Abstract
Concerns regarding sex differences are increasingly pertinent in scientific and societal arenas. Although biological sex and socio-cultural gender are increasingly recognized as important modulators of renal function under physiological and pathophysiological conditions, gaps remain in our understanding of the mechanisms underlying sex differences in renal pathophysiology, disease development, progression and management. In this Perspectives article, we discuss specific opportunities for future research aimed at addressing these knowledge gaps. Such opportunities include the development of standardized core data elements and outcomes related to sex for use in clinical studies to establish a connection between sex hormones and renal disease development or progression, development of a knowledge portal to promote fundamental understanding of physiological differences between male and female kidneys in animal models and in humans, and the creation of new or the development of existing resources and datasets to make them more readily available for interrogation of sex differences. These ideas are intended to stimulate thought and interest among the renal research community as they consider sex as a biological variable in future research projects.
In 1993, the US Congress passed ‘The National Institutes of Health (NIH) Revitalization Act’, which mandated that women and minorities be included in clinical trials funded through the NIH1. Despite this mandate, women remain under-represented in clinical trials in the USA. For example, between 2005 and 2015, representation of women in cardiovascular disease trials fell below a participation-to-prevalence ratio of 0.8 (REFS2,3) (no comparable data for trials in renal diseases exist). Moreover, when women are included, gender-based reporting of results is often lacking4. In 2015, the NIH released a notice, “Consideration of Sex as a Biological Variable in NIH-funded Research”, which outlined the expectation that sex will be considered in research design, analyses and reporting in preclinical studies5.
These NIH policies and initiatives raised awareness regarding differences in human disease according to sex and gender2, but much remains to be learned about sex and gender differences in disease pathophysiology, including renal diseases.
Many controversies exist regarding the contributions of both biological sex and socio-cultural factors in the pathophysiology, prevalence and progression of renal diseases. For example, although it has been argued that premenopausal women are relatively protected from non-diabetic renal disease compared with age-matched men6–8, epidemiological and clinical studies over the past decade depict a more complex picture. Some studies have suggested that female sex and/or gender might adversely affect renal pathophysiology, disease progression and outcomes. Compared with men, more women progress to stage G3 chronic kidney disease (CKD), they have reduced access to deceased donor transplantation and poorer quality of life while on renal replacement therapy (RRT)9–11. Reports also exist of female sex as a protective factor in renal disease and studies in animal models have suggested that oestrogens might exert renoprotective effects in various models of non-diabetic and diabetic renal disease12–15. However, the influence of sex on disease development and progression might not be limited to sex hormone interactions — whether sex chromosomes or other genetic and epigenetic events influence renal function independently or in concert with sex hormones remains poorly understood.
Recognizing the need to address these controversies and the gaps related to the understanding of sex and gender differences in renal diseases, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) convened a workshop entitled “Sex and the Kidneys” in July 2017 at the NIH in Bethesda, MD, USA. The workshop gathered experts from the fields of nephrology, endocrinology, imaging and sex differences in renal and other diseases (including cardiovascular disorders, diabetes, obesity and hypertension) to discuss the current state of the science and identify opportunities for future research. In this Perspectives article, we highlight the opportunities for clinical, basic and translational research into sex differences in renal disease as well as the potential tools and resources for conducting this research that were identified during the workshop (BOX 1; FIG. 1).
Box 1 |.
Opportunities for the study of sex differences in renal disease
Clinical research
|
Basic research
|
Translational research
|
Tools and resources
|
Fig. 1 |. Strategies to aid understanding of sex differences in kidney health and disease.
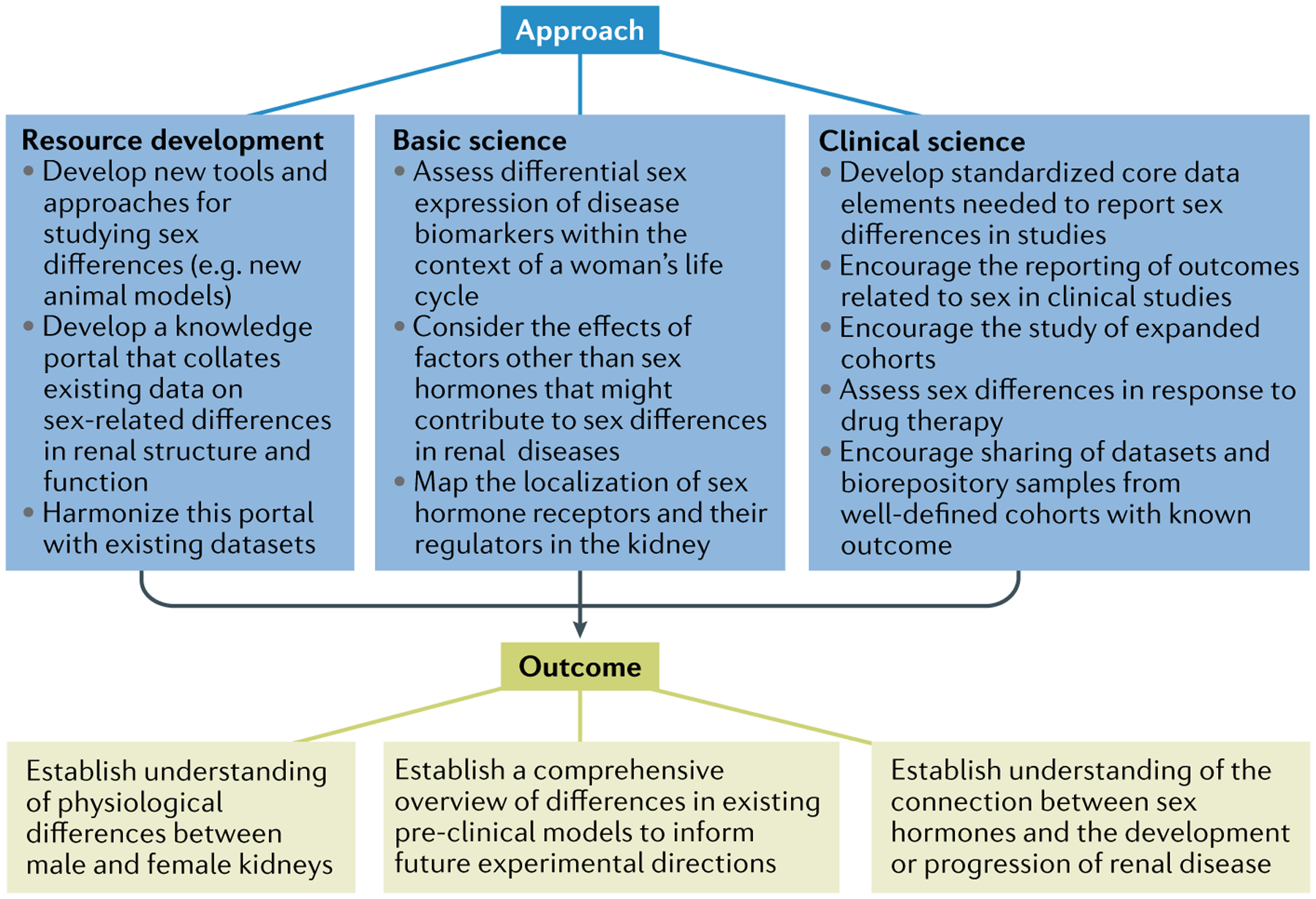
In recognition of the need to address controversies and knowledge gaps related to understanding the role of sex in kidney physiology and pathophysiology, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) asked leading experts in the field to discuss key considerations and identify opportunities for future research. They identified several challenges to basic and clinical science research related to sex differences, some of which could be overcome by developing new research tools and experimental models, and by expanding the availability of existing datasets for interrogation to answer relevant scientific questions around sex and gender differences that affect kidney health and disease. Improving our understanding of the complex interplay of factors that contribute to sex differences in kidney health and disease could contribute to improving the health of both women and men.
