale. Based on the optimizations described, the process was scaled to 100 mmol (∼35 g) and isolation conditions were explored to ultimately provide high-purity 6 as the succinate salt in 80% isolated yield (Table 1, entry 9).
Optimization of Workup, Isolation, and Salt Formation
Workup
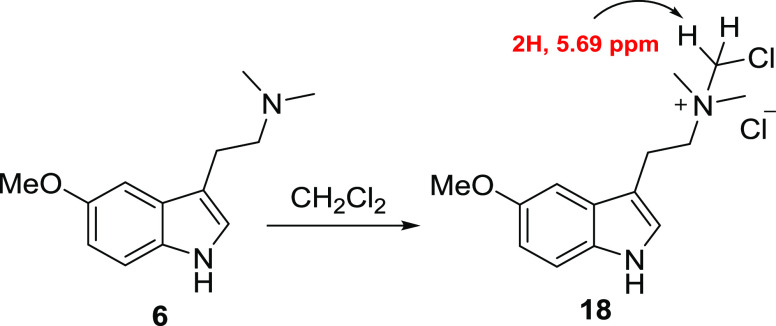
Crude freebase product was initially isolated by a routine acid/base workup procedure employing dichloromethane as both a washing solvent for the acidic crude reaction mixture and, upon basification, an extraction solvent for the freebase as well. On larger-scale reactions where extended hold times of the freebase product in methylene chloride were required, formation of a heavy insoluble oil impurity was encountered. Consistent with several literature reports on the chemical reactivity of DMT and other tertiary amines with methylene chloride,48−52 5-MeO-DMT was suspected to have undergone a similar reaction to form the quaternary ammonium byproduct 18 (Scheme 4). The crude heavy oil was analyzed by 1H NMR, which provided a singlet at 5.69 ppm that integrated to 2H; these data were consistent with the identity of structure 18 (Scheme 4 and Supporting Information S14). The apparent reactivity between product 6 and dichloromethane indicated that an alternative solvent should be used in the workup process, especially at larger scales where extended hold times may be required.
Scheme 4. Formation of Degradant 18 Annotated With 1H NMR Shift for the Suspected Dichloromethane Adduct.
2-Methyltetrahydrofuran (2-MeTHF) has been previously suggested as a good substitute for dichloromethane in biphasic aqueous workups.53 We found that freebase 6 was highly soluble, and 2-MeTHF formed a clean phase split with the acidic aqueous crude reaction mixture without the need for distillation of the acetonitrile cosolvent. Additionally, 2-MeTHF represented a greener solvent choice for process chemistry, as it is produced industrially by biorenewable processes. On smaller scales, the acetonitrile cosolvent was distilled prior to workup. On larger scales, this distillation was avoided and the workup proceeded directly into a liquid–liquid washing step. Subsequent data would indicate that some product loss occurred in the first washing step by being extracted into the organic phase, possibly related to increased partitioning due to the acetonitrile present.
Freebase Purification
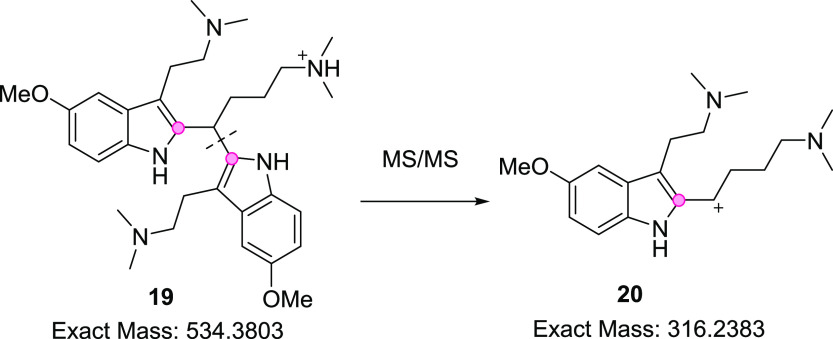
Analysis of the crude freebase extract by LC–UV–high-resolution mass spectrometry (HRMS) revealed the presence of several isomeric dimer-like products representing approximately 8% combined peak area for the crude reaction mixture. HRMS analysis provided m/z 534.3803 with MS/MS fragmentation to m/z 316.2383 for each of the isomers, supporting the putative structure 19 (Scheme 5 and Supporting Information S15), although different attachment points (denoted by red circles) for the dimer are also possible. Regardless of connectivity, the HRMS data supported the identity of a triamine for the isometric impurities corresponding to m/z 534.3803. Though ethanol was initially identified as a suitable recrystallization solvent for the succinate salt of 6, the isomeric dimers were found to co-crystallize with 6 at levels that exceeded impurity specifications. Alternatively, we speculated that a significant differential in retention would exist between monoamine 6 and triamine isomers of 19 on silica gel, such that a filtration through a small silica plug would be sufficient to remove the polar impurities while allowing the product 6 to readily elute. Mobile phase screening experiments with thin-layer chromatography revealed that 10% methanol in acetone provided such separation, with polar dimer impurities remaining adhered to the baseline and migration of the product spot for 6 with a retention factor (Rf) of about 0.3 (Supporting Information S16). While methanol/acetone is an atypical eluent with silica gel, dichloromethane, which is commonly used in separations with polar amines, was unacceptable given the reactivity concerns outlined above. On the preparative scale, filtration through a 5 wt % silica pad and washing the pad with 100 vol of 10% methanol in acetone was sufficient to recover 80–90% mass of the input crude freebase, while the polar dimeric impurities remained adhered to the baseline and were effectively removed.
