Abstract
Coronavirus disease 2019 (COVID-19) was discovered after unusual cases of severe pneumonia emerged in December 2019 in Wuhan Province (China). Coronavirus is a family of single-stranded RNA viruses. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is transmitted from person to person. Although asymptomatic individuals can transmit the virus, symptomatic patients are more contagious. The incubation period ranges from 3-7 d and symptoms are mainly respiratory, including pneumonia or pulmonary embolism in severe cases. Elevated serum levels of interleukins (IL)-2, IL-6, IL-7 indicate the presence of cytokine release syndrome, which is associated with disease severity. The disease has three main phases: Viral infection, pulmonary involvement, and hyperinflammation. To date, no treatment has proved to be safe or effective. Chest X-ray and computed tomography (CT) are the primary imaging tests for diagnosis of SARS-CoV-2 pneumonia, follow-up, and detection of complications. The main radiological findings are ground-glass opacification and areas of consolidation. The long-term clinical course is unknown, although some patients may develop pulmonary fibrosis. Positron emission tomography-computed tomography (PET-CT) is useful to assess pulmonary involvement, to define the affected areas, and to assess treatment response. The pathophysiology and clinical course of COVID-19 infection remain poorly understood. However, patterns detected on CT and PET-CT may help to diagnose and guide treatment. In this mini review, we analyze the clinical manifestations and radiological findings of COVID-19 infection.
Keywords: COVID-19, Coronavirus, Natural history, Clinical features, Pathogenesis, Radiology images
Core Tip: Coronavirus is a family of single-stranded RNA viruses transmitted from person to person. Asymptomatic individuals can transmit the virus, although symptomatic patients are more contagious. The incubation period is 3-7 d. Symptoms are mainly respiratory, including pneumonia or pulmonary embolism in severe cases. Blood tests typically show elevated levels of interleukins, associated with disease severity. The disease has three phases: Viral, pulmonary, and hyperinflammatory. To date, no treatment has proved safe or effective. Chest X-ray and computed tomography are the main imaging tests, used to diagnose pneumonia, for follow-up, and to detect complications. The most important radiological findings are ground-glass opacities and areas of consolidation. Some patients may develop pulmonary fibrosis. In patients with pulmonary involvement, positron emission tomography-computed tomography is useful to define the affected areas and to evaluate treatment response.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) was first discovered when unexplained cases of viral pneumonia began to emerge in Wuhan, China early December 2019[1,2]. The coronavirus belongs to the Coronaviridae family of single-stranded RNA viruses, which together with the Roniviridae and Arteriviridae families, comprise the Nidoviruses order. Coronavirus owes its name to the crown-like glycoprotein spikes on its surface, evident on examination with electron microscope[3].
Coronaviruses are found in various animal species, including birds, livestock, and other mammals such as camels, bats, mice, cats, and dogs[2]. Coronaviruses are widely distributed and highly infectious, making it a potent pathogen. Human pathogenic subtypes of coronavirus are associated with mild clinical symptoms; however, two notable exceptions are the coronaviruses associated with Middle East Respiratory Syndrome (MERS-CoV) and severe acute respiratory syndrome (SARS-CoV)[4].
The current outbreak began as pneumonia of unknown etiology in Wuhan, China. Laboratory studies identified the causal factor as a new strain of coronavirus[5], initially called 2019-nCoV; later the International Committee on Taxonomy of Viruses changed the name to SARS-CoV-2[4]. Subsequently, on 11 February 2020, the World Health Organization (WHO) denominated the disease caused by this new virus COVID-19. The ongoing outbreaks of coronavirus around the world represent an important public health threat. Moreover, due to changes in the world’s ecosystem and climate in recent decades, new outbreaks are more likely to occur in the future[5].
From December 30, 2019 to March 20, 2020, more than 200000 people were diagnosed with COVID-19 worldwide. Disease progression is rapid and some patients develop severe respiratory failure shortly after disease onset. Our understanding of COVID-19, including clinical manifestations, pathogenesis, and treatment comes mainly from recently-published research studies and clinical observation during the period of acute infection.
SARS-CoV-2 is primarily transmitted from person to person. Although asymptomatic individuals can transmit the virus[6], symptomatic individuals are the most common source of transmission, which occurs mainly through the propagation of respiratory droplets by means of coughing or sneezing[6]. The virus can be transmitted between individuals who are in close contact[7], with a greater risk in enclosed spaces due to high concentrations of aerosols[6]. Given that aerosol droplets generally fall to the ground within a few meters, there is a lower likelihood of transmission when people maintain a distance of at least 2 metres. SARS-CoV-2 has a basic reproduction factor of 2.2[6], meaning that one patient will, on average, infect an additional two people. Current data suggest that the incubation period for the virus ranges from three to seven days[8]. In the present mini review, we evaluate the clinical manifestations and imaging findings of COVID-19 infection.
NATURAL HISTORY OF COVID-19 DISEASE
Symptoms
The COVID-19 pandemic has numerous psychological, socioeconomic, and medical consequences. COVID-19 is among the most significant threats that society has had to confront in the current century to date. Therefore, it is crucial to understand the pathophysiology and clinical implications of this disease, as well as the development of novel preventive and therapeutic strategies.