For the purposes of this article, we define sex and gender according to the US Institute of Medicine guidelines, which describe sex differences as biological differences between men and women, such as differences in sex chromosomes, sex-specific gene expression and sex hormones. Gender refers to one’s sense of self as male or female in society, with differences including factors such as lifestyle, environmental influences and nutrition16. Although differences in gender and sex were considered equally important, discussions during the NIDDK workshop were predominantly centred around sex (that is, biological) differences. However, it should be noted that the effects of sex and gender are often intertwined and thus a clear distinction between the two might not be possible.
Clinical research
Numerous inconsistencies exist in findings regarding the association between biological sex and renal disease, and many attempts have been made to determine the nature of this association. Complex interactions among biological, cultural and socio-economic factors are thought to underlie some of these differences, but the precise mechanisms are not fully understood. Examining and reporting sex and gender differences is vitally important as they provide a foundation and scientific premise for studies in humans and animals. Furthermore, a better understanding of how sex contributes to disease aetiology, mechanisms and epidemiology might guide future clinical research on potential sex-specific therapies for renal diseases. In discussing these issues, the workshop participants identified the following priorities for future research.
Epidemiology of sex differences.
CKD (defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2) has been reported to be more common in women than in men17–21; however, the validity of this conclusion has been questioned. Although the sex difference in bias in the equations used to estimate GFR is relatively small, the use of a single eGFR cut-off to define CKD might lead to over-diagnosis of CKD in women without some adjustment for normal gender-specific decline in eGFR with ageing22,23. Nearly two decades ago a meta-analysis suggested that sex influences the rate of progression of non-diabetic CKD and that female sex is reno-protective8. Experimental models of non-diabetic CKD clearly show a protective effect of oestrogen and a deleterious effect of testosterone, but the data in humans are less clear24. Reports of a higher incidence of CKD in women than in men also contrast with the observation that incident end-stage renal disease (ESRD) is more common in men than in women25–27. Possible explanations for this apparent paradox are faster progression of renal disease in men than in women and/or disparities in health care delivery that lead to earlier initiation of dialysis in men, meaning that more women than men with stage 5 CKD die without having initiated RRT. The authors of a 2019 systematic review of more than 30 studies that reported sex-stratified data concluded that renal disease progresses more rapidly in men than in women23. However, alternative interpretations of these data are reasonable, because many of the studies included used ESRD as an end point, which is a subjective outcome measure that may be influenced by non-biological factors. Moreover, even among studies that used the change in the slope of eGFR as an end point, progression of CKD might not only reflect biological differences between the sexes but also differences in lifestyle, cultural and socio-economic factors that are affected by gender28. Currently available data are thus insufficient to ascertain how sex and gender-related differences in disease progression or delivery of RRT might contribute to the observed sexual dimorphism in the incidence of ESRD.
The conflicting data in reports related to sex and the progression of CKD underscore the need for more rigorous, well-designed observational studies that focus on defining sex differences in disease development and progression, as well as considering the sex-specific categorization of renal disease severity. These studies should address the role of sex-related biological differences and differences in psychosocial, lifestyle and other factors, and take into account the menopausal status of the patients, hormone therapy use and history of the use of oral contraceptives, which may also affect renal disease progression. However, capturing data on gender identity, which would help to isolate the effects of biological processes related to sex chromosome endowment at birth from subsequent complex inputs that result in gender identity, is not common in clinical studies. Such epidemiological studies might generate novel hypotheses related to the mechanisms underlying sex differences in disease pathophysiology that could be explored in future clinical and basic research.
Core data elements.
The presence of ovarian hormones and/or the absence of testosterone have been proposed as potential mechanisms underlying the perceived relative protection of females against the progression of renal disease. This theory is supported by the observation that younger women have a lower incidence of ESRD and slower progression of CKD than men8, but this advantage dissipates, at least according to some studies, after menopause6. Moreover, premenopausal women who undergo bilateral oophorectomy are at a higher risk of developing CKD than premenopausal women with intact ovaries29, an observation that is also supported by studies in animals30,31. In addition, some studies have suggested that the selective oestrogen receptor modulator (SERM), raloxifene, might slow CKD progression32, thus supporting the notion that oestrogens are renoprotective. These observations suggest a possible link between sex hormones and renal disease development or progression. However, studies examining this link are limited by the scarcity of relevant data, including age at menarche, menopausal status and use of oral contraceptives or hormone replacement therapy in female participants. This lack of data highlights the need to develop standardized core data elements and outcomes that incorporate key life cycle components for both women and men who participate in clinical studies.