Scheme 5. Putative Dimer Impurity Structure and MS/MS Fragmentation.
Red circles indicate alternate attachment points.
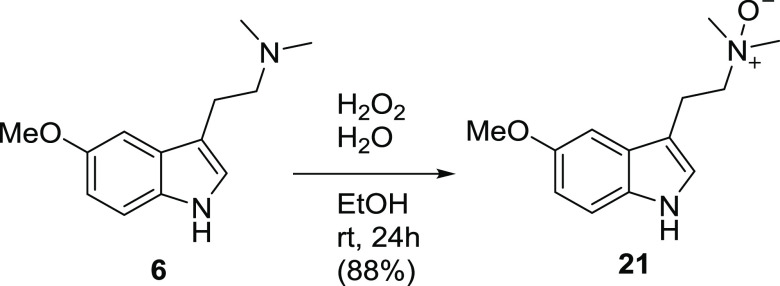
Following the silica filtration step during concentration of the resulting eluent, a previously unobserved degradant appeared in up to 3% peak area by HPLC. The degradant was conclusively identified as oxidation degradant 21, the N-oxide of 6. The structure was supported by HRMS initially (Supporting Information S17) and later chemical synthesis with additional characterization by 1H and 13C NMR (Scheme 6 and Supporting Information S18 and S19) conclusively characterized 21. Previous in vitro and in vivo metabolism data has indicated that N-oxide 21 is a metabolite of 6(54) and would therefore afford some flexibility in the allowable levels of this degradant in the API.
Scheme 6. Synthesis of N-Oxide 21.
Succinate Salt Formation
With smaller-scale development reactions, the succinic acid salt of 6 was readily isolated by adding 1 equiv of succinic acid to a solution of freebase 6 in acetone and collecting the resulting insoluble crystalline precipitate by filtration. We later found that the inclusion of a washing step using activated charcoal helped to minimize slight variability in color observed in the final isolated product. The color variation was found to be correlated with the use of different commercial sources of phenylhydrazine 13, even though all lots were tested upon receipt to >98% purity. The procedure was modified to form the succinate salt in a solution of methanol at a volume that did not initially induce precipitation. The resulting solution was stirred with activated charcoal, filtered, and then concentrated. The resulting solid succinate salt was slurried in acetone, filtered, and dried to provide a crystalline solid consistent with the desired polymorphic form. The process provided a net yield of 49% to produce 136 g of isolated succinic acid salt of 6 with HPLC purity of 99.86% peak area. The identified N-oxide degradant 21 was the only detectable impurity at 0.14% peak area. Though not reported in the larger-scale synthesis, as the required purity specifications were met, following salt formation, ethanol was found to perform well as a recrystallization solvent for further purification of the succinate salt of 6 if necessary.