Based on currently available data, males and older people have a higher risk of infection and mortality. The virus has a high capacity to enter and infect lung cells, where it causes interstitial pneumonitis. It can also affect other organs, potentially leading to multiorgan failure, in very severe cases, potentially involving the cardiovascular system.
Individual response to the virus is highly heterogenous, suggesting that COVID-19 should probably not be considered, phenotypically, a single disease. It is more probable that certain, still unknown characteristics of the host explain the variable course of disease, which can range from mild to severe, which may include cytokine release syndrome (CRS) and multiorgan failure.
The main clinical symptoms are respiratory, frequently associated with severe pneumonia. However other organs are commonly affected. The patient may present disseminated intravascular coagulation and/or pulmonary embolism, as evidenced by elevated levels of D-dimers and fibrin breakdown products[9,10].
COVID-19 primarily affects the lungs but can also involve the immune system. Pathologic examination of COVID-19 victims reveals the presence of splenic atrophy in which lymphocytes and neutrophils are significantly reduced, with the presence of necrosis and hemorrhage[11].
Blood tests reveal elevated levels of several ILs (IL-2, IL-6, IL-7), granulocyte colony-stimulating factor (G-CSF), chemokine 10, and tumor necrosis factor-α alpha (TNF-α), the presence of which is characteristic of cytokine release syndrome[12-14]. The development of CRS in COVID-19 is associated with disease severity, reminiscent of the cytokine profile observed in hemophagocytic lymphohistiocytosis[15]. Patients who develop CRS frequently present high levels of serum IL-6. In fact, a retrospective, multi-site analysis in Wuhan found that IL-6 Levels were predictive of COVID-19-associated mortality[16].
The cytokine storm and elevated IL-6 signalling detected in these patients could have severe consequences for the cardiovascular system, potentially leading to tachycardia, left-ventricular dysfunction, and hypertension. CRS-associated cardiotoxicity-mainly conduction abnormalities, atrial fibrillation, and elevated levels of brain natriuretic peptide and troponins-has also been described[17].
Disease spread
The incubation period between contact and symptom onset generally ranges from 1 to 14 d, although this can reach 24 d in some cases. The median (mean) time between recorded exposure and symptom onset is 5.1 (6.1) d. Elimination of viral nucleic acids requires from 8 to 34 d (median, 20 d) after the appearance of the initial symptoms[11,18].
Seven different coronaviruses can infect humans: HCoV-NL63 and 229E are alpha-coronaviruses associated with mild disease in adults; MERS-CoV and SARS are betacoronaviruses associated with severe respiratory disease; and OC43 and HKU1, associated with mild disease. COVID19 is caused by a new betacoronavirus that likely originated in bats after gain-of-function mutations in the receptor-binding domain[19].
Coronavirus receptors bind through the spike (S) protein, which is encoded by structural gene S. This has two subunits [subunits 1 (S1) and subunits 2 (S2)]. S1 mediates binding while S2 (a trimeric stem) mediates fusion with the infected cell. The receptor-binding S1 contains the N-terminal domain and the C-terminal domain. These two domains are capable of mediating binding to a variety of cell receptors that contain carbohydrates and proteins. SARS-CoV, SARS-CoV-2, and alpha-coronavirus HCoV-NL63 bind through S1-CTD to angiotensin-converting enzyme 1 (ACE1)[19]. Compared to SARS-CoV, the novel SARS-CoV-2 has a higher affinity for ACE2, involving a greater number of interaction sites in the binding process[20,21]. For the SARS-CoV-2 to bind ACE2, the S protein must first be cleaved by the transmembrane protease, serine 2 (TMPRSS212). Replication occurs by means of RNA-dependent polymerase RNA and involves discontinuous transcription of the subgenomic mRNA that code for 6 key open reading frameworks common to all known coronaviruses.
Importantly, transmission of SARS-CoV-2 is associated with infected persons with a high viral load (up to 1 billion copies of RNA per millilitre of sputum) and with the virus’ capacity to remain viable for long periods of time on contaminated surfaces. SARS-CoV-2 is more stable on certain surfaces (e.g., stainless steel, plastic) than on others (e.g., cardboard), and the virus may be present and viable for three days (72 h) on these surfaces[22]. The viral load tends to be higher in severe cases, who require an extended period to eliminate the virus[23].
Morbidity and mortality
A recent study in China (through January 28, 2020) found that 16% of cases may present severe disease, with an estimated overall mortality rate of 1.4% for reported cases, vs the 4.61% rate reported by the WHO. In some geographic regions, for reasons that remain unclear, the mortality rate may be even higher, with current estimated rates in Italy of 11.9% and 9.0% in Spain[24,25]. In this regard, it is important to be cautious when attempting to calculate mortality rates based on current data, as these may be overestimated-due to inadequate community testing-or underestimated as a consequence of to the prolonged time period between a positive test and death, or to major differences between countries in the criteria used to classify COVID-related mortality. Healthcare systems that have been overwhelmed by a surge in patients requiring invasive or non-invasive mechanical ventilation must also be considered as a potential source of inter-country discrepancies in mortality rates.