Basic science research
Despite evidence that biological sex influences the course, prevalence and incidence of several renal diseases, little is known about the cellular and molecular mechanisms underlying these differences. One reason for this lack of knowledge is that most basic science studies are conducted using male kidneys. Indeed, in three leading journals that publish research on kidney physiology and pathophysiology, male:female ratios in basic science studies are 5:1 (American Journal of Physiology-Renal Physiology), 11:1 (Kidney International) and 16:1 (Journal of the American Society of Nephrology)33.
In the few studies that have examined sex differences in renal diseases, the focus has predominantly been on examining the renoprotective effects of female sex hormones and SERMs12–15. In females, administration of SERMs or 17β-oestradiol (E2), which binds oestrogen receptor-α (ERα), attenuates albuminuria, podocytopathy and tubulointerstitial injury, and improves renal function in several rodent models of renal disease14,34–40. By contrast, inhibition of oestrogen synthesis with the aromatase inhibitor anastrozole partially attenuates renal injury in male streptozotocin-induced diabetic rats41. Moreover, in the streptozotocin-induced model of diabetic kidney disease in males, treatment with testosterone is renoprotective, an effect mediated through the androgen recepto42. By contrast, in experimental models of hypertension-associated renal injury in male rats, castration decreases blood pressure and renal vascular resistance while increasing glomerular filtration rate43,44. These studies demonstrate that sex hormones have an important role in the development and progression of renal diseases, at least in experimental models.
Although additional studies to increase understanding of the role of sex hormones in renal pathophysiology should be encouraged, the effects of other factors that might contribute to sex differences in renal diseases, such as sex chromosomes and the effects of sex-specific gene expression on autosomes, also need to be investigated. In the context of hypertension, the few studies that have examined these factors showed that, in addition to the effects of gonadal hormones on blood pressure, X-linked and Y-linked genes, parental imprinting and X chromosome mosaicism contribute to sex differences in hypertension-associated renal injury45. The following section articulates some specific opportunities for future research related to sex differences in renal pathophysiology.
Renal physiology.
Over the past three decades, many studies have reported sex differences in basic renal structure and function in experimental models and in humans (TABLE 1). For example, studies in humans and rodents have demonstrated that total renal mass and the renal cortex and proximal tubules are larger in males than in females46–48. By contrast, the renal medulla seems to be larger in female than in male Wistar-Kyoto rats48. The contribution of these structural differences to sex-related variation in renal physiology or progression of CKD remains unclear. For example, although the medulla is larger and thick ascending limbs are longer in females, urine concentration is greater in males; maximum urinary concentrating ability after 48 h of dehydration does not differ between sexes48. Total vascular resistance per glomerulus is greater in females, whereas urine protein excretion is higher in males49–51. The higher renovascular resistance in female Munich-Wistar rats might protect the glomerulus from hyperfiltration-induced injury by preventing fluctuations in glomerular blood pressure50.
Table 1 |.
Sex differences in renal structure and function in experimental models
| Parameter | Sex differences | Species | Refs |
|---|---|---|---|
| Kidney size and morphology | Renal mass, size of renal cortex and proximal tubules are greater in males than in females | Mice, rat, human | 46–48 |
| Renal haemodynamics | Male kidney is vasodilated relative to the female; total vascular resistance per glomerulus is greater in females; urine protein excretion is higher in males; proximal sodium transport is lower in females | Rat, mouse | 49–51,53 |
| Sodium transport | Phosphorylation of sodium–hydrogen exchanger 3 (NHE3) is greater in females; Na+–Pi cotransporter 2 protein expression is lower in females; abundance of total and phosphorylated Na+/Ch− cotransporter (NCC) and ENaC (α- and γ-subunits) is greater in females | C57BL/6 mice | 53 |
| Glucose transport | Sodium–glucose cotransporter 2 (SGLT2) protein expression is higher in females; no sex differences at the mRNA level | Wistar rat | 52 |
| Water channel | Aquaporin 1 (AQP1) protein and mRNA expression are ~80% and ~40% higher, respectively, in males than in females | Wistar rat | 53,54 |
| Organic anion transport | Solute carrier family 22 member 6 (mOat1) protein expression is higher in males; solute carrier family 22 member 8 (mOat3) protein expression is lower in males; solute carrier family 22 member 19 (mOat5) protein expression is lower in males | C57Bl/6 mice | 55,56 |
Sex differences in the expression of renal glucose52 and sodium transporters53, water channels53,54 and organic anion transporters55,56 have also been reported (TABLE 1). A 2018 study developed sex-specific computational models of solute and water transport in the proximal convoluted tubule of the rat kidney to investigate the functional implications of sexual dimorphism in transporter patterns57. Simulations from this study predicted that 70.6% and 38.7% of the filtered volume are reabsorbed by the proximal tubule of the male and female rat kidneys, respectively. This lower fractional volume reabsorption in females might result from their smaller transport area and lower expression of aquaporin 1. This study also showed sex differences in sodium reabsorption and sodium-glucose cotransporter 2 (SGLT2) expression levels57. The reportedly greater SGLT2 expression in females than in males might compensate for their lower tubular transport area and enable them to achieve a hyperglycaemic tolerance similar to that of males57.
A single, easily accessible reference portal that collates existing data on sex-related differences in normal renal structure and function would be a valuable research resource. Such a virtual library, which could be integrated, for example, into the existing NIDDK Information Network (dknet) or other existing data portals, would provide basic investigators with a comprehensive overview of differences reported in existing preclinical models and inform future experimental directions. The portal could also include relevant epidemiological data from large human cohorts to inform hypothesis-testing at the mechanistic level in preclinical models. Furthermore, information on animal age (for example, pre-pubertal or post-reproductive age), as well as the stage of oestrous cycle and parity in females, should also be included.
Sex hormone receptors in the kidney.