Future Optimization
The Fischer indole reaction to 6 readily provided API that met all set specifications. Achieving a high-purity product was the initial focus, and further optimization could improve the final yield without compromising final product purity. HPLC data indicated that product conversion was as high as 90%, yet isolated freebase recovery was 57%. Additionally, in the smaller-scale development reaction (Table 1, entry 9), an isolated yield of 80% was achieved. The key difference between the two processes was the distillation of the cosolvent prior to workup and much of the yield loss that occurred at the first liquid–liquid washing step, where approximately 10–20% of the product was extracted from the acidic aqueous layer in the first wash. In the future, the washes could potentially be back-extracted to recover this loss. With further scale-up, the elimination of the silica pad filtration step would be desirable. Vacuum distillation of the crude freebase could be an acceptable alternative for the separation of the freebase product from high-MW dimers such as 19. Though dimer impurities present in succinate salt were not readily purged by recrystallization approaches, exploration of the recrystallization of alternate salt forms prior to generation of the succinate salt may also circumvent the silica pad filtration. As an alternative to purification approaches, additional optimization of reaction conditions could be explored to further improve the specificity of the reaction toward formation of 6 and minimize side reactions.
Conclusions
The first production run has provided sufficient API to meet current clinical and nonclinical needs to enable first-in-human clinical trials with 6. The key features of the developed process were an optimized Fischer indole reaction with advantageous inclusion of acetonitrile cosolvent to provide crude freebase 6. The workup featured greener solvent choices with an intermediate purification via filtration through a silica pad. The 1:1 succinic acid salt was subsequently prepared from methanol with an activated charcoal decolorizing step followed by final purification by acetone slurry. A minor API degradation product, the corresponding N-oxide 21, was identified, synthesized, and characterized. The final product was isolated in 49% overall yield to provide 136 g of API with 99.86% HPLC purity. The controllability and scalability inherent to the developed process will ensure that current and future clinical demands for 6 are met.
Experimental Section
General Experimental Methods
Reactions were performed using commercially obtained raw materials and solvents. Unless otherwise stated, all commercially obtained reagents were identity tested and used as received. Reactions were conducted in a Borosilicate Glass 3.3 jacketed glass reactor (5 L) with a Julabo FPW91-SL Ultra-Low Refrigerated-Heating circulator for temperature control. Distillations (>5 L) were performed with a Buchi Rotavapor R-220 Pro. Reactions were monitored by thin-layer chromatography (TLC) using EMD/Merck silica gel 60 F254-precoated plates (0.25 mm). Flash column chromatography was performed using prepackaged RediSepRf columns on a CombiFlash Rf system (Teledyne ISCO Inc.). 1H and 13C NMR spectra were recorded on a Bruker Avance 400 (at 400 and 101 MHz, respectively) and a Bruker Avance 500 (at 500 and 126 MHz, respectively). Process development and reaction monitoring was performed with a Waters Acquity I-Class UPLC utilizing a Waters HSS T3 column (2.5 μm, 2.1 mm × 30 mm) run in gradient mode with H2O (0.1% formic acid) and acetonitrile (0.1% formic acid) mobile phases at 0.6 mL/min. Samples were diluted in acetonitrile or water to approximately 1 mg/mL and 0.1 μL was injected. Chromatographic peaks were detected by a diode array detector at 269 nm. High-resolution mass spectra were acquired in line with UV on a Waters Xevo G2-XS QTof in ESI-positive mode. Low and high collision energy mass spectra were acquired using a Waters MSe experiment.
2-(5-Methoxy-1H-indol-3-yl)-N,N-dimethylethanamine (6-freebase)