PATHOPHYSIOLOGIC FRAMEWORK OF SARS-COV-2 AND THERAPEUTIC STRATEGY
Various studies have established that the pathogenesis of COVID-19 begins with the recognition of an angiotensin receptor (ACE2), especially common on the surface of type 2 alveolar cells and capillary endothelial cells. These cells are infected by the SARS-CoV-2 virus, assisted by the viral protease TMPRSS212, thus allowing the virus to replicate easily[26-28].
The optimal therapeutic strategy remains an open question, in part because this depends on the stage of the infection. Most of the studies published to date have focused on antiviral drugs (e.g., remdesivir, lopinavir/ritonavir, favipiravir, oseltamivir) or immunomodulators (corticosteroids, immunoglobulins, monoclonal antibodies), as well as other treatments such as chloroquine, nitric oxide, extracorporeal membrane oxygenation, among others. To date, no treatment has proven to be safe and effective against COVID-19, thus management of these patients should focus on preventing infection and life support measures.
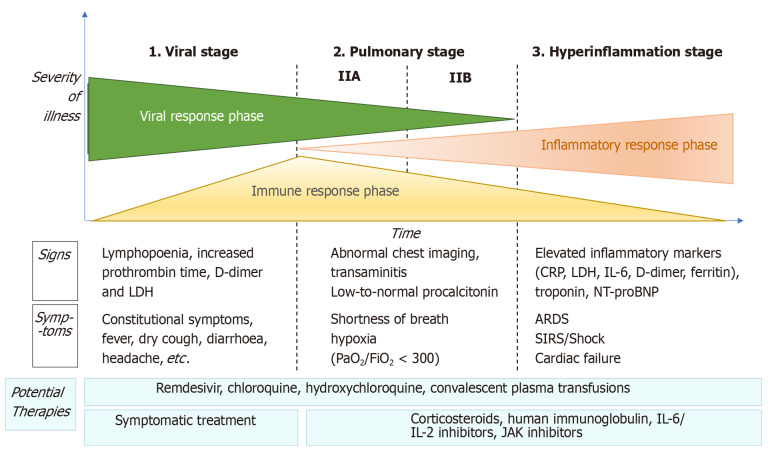
COVID-19 disease can be considered to have three distinct phases, in which the therapeutic strategy will vary according to the phase and disease severity (Figure 1).
Figure 1.
Adaptation of Siddiqi et al[29] the phases of coronavirus disease 2019 disease, including signs, symptoms, and potential therapeutic targets for each phase. CRP: C-reactive protein; LDH: Lactate dehydrogenase; BNP: Brain natriuretic peptide; ARDS: Acute respiratory distress syndrome; SIRS: Systemic inflammatory response syndrome; IL: Interleukin; JAK: Just another kinase.
Viral phase
The initial phase begins at infection, and includes the incubation period and the onset of non-specific symptoms (general malaise, fever, nonproductive cough). During this phase, SARS-CoV-2 uses the ACE2 receptor to enter and establish itself in the host cell-mainly alveolar cells and vascular endothelial cells-after which it begins to replicate, leading to a mild respiratory infection and general symptoms[29,30].
Diagnosis in this phase is made by reverse transcription polymerase chain reaction (RT-PCR), serology for SARS-CoV-2 (IgG and IgM), radiology [computed tomography (CT) or, if unavailable, chest X-ray], and blood tests, including a complete blood count (in which lymphopenia and neutrophilia are common), liver function markers, and acute-phase reactants. The aim of treatment during this phase is symptom control.
Pulmonary phase
In this phase, the virus continues to replicate, leading to pulmonary involvement (viral pneumonia with or without hypoxemia, 2A or 2B respectively). CT and chest X-ray imaging reveal bilateral infiltrates and ground-glass opacities, while blood test findings are characterized by lymphopenia, elevated transaminases, and markers of systemic inflammation[29]. Most patients in this stage require hospitalization, whose main purpose is to closely monitor the patient to ensure that early treatment will be available in case of significant clinical worsening. Treatment consists of life-support measures and anti-viral agents, such as remdesivir[31], which has been available for compassionate use.
Patients in stage 2 who do not present significant hypoxia should not receive corticosteroids due to the risk of bacterial superinfection[32]. However, in patients with significant hypoxemia and/or the need for non-invasive respiratory support, anti-inflammatory treatment (corticosteroids) is recommended.
Hyperinflammatory phase
Only a few patients with mild to moderate infection will develop a severe form of the disease, manifesting as extrapulmonary systemic inflammatory response syndrome. These patients will present elevated serum levels of markers of systemic inflammation such as C-reactive protein or fibrinogen[29]. Various studies have demonstrated that inflammatory markers such as cytokines, ILs (IL-2, IL-6, IL-7), G-CSF, TNF-α, PCR, ferritin, and/or D-dimer are significantly higher in patients with more severe forms of the disease[33].