Sex hormone receptors, namely the oestrogen and androgen receptors, have been extensively studied in the reproductive, cardiovascular and central nervous systems58–60, but a full appreciation of the cell types that express these receptors in the kidney has not been achieved. Sex hormones are thought to exert their effects through their classical, intranuclear receptors. However, studies from the past few years have shown that oestrogens can also bind to membrane-associated receptors61. Data on the localization and function of sex hormone receptors, especially the extranuclear steroid receptors in the kidney, are scarce. This information is crucial to the design of mechanistic studies that focus on the renal compartments that are most likely to mediate sex-specific effects on disease phenotypes. Current molecular probes for oestrogen and androgen receptors, as well as their co-activators and repressors, need to be validated in renal tissue and the results comprehensively reported. If existing probes are inadequate, then new ones need to be developed that recognize these receptors in different kidney cell types.
Many sophisticated genetic models of sex hormone receptor insufficiency already exist62,63, but they have not been carefully phenotyped for renal end points. For example, although many global sex hormone receptor knockouts in mouse models lack renal phenotypes, it is conceivable that abnormalities may only become evident under physiological stress or with comorbid disease or age. Investigating renal cell type-specific sex hormone receptor knockouts in male and female animals will be crucial to understanding sex differences attributed to sex hormone responsiveness in various renal diseases. Similar genetic studies for known activators and repressors of sex hormone action should be pursued if initial expression and mechanistic studies imply a role in cellular responsiveness.
Effects of sex chromosomes.
Current models of sex differences can be used to distinguish the effects of factors other than sex hormones (for example, sex chromosomes) on kidney phenotypes. For example, the four core genotype (FCG) mice, in which the sex-determining region of the Y chromosome gene (Sry) is deleted and inserted into an autosome, enables differentiation between the effects of sex chromosomes and sex hormones64. Studies using FCG mice have provided evidence of sex chromosome effects on hypertension45, endothelial function65 and bradycardic baroreflex responses66 that were independent of sex hormone effects. However, the FCG model has not been extensively used to distinguish sex hormone receptor effects from the genomic influence of sex chromosomes in renal diseases. Additional animal models of varying X chromosome dosage are also available, in which renal function can be studied under both physiological and pathophysiological conditions67.
Translational research
Given the wide gaps in knowledge of sex differences in renal physiology and pathophysiology, it may seem premature to think about translational research, defined here as the process of applying basic and clinical science knowledge to address critical medical needs and improve health outcomes. However, several areas in sex difference research warrant consideration when planning future translational studies. For example, it is important to assess whether promising disease biomarkers are differentially expressed in males and females, or whether sex differences in renal responses to drug therapies exist. Another important area for consideration is the study of individuals who have undergone significant changes in hormonal status in their lifetime (for example, transgender individuals) to further explore the physiology and implications of sex on varying disease states.
Sex-specific disease biomarkers.
A growing body of literature supports the importance of sex-specific biomarkers in predicting disease progression and outcome. A lack of controlling for sex is proposed to lead to up to 40% false discoveries in biomarker studies68 and to under-recognition of potential pathophysiology. For example, in ischaemic heart disease and heart failure, evidence suggests that ‘unisex’ troponin reference intervals might underdiagnose myocardial infarction in females, as baseline circulating concentrations of troponin are lower than in males69. Women with acute coronary syndromes present with lower troponin levels than men and those who do reach the standard troponin threshold might have suffered more severe myocardial damage at the time of detection than their male counterparts69. Another common biomarker with known sexual dimorphism is C-reactive protein, a marker of inflammation that may be up to 60% higher, on average, in women than in men70. Biomarkers may not only vary with sex but also with hormonal status among women, which is influenced for example, by the use of oral contraceptives, postmenopausal hormonal changes and stage of menstrual cycle68. For example, excretion of urinary fructose-1,6-bisphosphatase and glutathione-S-transferase peaked in women after ovulation and the onset of menses, whereas men and postmenopausal women showed consistently low levels of urinary fructose-1,6-bisphosphatase excretion over comparable time periods71. Despite these observations, potential sex-based differences in biomarkers are often overlooked.
Biomarkers of kidney function and disease may also differ by sex, but this possibility has not yet been rigorously studied. Studies in rats that demonstrated sex differences in the excretion levels of traditional and novel biomarkers of nephrotoxicity (such as alkaline phosphatase, cystatin C, liver-type fatty acid-binding protein (L-FABP), γ-glutamyl transpeptidase (γGTP) and β2-microglobulin (β2M)) support this notion72. Databases of biomarkers should therefore include detailed phenotyping so that sex-based differences in body composition and kidney function can be taken into account73. The use of biorepository samples from well-defined cohorts with known outcomes and shared databases should be encouraged — these resources might foster collaboration between basic scientists and clinicians and aid in the formulation of valid questions about how sex affects kidney function and disease progression. Existing information platforms, such as electronic health records, or the pairing of study cohorts with the Centers for Disease Control and Prevention, the World Health Organization and renal databases (for example, the US Renal Data System; USRDS) might be used to store and connect data from various cohort studies. Finally, given the potential implications of female hormonal status, as described in cardiovascular disease, it is important to examine biomarker repositories within the context of a woman’s life cycle, accounting for menopausal status73.
Renal responses to drug therapy.
Men and women differ in their responses to drug treatment owing to physiological differences, such as body weight, height and body surface area, as well as potential differences in pharmacokinetics or pharmacodynamics74. Unfortunately, sex is rarely considered when prescribing the type or dose of medication for the treatment of chronic diseases. In the context of renal diseases, many questions remain unanswered.
Studies in experimental models suggest an interaction between oestrogens and the renal expression and activity of components of the renin-angiotensin system75,76. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are recommended for the treatment of patients with diabetic and non-diabetic proteinuric renal disease, regardless of sex77. However, a 2019 population-based study suggested an increased hazard for the composite outcome of cardiovascular death, hospitalization for myocardial infarction or unstable angina in female (but not male) patients prescribed an ACEI compared with an ARB after a first myocardial infarction78. Likewise, all-cause mortality was higher in elderly women (but not men) with congestive heart failure who were prescribed ACEIs compared with ARBs79. These observations suggest possible sex differences in the response to ACEIs or ARBs, but data on such differences are not commonly analysed or reported and these issues require further study.