To a clean and dry 5 L reactor was charged 4-methoxyphenylhydrazine hydrochloride (145.0 g; 0.83 mol, 1.0 equiv, purity >98% confirmed by HPLC) followed by water (1.45 L, 10 vol) under a nitrogen atmosphere at 20–25 °C. The contents of the reactor were then stirred at 30–35 °C and a dark red colored suspension was observed. To the suspension, concentrated H2SO4 (47.7 mL, 0.91 mol, 1.1 equiv) was cautiously added dropwise under a nitrogen atmosphere over 10 min while maintaining the temperature below 40 °C. (Note: This addition is slightly exothermic.) The brown/red solution was heated to 35–40 °C (with a target temperature of 37 °C) and stirred for an additional 10 min. A solution of 4,4-diethoxy-N,N-dimethylbutan-1-amine (14) (165.0 g, 0.87 mol, 1.05 equiv) was prepared in acetonitrile (0.58 L, 4.0 vol) and added dropwise to the reactor under a nitrogen atmosphere over approximatively 60 min while maintaining the temperature between 35 and 40 °C. The addition funnel was rinsed with acetonitrile (145 mL, 1.0 vol) and added dropwise to the reactor. The temperature was maintained at 40 °C and the contents were agitated for an additional 4 h. A sample of the reaction mixture was aliquoted for HPLC analysis and reaction completion with a target limit of ≤2% peak area for the limiting reagent. (Result: 4-Methoxyphenylhydrazine: 1.86% area.) The mixture was cooled to 20–25 °C and the contents were transferred to a 10 L reactor. The acidic aqueous solution was washed with 2-MeTHF (2 × 2.03 L, 14.0 vol). After each wash, the layers were allowed to settle for 15 min. The lower acidic aqueous layer was collected and the upper 2-MeTHF wash was discarded. The acidic aqueous layer was recharged to the reactor and sodium hydroxide solution (4 M, 0.65 L, 4.5 vol) was added dropwise while maintaining the temperature at 20–25 °C to bring the pH to 11–12 providing a milky suspension. The suspension was extracted with 2-MeTHF (3 × 1.45 L, 10.0 vol); following each extraction, the layers were allowed to settle for 15 min, the lower alkaline water layer was separated into a drum, and the upper organic layer was collected. The lower aqueous layer was discarded and the combined 2-MeTHF organic layers were transferred to a 20 L-flask. The solution was concentrated in vacuo to an oily amber residue. Residual water was removed azeotropically by redissolving the residue with fresh 2-MeTHF (1.45 L, 10 vol) and repeating the concentration step. This oily residue was dried on the rotatory evaporator under vacuum (10–20 mbar) for 1 h at 40–45 °C to provide 117.68 g (64.9% theoretical yield) of crude 5-MeO-DMT freebase. The crude freebase was dissolved in acetone (1.45 L, 10.0 vol) and poured through a pad of silica (230–400 mesh, 725 g, 5 wt). The pad was eluted with acetone/MeOH (9:1, v-v, 14.5 L, 100.0 vol). The combined filtrates were concentrated to provide 102.94 g of purified 5-MeO-DMT freebase (56.8% yield, 98.27% area by HPLC) as a pale clear orange oil that slowly solidified on standing.
2-(5-Methoxy-1H-indol-3-yl)-N,N-dimethylethanamine (6-succinate (1:1))
To the 20 L-flask containing purified 5-MeO-DMT freebase from the previous step (101.1 g, 0.46 mol, 1.0 equiv) was charged fresh MeOH (1.01 L, 10.0 vol). The flask was attached to a rotary evaporator and rotation was started without applying vacuum until the material dissolved. The methanolic solution was then transferred to a 5 L-RBF fitted with an overhead mechanical stirrer. Additional MeOH (2.02 L, 20.0 vol) was charged to the RBF, under a nitrogen atmosphere, at 20–25 °C. Succinic acid (57.4 g, 0.48 mol, 1.05 equiv) was added portion wise and the solution was stirred at 20–25 °C for 48 h under a nitrogen atmosphere. Charcoal (NORIT SX1, 31.2 g, ∼20% w/w) was charged to the flask, under a nitrogen atmosphere, at 20–25 °C. The resulting dark suspension was stirred at 20–25 °C for 2.5 h under a nitrogen atmosphere and then filtered on a Celite pad. The Celite pad was rinsed with additional MeOH (3.03 L, 30.0 vol). The collected filtrate (5.05 L) was then concentrated under reduced pressure. Acetone was charged in portions to the rotatory evaporator containing the solid 5-MeO-DMT succinate salt and the solvent concentrated until no more distillate was observed to ensure that most of the residual MeOH had been distilled. Fresh acetone (505.5 mL, 5.0 vol) was added to the flask and the resulting suspension was slurried at ambient temperature for 1 h. The suspension was cooled to 0–5 °C on an ice bath and was filtered over a sintered funnel. The filter cake was washed with ice-cold acetone (2 × 101.1 mL, 1.0 vol) and the solids were pulled dry on the filter for approximately 30 min. The solid was dried in a vacuum oven at 40–45 °C to a constant weight to provide 136.0 g (86.0% yield, 48.8% overall yield, 99.86% area) of 5-MeO-DMT succinate salt (6). TG/DTA Melt onset: 140 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.66 (s, 1H), 7.22 (d, J = 9 Hz, 1H), 7.06 (d, J = 2 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.72 (dd, J = 9 Hz, 2 Hz, 1H), 3.76 (s, 3H), 2.85 (m, 2H), 2.77 (m, 2H), 2.42 (s, 6H), 2.34 (s, 4H); 13C NMR (126 MHz, DMSO-d6): δ 175.1, 153.5, 131.8, 127.8, 123.9, 112.5, 111.5, 111.2, 100.7, 58.8, 55.8, 44.1, 30.9, 22.2.