This phase is characterized by the following: (1) development of severe respiratory failure requiring respiratory support; (2) shock; and (3) organ failure (including myocarditis) due to severe systemic inflammation. To reduce the effects of the systemic inflammatory cascade and the risk of multiorgan failure in patients at this stage, the following treatments are recommended: Immunomodulating agents such as high-dose corticosteroids, tocilizumab (an IL-6 inhibitor), anakinra (an IL-1 receptor antagonist), or intravenous immunoglobulins[15]. The prognosis and likelihood of recovery in patients at this stage are poor, which is why early diagnosis and treatment are essential.
Radiologic manifestations (chest X-ray and CT)
Simple chest X-ray and CT both play a key role in the diagnosis of SARS-CoV-2 pneumonia, follow-up, and detection of complications. These imaging tests can also help to determine disease severity[34]. The main radiologic manifestations are ground-glass opacities and areas of consolidation (generally bilateral), similar to the characteristics observed in other coronaviruses such as SARS-CoV and MERS-CoV[35].
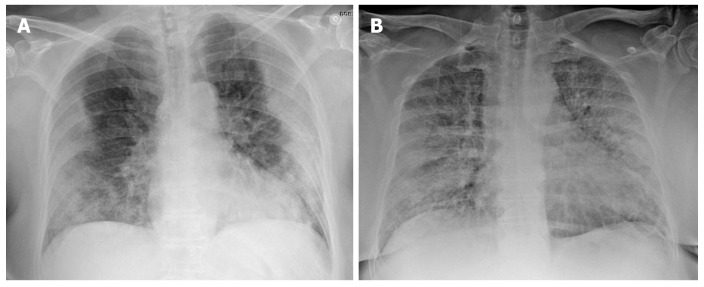
Chest X-ray has a low sensitivity to detect pulmonary infiltrates during the initial phases of infection and in mild forms of the disease. Nonetheless, the wide availability of X-ray makes it a valuable tool to establish the radiologic suspicion of pneumonia, especially in limited resource settings. The main role of chest X-ray is to monitor the course of pulmonary lesions in patients with severe disease, including those admitted to intensive care[36]. A study involving 64 patients diagnosed with SARS-CoV-2 found that basal consolidations were the most common finding on X-ray, which were normal in 20% of cases[37] (Figure 2).
Figure 2.
Chest X-ray findings in two patients with confirmed severe acute respiratory syndrome coronavirus 2 pneumonia (positive reverse transcription polymerase chain reaction test). A: 73-year-old diabetic woman. General malaise, myalgia and diarrhea of 8 d clinical course. Dyspnea in the last 2 d, no fever. AP X-ray: Peripherally-distributed bilateral lung opacities; B: 60-year-old man, fever and cough, progressive dyspnea. The patient presented at the emergency department with acute respiratory failure requiring admission to intensive care. AP X-ray: Alveolar infiltrates and diffusely-distributed, bilateral ground-glass opacities.
The mains signs of COVID-19 on CT (Table 1) have been widely described. There are several common signs, which are usually most evident from 10-12 d from symptom onset. The most common and characteristic manifestation is multifocal ground-glass opacities, of variable extension and morphology, with peripheral/ subpleural distribution (including perifissural regions)[38,39].
Table 1.
Computed tomography findings, the most common findings are bolded
|
CT findings
|
Frequency
|
| Ground-glass opacities: Circumscribed margin; rounded/poorly-defined | ++++ |
| Consolidations | + |
| Ground-glass opacity + consolidation | +++ |
| Crazy-paving pattern | +++ |
| Elongated/linear/curved consolidation | +++ |
| Pattern perilobular pattern | +++ |
| Reverse halo sign | + |
| Other signs: Vascular dilation + ground-glass opacity | ++ |
| Other signs: Dense subpleural line | ++ |
| Other signs: Subpleural parenchyma normal | ++ |
| Other signs: “Tree-in-bud pattern”/centrilobular nodules | - |
| Distribution: Peripheral/subpleural | ++++ |
| Distribution: Central/perihilar | + |
| Distribution: Multifocal bilateral | ++++ |
| Extrapulmonary findings: Nodal involvement | - |
| Extrapulmonary findings: Pleural effusion | - |
CT: Computed tomography.
The following findings will vary according to the stage of the infection: Asymptomatic patients may present pulmonary opacities but with limited extension[40]. In the first two days from symptom onset, CT findings are normal in just over 50% of cases[41].