Sex-specific differences in pharmacokinetics and pharmacodynamics have also been proposed to exist in kidney transplant recipients treated with immunosuppressive drugs. Clearance of calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors is thought to be higher in women than in men80,81, whereas clearance of glucocorticoids and mycophenolate mofetil might be lower in women82,83. Of note, prolonged gastrointestinal transit time and lower gastric acid secretion might decrease the absorption of drugs in females compared with males, rendering certain medications less effective in women84.
Another aspect to consider is the effect of sex on renal drug metabolism. Differences between the sexes in the filtration, renal tubular transport and excretion of some drugs might lead to greater toxicity in women than in men85. Thus, medications excreted by the kidney, either as active drugs or metabolites, might have differential effects on women and men.
New study cohorts.
The dichotomous study of outcomes in males versus females may be an oversimplification — new cohorts might elucidate the physiology and implications of sex in varying disease states. Such cohorts might include transgender individuals, body builders, patients with androgen insensitivity, individuals with infertility or who are undergoing fertility therapy, persons with genetic variations that result in sexual dimorphism and pregnant women with medical conditions. Studies in such individuals might lead to a better understanding of how sex hormones contribute to disease mechanisms independently of sex chromosomes in humans.
The differential and respective effect of genetic, hormonal and environmental factors may be explored in transgender individuals who undergo gender-affirming medical intervention with sex hormones86. For example, cross-sex treatment with oestrogens has been associated with a higher risk of venous thromboembolism and ischaemic stroke in transfeminine persons undergoing male-to-female transformation than in age-matched cisgender male and female controls87.Renal metabolic and functional changes in transgender individuals have not yet been explored in detail. The Gender Dysphoria Treatment in Sweden study is creating a dataset with information on patients with gender dysphoria undergoing cross-sex hormone treatment86 and the European Network for the Investigation of Gender Incongruence, the largest study of transgender individuals to date, includes over 2,600 participants88. Investigation of such cohorts might enable researchers to obtain data regarding sex, gender and selected renal parameters in the context of CKD to evaluate how physiological changes driven by exogenous hormones affect the renal phenotype.
Tools, approaches and resources
To date, the majority of renal physiology studies have been conducted in males89. One reason for this sex bias might be the belief that females are more intrinsically variable than males, owing to their hormonal cycles90. Although future studies should certainly include females, such a change would be insufficient to address the existing knowledge gap. First, many existing experimental models of renal disease have not been adapted for females. This difference is consequential, because observations in animal models, as well as in selected human populations, demonstrate that females commonly seem to be protected, or at least develop a less severe form of the renal disease being studied91. This discrepancy highlights the importance of either developing new models or adapting existing models to induce a phenotype in females. Second, studying a single sex in isolation often masks important research questions that might only be answered through a direct comparison of the two sexes in adequately powered studies. Third, both preclinical and clinical studies are needed to appropriately address specific research questions concerning sex and the kidney92. In addition to experimental models, there seems to be a need to develop appropriate tools and approaches for studying sex differences, some of which are outlined below.
New animal models.
A study that analysed high-throughput phenotype data from 14,250 wild-type and 40,192 mutant mice (representing 2,186 knockout lines) showed that up to 56.6% of quantitative traits in wild-type mouse models exhibit a degree of sexual dimorphism93. These findings indicate that extrapolating data from a sample in which males are over-represented to the general population may be misleading and underscores the need to avoid the exclusive study of male animals. In the context of renal disease, the lack of animal models that adequately recapitulate the human condition, especially for women, needs to be addressed. Models of renal disease in which the female kidney is protected, including hypertension, acute kidney injury (AKI), ischaemia-reperfusion and diabetic kidney disease94–98, represent an opportunity to investigate the mechanisms underlying resistance to renal disease in women. Such insights might contribute to the development of novel therapeutic approaches for both women and men.
Animal models in which gonadal hormones or sex hormone receptors have been manipulated, such as the aromatase-deficient mouse or the ERα- and ERβ-deficient mouse, are widely available, but have not been as extensively used by the renal research community as in other fields. Superimposing gonadal hormones and sex hormone receptor deletions on existing experimental models of renal disease might create useful tools for the study of sex differences.
As discussed above, the FCG model can be used to separate the contribution of sex hormones from the effects of sex chromosomes64. Moreover, studies in women with Turner’s syndrome (who have a single X chromosome; XO) show that those with maternal X chromosome inheritance (XmO) have a stiffer aorta99 and a greater atherogenic profile100 than those with paternal X chromosome inheritance (XpO). Haploinsufficiency for genes encoded in the short arm of the X chromosome (Xp) also increases the risk of type 2 diabetes mellitus101. However, the importance of X inheritance and X inactivation in kidney disease has yet to be investigated. Unfortunately, the poor survival of XpO animals hinders the use of XmO and XpO mice in parallel to differentiate the effects of maternal versus paternal inheritance of the X chromosome102. Thus, additional animal models are needed to further evaluate the role of sex chromosome dosage, parental imprinting and escape from X-inactivation in renal physiology and pathophysiology.
Datasets from experimental models that use large animals constitute another rich resource for sex difference research. Unlike typical breeding practices in mice and rodents, in which frequently only the male pups are kept for further study, the expense of breeding large animals promotes investigations using both sexes. Collating renal data from these large animal datasets might enable comparative genomics studies between the sexes.
Existing cohorts.
The NIDDK supports several centres that can be used to promote tool development and provide necessary resources to the renal research community interested in sex differences research. For example, the O’Brien Kidney Center and Polycystic Kidney Disease Centers provide core services and funds for pilot and feasibility projects103. These programmes can provide an avenue for investigators to initiate studies in sex differences. The core services vary between centres but can either serve as universal phenotyping resources or provide needed reagents for the investigative community. Furthermore, the development of novel hypotheses that inform future epidemiological, clinical and basic mechanistic studies on sex differences in renal disease could be facilitated by developing data mining tools and encouraging investigators to efficiently utilize existing data from human cohorts, including data from the Chronic Renal Insufficiency Cohort study (CRIC)104, Chronic Kidney Disease in Children Cohort study (CKiD)105 and the USRDS106.