2-(5-Methoxy-1H-indol-3-yl)-N,N-dimethylethan-1-amine oxide (21)
Freebase 6 (500 mg, 2.3 mmol) was suspended in 30% w/w H2O2 (1.2 mL, 11.5 mmol, 5 equiv) and stirred. Ethanol (ca. 3 mL) was added dropwise to the suspension until a homogeneous solution was achieved. Stirring continued for 48 h whereupon thin-layer chromatography (100:10:1; CHCl3/MeOH/NH4OH) indicated complete conversion of the starting material to a new slightly more polar spot. Without concentration, the reaction mixture was applied directly to a preparative C18 column (130 g) and gradient eluted at 85 mL/min with MeOH and H2O, both containing 1% NH4OH. Collected fractions were combined and concentrated to provide the target compound as a yellow deliquescent solid, (470 mg, 88%). HRMS (ESI+): calcd for [C13H18N2O2] [M+H]+: 235.1441; found: 235.1426. 1H NMR (400 MHz, DMSO-d6): δ 11.29 (s, 1H), 7.24 (d, 1H, J = 8.7 Hz), 7.13 (s, 1H), 7.05 (1H, s), 6.71 (d, 1H, J = 8.7 Hz), 3.75 (s, 3H), 3.45–3.36 (m, 2H), 3.25–3.16 (m, 2H), 3.12 (s, 6H); 13C NMR (101 MHz, DMSO-d6): δ 153.0, 131.5, 127.4, 123.6, 112.1, 111.1, 109.9, 100.2, 69.9, 58.5, 55.4, 19.1.
Acknowledgments
The authors wish to thank William Linton for his vision and support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05099.
Certificate of analysis for 5-MeO-DMT succinate salt; solubility data; characterization data; polymorph screen summary and results; HPLC methodology and chromatograms; impurity identification and characterization (PDF)
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Garcia-Romeu A.; Kersgaard B.; Addy P. H. Clinical Applications of Hallucinogens: A Review. Exp. Clin. Psychopharmacol. 2016, 24, 229–268. 10.1037/pha0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A. M.; Prisinzano T. E. Novel Psychotherapeutics–a Cautiously Optimistic Focus on Hallucinogens. Expert Rev. Clin. Pharmacol. 2018, 11, 1–3. 10.1080/17512433.2018.1415755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; Bolstridge M.; Day C. M. J.; Rucker J.; Watts R.; Erritzoe D. E.; Kaelen M.; Giribaldi B.; Bloomfield M.; Pilling S.; Rickard J. A.; Forbes B.; Feilding A.; Taylor D.; Curran H. V.; Nutt D. J. Psilocybin with Psychological Support for Treatment-Resistant Depression: Six-Month Follow-Up. Psychopharmacology 2018, 235, 399–408. 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; Cosimano M. P.; Klinedinst M. A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ot’alora G M.; Grigsby J.; Poulter B.; Van Derveer J. W.; Giron S. G.; Jerome L.; Feduccia A. A.; Hamilton S.; Yazar-Klosinski B.; Emerson A.; Mithoefer M. C.; Doblin R. 3,4-Methylenedioxymethamphetamine-Assisted Psychotherapy for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Phase 2 Controlled Trial. J. Psychopharmacol. 2018, 32, 1295–1307. 10.1177/0269881118806297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.; Bossis A.; Guss J.; Agin-Liebes G.; Malone T.; Cohen B.; Mennenga S. E.; Belser A.; Kalliontzi K.; Babb J.; Su Z.; Corby P.; Schmidt B. L. Rapid and Sustained Symptom Reduction Following Psilocybin Treatment for Anxiety and Depression in Patients with Life-Threatening Cancer: A Randomized Controlled Trial. J. Psychopharmacol. 2016, 30, 1165–1180. 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aday J. S.; Mitzkovitz C. M.; Bloesch E. K.; Davoli C. C.; Davis A. K. Long-Term Effects of Psychedelic Drugs: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 113, 179–189. 10.1016/j.neubiorev.2020.03.017. [DOI] [PubMed] [Google Scholar]
- Barsuglia J.; Davis A. K.; Palmer R.; Lancelotta R.; Windham-Herman A. M.; Peterson K.; Polanco M.; Grant R.; Griffiths R. R. Intensity of Mystical Experiences Occasioned by 5-MeO-DMT and Comparison with a Prior Psilocybin Study. Front. Psychol. 2018, 9, 2459 10.3389/fpsyg.2018.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. K.; So S.; Lancelotta R.; Barsuglia J. P.; Griffiths R. R. 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) Used in a Naturalistic Group Setting Is Associated with Unintended Improvements in Depression and Anxiety. Am. J. Drug Alcohol Abuse 2019, 45, 161–169. 10.1080/00952990.2018.1545024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; McCann U.; Jesse R. Psilocybin Occasioned Mystical-Type Experiences: Immediate and Persisting Dose-Related Effects. Psychopharmacology 2011, 218, 649–665. 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil A. T.; Davis W. Bufo alvarius: A Potent Hallucinogen of Animal Origin. J. Ethnopharmacol. 1994, 41, 1–8. 10.1016/0378-8741(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Agurell S.; Holmstedt B.; Lindgren J. E.; Schultes R. E.; et al. Alkaloids in Certain Species of Virola and Other South American Plants of Ethnopharmacologic Interest. Acta Chem. Scand. 1969, 23, 903–916. 10.3891/acta.chem.scand.23-0903. [DOI] [PubMed] [Google Scholar]