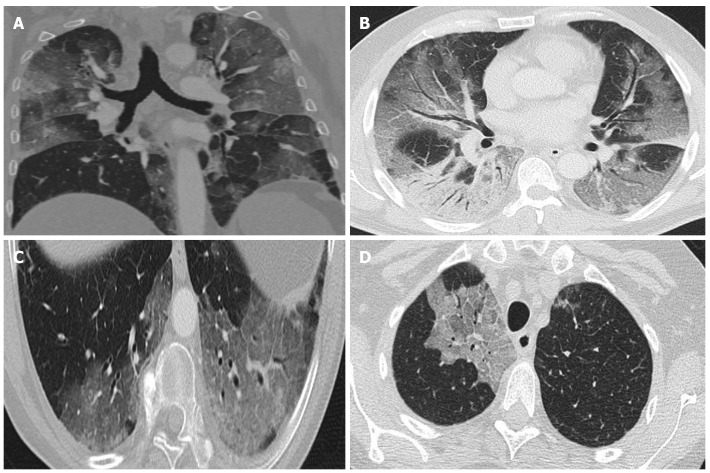
During the early phase (first week) ground-glass opacities are common, with or without vascular dilatation. Rapid progression of these opacities and/or consolidations is common[42]. Ground-glass opacities with reticulation (“crazy-paving pattern") have been associated with more advanced disease (Figure 3).
Figure 3.
Computed tomography findings. A: Extensive bilateral ground-glass opacities, one with poorly-defined margins and another with clearly defined borders; B: Multilobar ground-glass opacities and consolidation with air bronchogram in the right lower lobe. Band of subpleural parenchyma respected in the left lung; C: Microvascular dilation sign in the middle of ground-glass opacity in the left lower lobe; D: Crazy-paving pattern in the right upper lobe.
Later (at 2-3 wk from infection) signs of organized pneumonia can be seen (linear/curved consolidation patterns, perilobular pattern, reverse halo sign) (Figure 3). As the disease begins to resolve, there is a decrease in alveolar opacities and subpleural lines begin to emerge[36].
The long-term clinical course is unknown, but it is expected that some patients with severe pneumonia will develop pulmonary fibrosis, as occurred with SARS (2003), in which a small percentage of patients presented signs of fibrosis with reticulation associated with traction bronchiectasis[43]. Some findings are rare, including nodal involvement, tree-in-bud pattern, cavitation, lobar/segmental consolidation, and significant pleural effusion. The presence of such findings should suggest a different diagnosis or the presence of complications, such as a bacterial superinfection[44].
The differential diagnosis includes infectious and non-infectious processes. To discriminate SARS-CoV-2 pneumonia from other viral pneumonias, the most common findings are subpleural pulmonary opacities and the microvascular dilation sign[45].
The Radiological Society of North America (RSNA) proposes standardizing the radiologic report to improve communication with clinicians to facilitate patient management. The RSNA suggests that there are four main CT findings relative to the diagnosis of COVID-19: (1) compatible with viral pneumonia; (2) indeterminate; (3) atypical (suggestive of other diagnoses); and (4) no evidence of pneumonia[46].
CT allows us to determine the extent of the lung disease, which correlates with clinical severity. The presence of extensive areas of consolidation usually indicates a poor prognosis, especially in the elderly[47]. CT is also useful to assess suspected complications, particularly pulmonary thromboembolism, whose incidence is higher in patients with severe disease[48].
Various guidelines have described the indications for performing imaging tests in patients with suspected SARS-CoV-2[49]. However, the role of CT for diagnosis and screening for SARS-CoV-2 has been questioned, although some authors have emphasised its diagnostic value, especially when RT-PCR is unavailable[50]. However, in a review of the literature, Raptis et al[51] found that the limited data (mostly from retrospective studies) do not substantiate the use of CT as a diagnostic test for COVID-19. In addition, in the meta-analysis carried out by Kim et al[52], the sensitivity of CT was greater than 90% but the specificity was low, leading those authors to conclude that, given the high false positive rate of CT, this imaging technique should not be used for screening in geographic regions where the disease prevalence is low (< 10%). Therefore, although certain radiologic findings and patterns are common in SARS-CoV-2 pneumonia, the definitive diagnosis requires a positive RT-PCR test result[53].
The Fleischner Society recommends the following that imaging tests not be performed in cases of community transmission and mild infection (no hypoxemia, mild or no dyspnea), except in patients with risk factors (diabetes, obesity, hypertension, etc). By contrast, this society recommends performing imaging tests in cases with moderate to severe infection (hypoxemia, moderate-severe dyspnea), regardless of the findings of RT-PCR. CT is indicated in patients who present functional abnormalities and/or hypoxemia after the recovery phase, while RT-PCR is indicated if incidental findings on CT suggest the presence of viral pneumonia[54].
FLUORODEOXYGLUCOSE-POSITRON EMISSION TOMOGRAPHY METABOLIC IMAGING IN COVID-19
Positron emission tomography-CT (PET-CT) imaging with fluorodeoxyglucose (FDG) (18F-fluorodeoxyglucose) is a highly useful diagnostic tool for inflammatory or infectious pulmonary diseases in general, as it can not only demarcate the affected areas, but it can also be used to evaluate treatment response and monitor the clinical course[55]. However, only a few published studies have assessed the utility of 18F-FDG-PET-CT metabolic imaging in COVID-19 patients, although the first reports support the value of this FDG-PET to evaluate lung areas with inflammation/infection[56,57].