The NIDDK initiated the Kidney Precision Medicine Project (KPMP) in response to recognition that advances in multi-scale interrogation of human tissue and single cells have paved the way for precision medicine in nephrology107. This project is aimed at collecting and analysing human kidney biopsy samples from participants with AKI and CKD to create a kidney tissue atlas, define disease subgroups and identify crucial cells, pathways and targets for novel therapies. The KPMP also creates a timely opportunity to collect sex and gender data elements on a large cohort of patients with renal disease. Specifically, it would be useful to collect data on hormonal and reproduction status, number of pregnancies, as well as psychosocial and psychological variables that affect gender identity for all consenting KPMP participants. Such a valuable dataset would not only benefit the renal community but could also serve as a blueprint for similar projects in other areas of medicine; collecting such data in all prospective cohorts should be encouraged.
Multidisciplinary research.
The future of sex differences research will require scientific partnership between the renal research community and investigators outside traditional nephrology. Contributions from endocrinologists, sex hormone receptor biologists, reproductive physiologists and geneticists will be essential to provide context and ensure appropriate interpretation of emerging data. Chemists and structural biologists must also be engaged to develop novel SERMs and selective androgen receptor modulators that enable finer experimental manipulation of sex hormone receptors. Establishing teams that include such experts will be crucial to much-needed research.
Multidisciplinary and collaborative studies should be encouraged to prospectively address issues regarding the physiology and implications of sex differences in kidney health and disease. These research teams should include basic, clinical and statistical expertise, and use systems approaches, rather than organ-specific investigations. To foster a community of collaboration between basic and clinical scientists, the potential to increase support for specific training programmes and early career awards to develop the next generation of translational investigators (both clinicians and basic scientists) should be explored.
Conclusions
Accumulating evidence from epidemiological and clinical studies suggests that sex-related differences might exist in the prevalence, course and severity of renal diseases. Experimental studies have provided some evidence that female sex hormones might be renoprotective, but a better understanding of how sex hormones and sex chromosomes regulate renal function under both normal physiological and pathophysiological conditions is needed. The opportunities for future research outlined in this report, directed at addressing some of these knowledge gaps, also highlight the need to develop new resources and appropriate training. These opportunities are not intended to limit or direct future research related to sex differences in renal disease, but rather to stimulate thought and interest in the research community as it considers sex as a biological variable108.
Acknowledgements
The authors thank all the invited speakers: K. Korach, S. Hammes, C. Smith, C. Disteche, F. Mauvais-Jarvis, A. Ricardo, J. Morton, J. Fuscoe, V. Garovic, J. Charlton and V. Miller, and all workshop participants for their thoughtful comments and ideas offered during the workshop. The authors thank the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for providing funding for the workshop. The views expressed in this article are those of the authors and do not necessarily represent the views of the NIDDK, the US National Institutes of Health (NIH) or the United States Department of Health and Human Services.
Glossary
- Parental imprinting
A process that results in allele-specific differences in transcription, DNA methylation and DNA replication timing
- X chromosome mosaicism
The presence of two populations of cells in the body: some cells have two X chromosomes whereas others have only one X chromosome
Footnotes
Competing interests
The authors declare no competing interests.
RELATED LiNKS
NIDDK Information Network: https://dknet.org/
References
- 1.National Institutes of Health. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Fed. Register 59, 14508–14513 (1994). [Google Scholar]
- 2.Scott PE et al. Participation of women in clinical trials supporting fda approval of cardiovascular drugs. J. Am. Coll. Cardiol 71, 1960–1969 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Pilote L & Raparelli V Participation of women in clinical trials: not yet time to rest on our laurels. J. Am. Coll. Cardiol 71, 1970–1972 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Geller SE, Koch A, Pellettieri B & Carnes M Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J. Womens Health 20, 315–320 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health. Consideration of sex a biological variable in NIH-funded research. NIH; https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (2015). [Google Scholar]
- 6.Silbiger S & Neugarten J Gender and human chronic renal disease. Gend. Med 5, S3–S10 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Silbiger SR & Neugarten J The role of gender in the progression of renal disease. Adv Ren. Replace. Ther 10, 3–14 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Neugarten J, Acharya A & Silbiger SR Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J. Am. Soc. Nephrol 11, 319–329 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Carrero JJ, Hecking M, Chesnaye NC & Jager KJ Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol 14, 151–164 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Cobo G et al. Sex and gender differences in chronic kidney disease: progression to end-stage renal disease and haemodialysis. Clin. Sci. (Lond.) 130, 1147–1163 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Ricardo AC et al. Sex-related disparities in CKD progression. J. Am. Soc. Nephrol 30, 137–146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka R et al. Protective effect of 17beta-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur. J. Pharmacol 683, 270–275 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Delle H et al. Antifibrotic effect of tamoxifen in a model of progressive renal disease. J. Am. Soc. Nephrol 23, 37–48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mankhey RW, Bhatti F & Maric C 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am. J. Physiol. Ren. Physiol 288, F399–F405 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman MA et al. Long- but not short-term estradiol treatment induces renal damage in midlife ovariectomized Long-Evans rats. Am. J. Physiol. Ren. Physiol 312, F305–F311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences. Exploring the Biological Contributions to Human Health: Does Sex Matter? Vol. 10 (eds Wizemann TM & Parde M-L) 433–439 (National Academies Press, 2001). [PubMed] [Google Scholar]
- 17.Hill NR et al. Global prevalence of chronic kidney disease — a systematic review and meta-analysis. PLOS ONE 11, e0158765 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills KT et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 88, 950–957 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang QL & Rothenbacher D Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 8, 117 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy D et al. Trends in prevalence of chronic kidney disease in the united states. Ann. Intern. Med 165, 473–481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Renal Data System. Atlas of chronic disease and end-stage renal disease in the United States. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: 2012). [Google Scholar]