- Mckenna D. J.; Towers G. H. N.; Abbott F. S. Monoamine Oxidase Inhibitors in South American Hallucinogenic Plants Part 2: Constituents of Orally-Active Myristicaceous hallucinogens. J. Ethnopharmacol. 1984, 12, 179–211. 10.1016/0378-8741(84)90048-5. [DOI] [PubMed] [Google Scholar]
- Schultes R. E. Fifteen Years of Study of Psychoactive Snuffs of South America: 1967-1982- a Review. J. Ethnopharmacol. 1984, 11, 17–32. 10.1016/0378-8741(84)90093-X. [DOI] [PubMed] [Google Scholar]
- Torres C. M.; Repke D. B.. Anadenanthera: Visionary Plant of Ancient South America; Haworth Herbal Press: New York, 2014. [Google Scholar]
- Davis A. K.; Barsuglia J. P.; Lancelotta R.; Grant R. M.; Renn E. The Epidemiology of 5-Methoxy-N, N-Dimethyltryptamine (5-MeO-DMT) Use: Benefits, Consequences, Patterns of Use, Subjective Effects, and Reasons for Consumption. J. Psychopharmacol. 2018, 32, 779–792. 10.1177/0269881118769063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaug M. V.; Lancelotta R.; van Oorsouw K.; Kuypers K. P. C.; Mason N.; Rak J.; Šuláková A.; Jurok R.; Maryška M.; Kuchař M.; Páleníček T.; Riba J.; Ramaekers J. G. A Single Inhalation of Vapor from Dried Toad Secretion Containing 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) in a Naturalistic Setting Is Related to Sustained Enhancement of Satisfaction with Life, Mindfulness-Related Capacities, and a Decrement of Psyc. Psychopharmacology 2019, 236, 2653–2666. 10.1007/s00213-019-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R.The Toad and the Jaguar: A Field Report of Underground Research on a Visionary Medicine: Bufo alvarius and 5-Methoxy-Dimethyltryptamine, 1st ed.; Regent Press: Berkeley, CA, 2013. [Google Scholar]
- Uthaug M. V.; Lancelotta R.; Ortiz Bernal A. M.; Davis A. K.; Ramaekers J. G. A Comparison of Reactivation Experiences Following Vaporization and Intramuscular Injection (IM) of Synthetic 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) in a Naturalistic Setting. J. Psychedelic Stud. 2020, 4, 104–113. 10.1556/2054.2020.00123. [DOI] [Google Scholar]
- Sepeda N. D.; Clifton J. M.; Doyle L. Y.; Lancelotta R.; Griffiths R. R.; Davis A. K. Inhaled 5-Methoxy-N,N-Dimethyltryptamine: Supportive Context Associated with Positive Acute and Enduring Effects. J. Psychedelic Stud. 2019, 4, 114–122. 10.1556/2054.2019.033. [DOI] [Google Scholar]
- Lancelotta R. L.; Davis A. K. Use of Benefit Enhancement Strategies among 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) Users: Associations with Mystical, Challenging, and Enduring Effects. J. Psychoact. Drugs 2020, 52, 273–281. 10.1080/02791072.2020.1737763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364–381. 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Nichols D. E.; Geyer M. A. Behavioral Effects of α,α,β,β-Tetradeutero-5-MeO-DMT in Rats: Comparison with 5-MeO-DMT Administered in Combination with a Monoamine Oxidase Inhibitor. Psychopharmacology 2012, 221, 709–718. 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Thomson K.; Ruiz E. M.; Masten V.; Buell M.; Geyer M. A. The Roles of 5-HT1A and 5-HT2 Receptors in the Effects of 5-MeO-DMT on Locomotor Activity and Prepulse Inhibition in Rats. Psychopharmacology 2006, 189, 319–329. 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K.; Geyer M. A. Evidence for a Functional Interaction between 5-HT(1A) and 5-HT2 Receptors in Rats. Psychopharmacology 1998, 140, 69–74. 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Krebs K. M.; Geyer M. A. Cross-Tolerance Studies of Serotonin Receptors Involved in Behavioral Effects of LSD in Rats. Psychopharmacology 1994, 113, 429–437. 10.1007/BF02245219. [DOI] [PubMed] [Google Scholar]
- Data from the NIMH Psychoactive Drug Screening Program.