Although FDG-PET imaging should not be routinely used in COVID-19 patients, the first published studies suggest that metabolic data provided by this imaging technique can provide important complementary data in these patients. In particular, FDG-PET metabolic imaging can be of value in the following areas: (1) as a diagnostic tool in asymptomatic patients for the differential diagnosis and to diagnosed patients with a normal CT scan; (2) to monitor treatment response, in combination with CT; (3) as a potential prognostic factor for recovery from the disease; and (4) to evaluate systemic extrapulmonary involvement.
Utility of metabolic imaging in asymptomatic patients
Several preclinical studies[58] have found that FDG-PET imaging may be useful to assess the immune response to viral infection. FDG-PET imaging can detect lymph node involvement by showing the presence of increased cellular activity in the mediastinum and axilla (drainage of damaged lung tissue), even in individuals without pathologic findings on CT or clinical manifestations after exposure to the virus. Studies in animal models have found a discreet increase in circulating monocytes in the affected lymph nodes, showing elevated cellular activity on day 5 of viral exposure. This finding is related to the role of monocytes in the immune response to viral infections. PET metabolic imaging with other non-FDG tracers[59] might provide information about which strains are involved in COVID-19 infection.
In animal models, some authors have found that FDG uptake is elevated in various lymph node stations before large-scale viral replication occurs[60]. This finding suggests that FDG-PET imaging could be an especially useful diagnostic tool to detect early changes in the immune response to infection in asymptomatic patients, potentially playing a particularly important role in early phases of the disease and in the differential diagnosis.
Monitoring metabolic response to treatment
Another potential application of FDG-PET in COVID-19 patients is the potential to monitor treatment response and predict recovery time[61]. Some correlation has been observed between high uptake of FDG in pulmonary lesions associated with SARS-CoV-2 and recovery time for these patients. For example, patients with a standard uptake value (SUV) of 4.6 have been shown to recover in 17 d after symptom onset, vs 26 d in patients with a higher SUV (SUV = 12.2). However, a larger series of patients is needed to confirm the predictive value of SUV values for clinical recovery. In this regard, it would be valuable to quantitatively determine the relation between the SUV value and the expected recovery time.
Evaluation of systemic involvement
FDG-PET imaging can also be particularly useful in patients with SARS-CoV-2 to detect the presence of alterations in other organs, including the gastrointestinal tract, heart, kidneys, brain, and/or bone marrow[62]. In some cases, a diffuse increase has been observed in bone marrow metabolic activity after COVID-19 infection. The chronic inflammation associated with COVID-19 has also been shown to affect both the brain and heart.
Complementary role of FDG-PET imaging to confirm morphologic abnormalities evidenced on CT
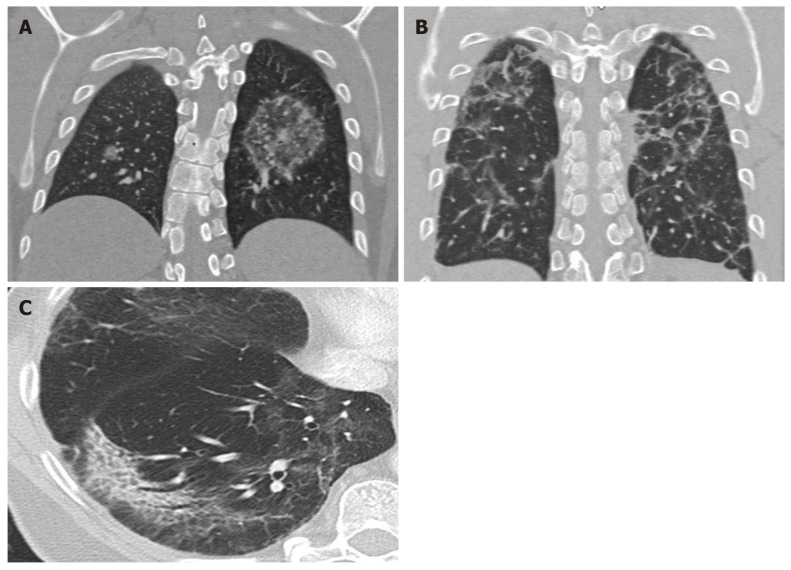
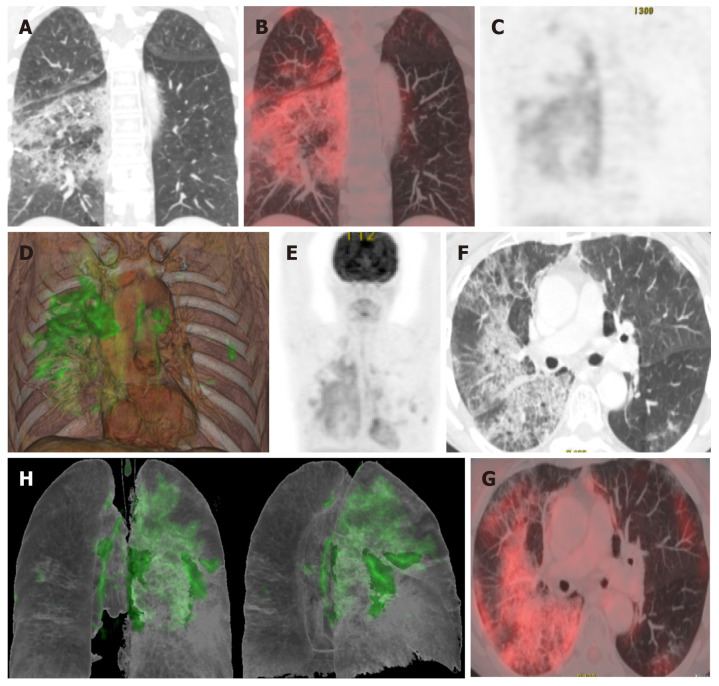
Various studies have found a correlation between morphologic abnormalities observed on CT with metabolic data obtained by FDG-PET imaging (Figure 4). However, the possible association between the topographic distribution of lesions observed on CT and the intensity of FDG metabolism needs to be evaluated in larger series of patients. It would be particularly useful to evaluate the possible relation between metabolic uptake and lung areas with greater or lesser ventilation (Figure 5)[63]. It appears that areas with greater ventilation are more likely to present higher infiltration of inflammatory cells.