- 22.Glassock RJ & Winearls C An epidemic of chronic kidney disease: fact or fiction? Nephrol. Dial Transpl 23, 1117–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Neugarten J & Golestaneh L Influence of sex on the progression of chronic kidney disease. Mayo Clin. Proc 94, 1339–1356 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Silbiger SR & Neugarten J The impact of gender on the progression of chronic renal disease. Am. J. Kidney Dis 25, 515–533 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Gilg J, Castledine C & Fogarty D Chapter 1 UK RRT incidence in 2010: national and centre-specific analyses. Nephron Clin. Pract 120, c1–c27 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Hecking M et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS). PLOS MED. 11, e1001750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iseki K et al. Increasing gender difference in the incidence of chronic dialysis therapy in Japan. Ther. Apher. Dial 9, 407–411 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Ricardo AC et al. Sex-related disparities in CKD progression. J. Am. Soc. Nephrol 30, 137–146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kattah AG et al. CKD in patients with bilateral oophorectomy. Clin J. Am. Soc. Nephrol 7, 1649–1658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliot SJ et al. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am. J. Pathol 162, 1441–1448 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reckelhoff JF & Baylis C Glomerular metalloprotease activity in the aging rat kidney: inverse correlation with injury. J. Am. Soc. Nephrol 3, 1835–1838 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Melamed ML et al. Raloxifene, a selective estrogen receptor modulator, is renoprotective: a post-hoc analysis. Kidney Int. 79, 241–249 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Sandberg K, Pai AV & Maddox T Sex and rigor: the TGF-beta blood pressure affair. Am. J. Physiol. Ren. Physiol 313, F1087–F1088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inada A et al. Adjusting the 17beta-estradiol-to-androgen ratio ameliorates diabetic nephropathy. J. Am. Soc. Nephrol 27, 3035–3050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon A & Maric C 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am. J. Physiol. Ren. Physiol 293, F1678–F1690 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doublier S et al. Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 79, 404–413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catanuto P et al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 75, 1194–1201 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Keck M, Romero-Aleshire MJ, Cai Q, Hoyer PB & Brooks HL Hormonal status affects the progression of STZ-induced diabetes and diabetic renal damage in the VCD mouse model of menopause. Am. J. Physiol. Ren. Physiol 293, F193–F199 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM & Pollock JS Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 293, R1573–R1579 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R & Reckelhoff JF Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am. J. Physiol. Heart. Circ. Physiol 295, H466–H474 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manigrasso MB, Sawyer RT, Marbury DC, Flynn ER & Maric C Inhibition of estradiol synthesis attenuates renal injury in male streptozotocin-induced diabetic rats. Am. J. Physiol. Ren. Physiol 301, F634–F640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manigrasso MB, Sawyer RT, Hutchens ZM Jr., Flynn ER & Maric-Bilkan C Combined inhibition of aromatase activity and dihydrotestosterone supplementation attenuates renal injury in male streptozotocin (STZ)-induced diabetic rats. Am. J. Physiol. Ren. Physiol 302, F1203–F1209 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortepiani LA, Yanes L, Zhang H, Racusen LC & Reckelhoff JF Role of androgens in mediating renal injury in aging SHR. Hypertension 42, 952–955 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Baltatu O et al. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J. Am. Soc. Nephrol 13, 2681–2687 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Ji H et al. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 55, 1275–1282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jean-Faucher C et al. Sex-related differences in renal size in mice: ontogeny and influence of neonatal androgens. J. Endocrinol 115, 241–246 (1987). [DOI] [PubMed] [Google Scholar]
- 47.Neugarten J, Kasiske B, Silbiger SR & Nyengaard JR Effects of sex on renal structure. Nephron 90, 139–144 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Oudar O et al. Differences in rat kidney morphology between males, females and testosterone-treated females. Ren. Physiol. Biochem 14, 92–102 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Baylis C Sexual dimorphism of the aging kidney: role of nitric oxide deficiency. Physiology 23, 142–150 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Munger K & Baylis C Sex differences in renal hemodynamics in rats. Am. J. Physiol 254, F223–F231 (1988). [DOI] [PubMed] [Google Scholar]
- 51.Remuzzi A, Puntorieri S, Mazzoleni A & Remuzzi G Sex related differences in glomerular ultrafiltration and proteinuria in munich-wistar rats. Kidney Int. 34, 481–486 (1988). [DOI] [PubMed] [Google Scholar]
- 52.Sabolic I et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol 302, C1174–C1188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veiras LC et al. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J. Am. Soc. Nephrol 28, 3504–3517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herak-Kramberger CM et al. Sex-dependent expression of water channel AQP1 along the rat nephron. Am. J. Physiol. Ren. Physiol 308, F809–F821 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breljak D, Brzica H, Sweet DH, Anzai N & Sabolic I Sex-dependent expression of Oat3 (Slc22a8) and Oat1 (Slc22a6) proteins in murine kidneys. Am. J. Physiol. Ren. Physiol 304, F1114–F1126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breljak D et al. Renal expression of organic anion transporter Oat5 in rats and mice exhibits the female-dominant sex differences. Histol. Histopathol 25, 1385–1402 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Li Q, McDonough AA, Layton HE & Layton AT Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am. J. Physiol. Ren. Physiol 315, F692–F700 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelletier G Localization of androgen and estrogen receptors in rat and primate tissues. Histol. Histopathol 15, 1261–1270 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Boese AC, Kim SC, Yin KJ, Lee JP & Hamblin MH Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart. Circ. Physiol 313, H524–H545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marrocco J & McEwen BS Sex in the brain: hormones and sex differences. Dialogues Clin. Neurosci 18, 373–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton M Position paper: the membrane estrogen receptor GPER — clues and questions. Steroids 77, 935–942 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Stefkovich ML, Arao Y, Hamilton KJ & Korach KS Experimental models for evaluating non-genomic estrogen signaling. Steroids 133, 34–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang C, Yeh S, Lee SO & Chang TM Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl. Recept. Signal 11, e001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnold AP & Chen X What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol 30, 1–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pessoa BS et al. Angiotensin II Type 2 receptor-and acetylcholine-mediated relaxation: essential contribution of female sex hormones and chromosomes. Hypertension 66, 396–402 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Caeiro XE, Mir FR, Vivas LM, Carrer HF & Cambiasso MJ Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertension 58, 505–511 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Arnold AP, Chen X & Itoh Y What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation. Handb. Exp. Pharmacol 67–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsey JM, Cooper JD, Penninx BW & Bahn S Variation in serum biomarkers with sex and female hormonal status: implications for clinical tests. Sci. Rep 6, 26947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sobhani K et al. Sex differences in ischemic heart disease and heart failure biomarkers. Biol. Sex Differ 9, 43 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogowski O et al. Gender difference in C-reactive protein concentrations in individuals with atherothrombotic risk factors and apparently healthy ones. Biomarkers 9, 85–92 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Seppi T et al. Sex differences in renal proximal tubular cell homeostasis. J. Am. Soc. Nephrol 27, 3051–3062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsuji S, Sugiura M, Tsutsumi S & Yamada H Sex differences in the excretion levels of traditional and novel urinary biomarkers of nephrotoxicity in rats. J. Toxicol. Sci 42, 615–627 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Lew J et al. Sex-based differences in cardiometabolic biomarkers. Circulation 135, 544–555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soldin OP & Mattison DR Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet 48, 143–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol. Sex Differ 1, 6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandberg K & Ji H Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv. Ren. Replace. Ther 10, 15–23 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Roberts MA Commentary on the KDIGO Clinical Practice Guideline for the management of blood pressure in chronic kidney disease. Nephrology 19, 53–55 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Ko D et al. Comparative effectiveness of ACE inhibitors and angiotensin receptor blockers in patients with prior myocardial infarction. Open Heart 6, 1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hudson M, Rahme E, Behlouli H, Sheppard R & Pilote L Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure — a population study. Eur. J. Heart Fail 9, 602–609 (2007). [DOI] [PubMed] [Google Scholar]
- 80.Kahan BD et al. Demographic factors affecting the pharmacokinetics of cyclosporine estimated by radioimmunoassay. Transplant. 41, 459–464 (1986). [DOI] [PubMed] [Google Scholar]
- 81.Zimmerman JJ Exposure-response relationships and drug interactions of sirolimus. AAPS J. 6, e28 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magee MH, Blum RA, Lates CD & Jusko WJ Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J. Clin. Pharmacol 41, 1180–1194 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tornatore KM et al. Influence of sex and race on mycophenolic acid pharmacokinetics in stable African American and Caucasian renal transplant recipients. Clin. Pharmacokinet 54, 423–434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stolarz AJ & Rusch NJ Gender differences in cardiovascular drugs. Cardiovasc. Drugs Ther 29, 403–410 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Abdel-Rahman AA Influence of sex on cardiovascular drug responses: role of estrogen. Curr. Opin. Pharmacol 33, 1–5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiik A et al. Metabolic and functional changes in transgender individuals following cross-sex hormone treatment: design and methods of the Gender Dysphoria Treatment in Sweden (GETS) study. Contemp. Clin. Trials Commun 10, 148–153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Getahun D et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann. Intern. Med 169, 205–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kreukels BP et al. A European network for the investigation of gender incongruence: the ENIGI initiative. Eur. Psychiatry 27, 445–450 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Zucker I & Beery AK Males still dominate animal studies. Nature 465, 690 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Becker JB, Prendergast BJ & Liang JW Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol. Sex Differ 7, 34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silbiger SR Raging hormones: gender and renal disease. Kidney Int. 79, 382–384 (2011). [DOI] [PubMed] [Google Scholar]
- 92.de Caestecker M et al. Bridging translation by improving preclinical study design in AKI. J. Am. Soc. Nephrol 26, 2905–2916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karp NA et al. Prevalence of sexual dimorphism in mammalian phenotypic traits. Nat. Commun 8, 15475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sullivan JC & Gillis EE Sex and gender differences in hypertensive kidney injury. Am. J. Physiol. Ren. Physiol 313, F1009–F1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boddu R et al. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am. J. Physiol. Ren. Physiol 313, F740–F755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Alencar Franco Costa D et al. Sex-dependent differences in renal angiotensinogen as an early marker of diabetic nephropathy. Acta Physiol. 213, 740–746 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Kang KP et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mo. Med. Rep 9, 2061–2068 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bloor ID, Sebert SP, Mahajan RP & Symonds ME The influence of sex on early stage markers of kidney dysfunction in response to juvenile obesity. Hypertension 60, 991–997 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Abd-Elmoniem KZ et al. X chromosome parental origin and aortic stiffness in turner syndrome. Clin. Endocrinol 81, 467–470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van PL, Bakalov VK & Bondy CA Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J. Clin. Endocrinol. Metab 91, 2867–2870 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Bakalov VK, Cheng C, Zhou J & Bondy CA X-chromosome gene dosage and the risk of diabetes in Turner syndrome. J. Clin. Endocrinol. Metab 94, 3289–3296 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He N et al. At Term, XmO and XpO mouse placentas show differences in glucose metabolism in the trophectoderm-derived outer zone. Front. Cell Dev. Biol 5, 63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.NIDDK. Kidney disease centers. NIH; https://www.niddk.nih.gov/research-funding/research-programs/kidney-disease-centers (2019). [Google Scholar]
- 104.NIDDK. Effects of chronic kidney disease in adults study: CRIC. NIH; https://www.niddk.nih.gov/about-niddk/research-areas/kidney-disease/effects-chronic-kidney-disease-adults-study-cric (2019). [Google Scholar]
- 105.CKID. Chronic kidney disease in children. John Hopkins University Bloomberg School of Public Health; https://statepi.jhsph.edu/ckid (2019). [Google Scholar]
- 106.USRDS. United states renal data system. USRDS; https://www.usrds.org (2019). [Google Scholar]
- 107.NIDDK. Kidney precision medical project. NIH; https://www.niddk.nih.gov/research-funding/research-programs/kidney-precision-medicine-project-kpmp (2019). [Google Scholar]
- 108.Clayton JA & Collins FS Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