- Pokorny T.; Preller K. H.; Kraehenmann R.; Vollenweider F. X. Modulatory Effect of the 5-HT1A Agonist Buspirone and the Mixed Non-Hallucinogenic 5-HT1A/2A Agonist Ergotamine on Psilocybin-Induced Psychedelic Experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Shulgin A.; Shulgin A.. TIHKAL: The Continuation; Transform Press: Berkeley, CA, 1997. [Google Scholar]
- Ly C.; Greb A. C.; Cameron L. P.; Wong J. M.; Barragan E. V.; Wilson P. C.; Burbach K. F.; Soltanzadeh Zarandi S.; Sood A.; Paddy M. R.; Duim W. C.; Dennis M. Y.; McAllister A. K.; Ori-McKenney K. M.; Gray J. A.; Olson D. E. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. 10.1016/j.celrep.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.; Erritzoe D.; Carhart-Harris R. Psychedelic Psychiatry’s Brave New World. Cell 2020, 181, 24–28. 10.1016/j.cell.2020.03.020. [DOI] [PubMed] [Google Scholar]
- Davis A. K.; Barrett F. S.; May D. G.; Cosimano M. P.; Sepeda N. D.; Johnson M. W.; Finan P. H.; Griffiths R. R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder A Randomized Clinical Trial. JAMA Psychiatry 2020, 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D.; Bhatia D.; Dave V.; Sutariya V.; Gupta S. V. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719 10.3390/molecules23071719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeayne A. R.; Golen J. A.; Manke D. R. Bis(4-Acetoxy- N, N -Dimethyltryptammonium) Fumarate: A New Crystalline Form of Psilacetin, an Alternative to Psilocybin as a Psilocin Prodrug. Acta Crystallogr., Sect. E 2019, 75, 900–902. 10.1107/S2056989019007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman R. J.; Qualls C. R.; Berg L. M. Differential Tolerance to Biological and Subjective Effects of Four Closely Spaced Doses of N,N-Dimethyltryptamine in Humans. Biol. Psychiatry 1996, 39, 784–795. 10.1016/0006-3223(95)00200-6. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J. W.; Wikström H.; Andersson T.; Norrby P. O. Degradation Caused by Incompatibility between Sodium Stearyl Fumarate (PRUV) and AZD7986 in the Drug Product. J. Pharm. Biomed. Anal. 2018, 158, 82–87. 10.1016/j.jpba.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Do J.; Kang J.; Lee Y.; Ok K. M.; Jacobson A. J. Copper(II) Complexes with N-Substituted Aspartic Acids: A New One-Pot Synthesis Method via in Situ Michael Addition of Amines to Fumaric Acid. Inorg. Chim. Acta 2015, 430, 280–287. 10.1016/j.ica.2015.03.020. [DOI] [Google Scholar]
- Brandt S. D.; Moore S. A.; Freeman S.; Kanu A. B. Characterization of the Synthesis of N,N-Dimethyltryptamine by Reductive Amination Using Gas Chromatography Ion Trap Mass Spectrometry. Drug Test. Anal. 2010, 2, 330–338. 10.1002/dta.142. [DOI] [PubMed] [Google Scholar]
- Speeter M. E.; Anthony W. C. The Action of Oxalyl Chloride on Indoles: A New Approach to Tryptamines. J. Am. Chem. Soc. 1954, 76, 6208–6210. 10.1021/ja01652a113. [DOI] [Google Scholar]