Figure 4.
Computed tomography findings late stage disease. A: Reverse halo or atoll sign: Rounded opacity in the left lung with ground-glass attenuation in the center demarcated by a denser, fine ring. Small, homogeneous rounded opacity in the right lung; B: Bilateral perilobular pattern: Polygonal (irregular, linear or band-like) peripheral opacities in secondary pulmonary lobules; C: Peripheral, elongated, curved consolidation in the right lower lobe containing dilated bronchi.
Figure 5.
A 65-year-old patient with a history of invasive lepidic-predominant adenocarcinoma (stage pT1bNxM0) treated with surgery, chemotherapy and radiotherapy. A: Coronal computed tomography showing the crazy-paving pattern (with interstitial septal thickening and increased density of ground-glass opacities) with a markedly asymmetric bilateral distribution, mainly affecting the right side; B: Positron emission tomography-computed tomography (PET-CT) coronal section; C: Metabolic PET; D: Volume rendering 3D PET-CT; E: MIP, PET; B-E: Reveals increased cellular activity [standard uptake value (SUV) 4-6] related to the associated inflammatory process. PET-CT pattern of bilateral coronavirus disease 2019 (COVID-19) viral pneumonitis, predominantly right-sided; F: Axial computed tomography showing crazy-paving pattern (with interstitial septal thickening and increased ground-glass density) with a bilateral, but markedly asymmetric distribution, predominant right-sided; G and H: Axial section and 3D volume rendering from PET-CT metabolic imaging that reveals increased cellular activity (SUV 4-6) related to the associated inflammatory process. PET-CT pattern of bilateral, predominantly right-sided, COVID-19 viral pneumonitis.
CONCLUSION
There is much we still do not know about the pathophysiology and clinical course of COVID-19 infection. However, the presence of certain patterns on CT and PET-CT scans may facilitate diagnosis and help to select the appropriate treatment.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: May 19, 2020
First decision: July 30, 2020
Article in press: October 26, 2020
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng BG S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
Contributor Information
Pedro Landete, Department of Pulmonology, H. U. La Princesa, Madrid 28006, Spain.
Carlos Andrés Quezada Loaiza, Department of P ulmonology, H. 12 de Octubre, Madrid 28041, Spain.
Beatriz Aldave-Orzaiz, Department of Pulmonology, H. U. La Princesa, Madrid 28006, Spain.
Susana Hernández Muñiz, Department of Radiologist, H. U. La Princesa, Madrid 28006, Spain.
Antonio Maldonado, Department of Nuclear Medicine, Hospital Universitario Quironsalud Madrid, Madrid 28223, Spain.
Enrique Zamora, Department of Pulmonology, H. U. La Princesa, Madrid 28006, Spain.
Allan Charles Sam Cerna, Department of Pulmonology, Hospital MD Anderson Madrid. España, Madrid 28033, Spain.
Elia del Cerro, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Department of Radiation Oncology, Universidad Europea de Madrid, Villaviciosa de Odón, Madrid 28670, Spain.
Raquel Cano Alonso, Department of Diagnostic Imaging, Hospital Universitario Quirón Madrid, Madrid 28223, Spain.
Felipe Couñago, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Pozuelo de Alarcón, Madrid 28223, Spain; Department of Radiation Oncology, Hospital La Luz, Madrid 28003, Spain; Department of Radiation Oncology, Universidad Europea de Madrid, Villaviciosa de Odón, Madrid 28670, Spain. landete.pedro@gmail.com.
References
- 1.Department of Error. Lancet. 2020;395:496. [Google Scholar]
- 2.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. : 2020. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Liu Q, Guo D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bchetnia M, Girard C, Duchaine C, Laprise C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status. J Infect Public Health. : 2020. doi: 10.1016/j.jiph.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Rubin R, Morales-Estrada S, Black SR, Pacilli M, Fricchione MJ, Chugh RK, Walblay KA, Ahmed NS, Stoecker WC, Hasan NF, Burdsall DP, Reese HE, Wallace M, Wang C, Moeller D, Korpics J, Novosad SA, Benowitz I, Jacobs MW, Dasari VS, Patel MT, Kauerauf J, Charles EM, Ezike NO, Chu V, Midgley CM, Rolfes MA, Gerber SI, Lu X, Lindstrom S, Verani JR, Layden JE Illinois COVID-19 Investigation Team. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan DY, Luo XY, Dong W, Zhang ZW. Current practice and potential strategy in diagnosing COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4548–4553. doi: 10.26355/eurrev_202004_21039. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketelhuth DFJ, Lutgens E, Bäck M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O'Neill L, Weber C, Evans PC. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res. 2019;115:1385–1392. doi: 10.1093/cvr/cvz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ketelhuth DFJ. The immunometabolic role of indoleamine 2,3-dioxygenase in atherosclerotic cardiovascular disease: immune homeostatic mechanisms in the artery wall. Cardiovasc Res. 2019;115:1408–1415. doi: 10.1093/cvr/cvz067. [DOI] [PubMed] [Google Scholar]
- 15.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Chen L, Zhong J, Gao P, Oudit GY. ACE2/Ang-(1-7) signaling and vascular remodeling. Sci China Life Sci. 2014;57:802–808. doi: 10.1007/s11427-014-4693-3. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur Radiol. 2020;30:3266–3267. doi: 10.1007/s00330-020-06748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanne JP. Chest CT Findings in 2019 Novel Coronavirus (2019-nCoV) Infections from Wuhan, China: Key Points for the Radiologist. Radiology. 2020;295:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W, Sirajuddin A, Zhang X, Liu G, Teng Z, Zhao S, Lu M. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020;30:4874–4882. doi: 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung TW, Lee EYP, Wan EYF, Hung IFN, Lam TPW, Kuo MD, Ng MY. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 39.Lomoro P, Verde F, Zerboni F, Simonetti I, Borghi C, Fachinetti C, Natalizi A, Martegani A. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KW, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am J Roentgenol. 2004;182:39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]
- 44.Revel MP, Parkar AP, Prosch H, Silva M, Sverzellati N, Gleeson F, Brady A European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30:4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie FF, Li S, Healey T, Atalay MK, Liao WH. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology. 2020;296:E46–E54. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 48.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair A, Rodrigues JCL, Hare S, Edey A, Devaraj A, Jacob J, Johnstone A, McStay R, Denton E, Robinson G. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75:329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raptis CA, Hammer MM, Short RG, Shah A, Bhalla S, Bierhals AJ, Filev PD, Hope MD, Jeudy J, Kligerman SJ, Henry TS. Chest CT and Coronavirus Disease (COVID-19): A Critical Review of the Literature to Date. AJR Am J Roentgenol. 2020;215:839–842. doi: 10.2214/AJR.20.23202. [DOI] [PubMed] [Google Scholar]
- 52.Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology. 2020;296:E145–E155. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao C, Liu X, Zhang H, Li Y, Liu J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J Am Coll Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, Schluger NW, Volpi A, Yim JJ, Martin IBK, Anderson DJ, Kong C, Altes T, Bush A, Desai SR, Goldin O, Goo JM, Humbert M, Inoue Y, Kauczor HU, Luo F, Mazzone PJ, Prokop M, Remy-Jardin M, Richeldi L, Schaefer-Prokop CM, Tomiyama N, Wells AU, Leung AN. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology. 2020;296:172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capitanio S, Nordin AJ, Noraini AR, Rossetti C. PET/CT in nononcological lung diseases: current applications and future perspectives. Eur Respir Rev. 2016;25:247–258. doi: 10.1183/16000617.0051-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juengling FD, Maldonado A, Wuest F, Schindler TH. The Role of Nuclear Medicine for COVID-19: Time to Act Now. J Nucl Med. 2020;61:781–782. doi: 10.2967/jnumed.120.246611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chefer S, Thomasson D, Seidel J, Reba RC, Bohannon JK, Lackemeyer MG, Bartos C, Sayre PJ, Bollinger L, Hensley LE, Jahrling PB, Johnson RF. Modeling [(18)F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015;5:65. doi: 10.1186/s13550-015-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallace M, Pyzalski R, Horejsh D, Brown C, Djavani M, Lu Y, Hanson JM, Mitchen JL, Perlman SB, Pauza CD. Whole body positron emission tomography imaging of activated lymphoid tissues during acute simian-human immunodeficiency virus 89.6PD infection in rhesus macaques. Virology. 2000;274:255–261. doi: 10.1006/viro.2000.0479. [DOI] [PubMed] [Google Scholar]
- 61.Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020;296:E118. doi: 10.1148/radiol.2020200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellani G, Messa C, Guerra L, Spagnolli E, Foti G, Patroniti N, Fumagalli R, Musch G, Fazio F, Pesenti A. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med. 2009;37:2216–2222. doi: 10.1097/CCM.0b013e3181aab31f. [DOI] [PubMed] [Google Scholar]