- Kargbo R. B.; Sherwood A.; Walker A.; Cozzi N. V.; Dagger R. E.; Sable J.; O’Hern K.; Kaylo K.; Patterson T.; Tarpley G.; Meisenheimer P. Direct Phosphorylation of Psilocin Enables Optimized CGMP Kilogram-Scale Manufacture of Psilocybin. ACS Omega 2020, 5, 16959–16966. 10.1021/acsomega.0c02387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota O.; Hakamata W.; Goda Y. Concise Large-Scale Synthesis of Psilocin and Psilocybin, Principal Hallucinogenic Constituents of “Magic Mushroom. J. Nat. Prod. 2003, 66, 885–887. 10.1021/np030059u. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin. Synthesis 1999, 1999, 935–938. 10.1055/s-1999-3490. [DOI] [Google Scholar]
- Crookes D. L.; Parry K. P.; Smith G. F. 2-(Indol-3′-Yl)-2-Hydroxy-N,N-Dimethylethylamine and 2-(Indol-3′-Yl)-2[3″-[2’’’-(N,N-Dimethylamino) Ethyl]Indol-2″-Yl]-N,N-Dimethylethylamine, by-Products in the LAH Reduction of 3-Indoleglyoxyl- N,N-Dimethylamide. Pol. J. Chem. 1979, 53, 73–78. [Google Scholar]
- Chen C.-y.; Senanayake C. H.; Bill T. J.; Larsen R. D.; Verhoeven T. R.; Reider P. J. Improved Fischer Indole Reaction for the Preparation of N,N-Dimethyltryptamines: Synthesis of L-695,894, a Potent 5-HT1D Receptor Agonist. J. Org. Chem. 1994, 59, 3738–3741. 10.1021/jo00092a046. [DOI] [Google Scholar]
- Baumann M.; Baxendale I. R.; Ley S. V.; Nikbin N. An Overview of the Key Routes to the Best Selling 5-Membered Ring Heterocyclic Pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. 10.3762/bjoc.7.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Martins C. P. B.; Freeman S.; Dempster N.; Riby P. G.; Gartz J.; Alder J. F. Halogenated Solvent Interactions with N,N-Dimethyltryptamine: Formation of Quaternary Ammonium Salts and Their Artificially Induced Rearrangements during Analysis. Forensic Sci. Int. 2008, 178, 162–170. 10.1016/j.forsciint.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Dunlap L. E.; Olson D. E. Reaction of N, N-Dimethyltryptamine with Dichloromethane under Common Experimental Conditions. ACS Omega 2018, 3, 4968–4973. 10.1021/acsomega.8b00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jaber-Vazdekis N.; Gutierrez-Nicolás F.; Ravelo Á. G.; Zárate R. Studies on Tropane Alkaloid Extraction by Volatile Organic Solvents: Dichloromethane vs. Chloroform. Phytochem. Anal. 2006, 17, 107–113. 10.1002/pca.893. [DOI] [PubMed] [Google Scholar]
- Brandt S. D.; Martins C. P. B.; Freeman S.; Dempster N.; Wainwright M.; Riby P. G.; Alder J. F. N,N-Dimethyltryptamine and Dichloromethane: Rearrangement of Quaternary Ammonium Salt Product during GC-EI and CI-MS-MS Analysis. J. Pharm. Biomed. Anal. 2008, 47, 207–212. 10.1016/j.jpba.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Nevstad G. O.; Songstad J.; Rodriguez B.; Mörch L.; Norin T. Solvent Properties of Dichloromethane. II. The Reactivity of Dichloromethane Toward Amines. Acta Chem. Scand. 1984, 38b, 469–477. 10.3891/acta.chem.scand.38b-0469. [DOI] [Google Scholar]
- Aycock D. F. Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions. Org. Process Res. Dev. 2007, 11, 156–159. 10.1021/op060155c. [DOI] [Google Scholar]
- Shen H.-W.; Jiang X.-L.; C. Winter J.; Yu A.-M. Psychedelic 5-Methoxy-N,N-Dimethyltryptamine: Metabolism, Pharmacokinetics, Drug Interactions, and Pharmacological Actions. Curr. Drug Metab. 2010, 11, 659–666. 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.