Abstract
Background
Many patients with alpha-1 antitrypsin deficiency (A1ATD) receive care in respiratory clinics without access to specialist hepatology expertise. Liver disease can develop asymptomatically, and non-invasive markers of fibrosis may help identify patients who require definitive assessment with liver biopsy. We evaluated the utility of non-invasive markers of liver fibrosis in A1ATD to guide testing in settings without ready access to hepatology expertise.
Methods
Patients attending the London A1ATD service undergo assessment using blood tests to calculate the ‘APRI’ and ‘FIB-4’ score, liver ultrasound and Fibroscan. Liver biopsy is offered to patients who have abnormal liver function tests with abnormal liver ultrasound and/or liver stiffness >6 kPa on Fibroscan. Liver biopsies were assessed for the presence of A1AT, steatosis, fibrosis and inflammation.
Results
75 patients with A1ATD had results for analysis, 56% were female, age 16–82 years. 75% of patients had Fibroscan <6 kPa, 19% had Fibroscan 6–7.9 kPa and 6%>8 kPa. There was a significant correlation between FIB-4 and Fibroscan (r=0.244, p=0.035). Fibroscan >6 kPa corresponded to a FIB-4 score of >1.26. However, FIB-4 >1.26 had poor sensitivity (47%), specificity (32%) and positive-predictive value (PPV; 36%) to identify Fibroscan >6 kPa. The negative-predictive value (NPV) was stronger at 81%. APRI data were similar. Twelve patients underwent liver biopsy, with 11 reports available for analysis. Six had FIB-4 scores<1.26 and five had Fibroscan of <6 kPa. A1AT was present in 64% of biopsies, steatosis in 82%, mild fibrosis in 36%, moderate fibrosis in 9% and severe fibrosis in 9%.
Conclusion
A combination of liver ultrasound and non-invasive fibrosis tests can help identify patients with A1ATD liver injury. However, APRI and FIB-4 scores alone had poor sensitivity and specificity to justify use as an independent tool for liver pathology in A1ATD.
Keywords: alpha1 antitrypsin deficiency
Key messages.
Which non-invasive tests should be used by respiratory specialists to assess liver disease in alpha-1 antitrypsin deficiency (A1ATD)?
More research is required to establish a test which accurately assesses liver disease in A1ATD patients.
This paper helps inform decisions for respiratory specialists managing patients with A1ATD in a setting without specialist hepatology expertise.
Introduction
Alpha-1 antitrypsin deficiency (A1ATD) is a genetic condition which affects the production of alpha-1 antitrypsin in the liver.1 The consequences of this can result both in liver and lung pathology. Previous work has identified the particular significance of the PiZZ genotype in the development of early onset emphysema (said to be the cause of 1%–2% of chronic obstructive pulmonary disease (COPD)),2 as well as liver fibrosis, cirrhosis and hepatocellular carcinoma.3
Developments in hepatology have included scoring systems for liver disease assessment and monitoring, such as the APRI (aspartate aminotransferase (AST) to platelet ratio) and FIB-4 score (see below).4 Other key non-invasive liver assessments include ultrasound scans5 and transient elastography6 (eg, Fibroscan), the use of which aims to replace or better inform need for invasive investigation with liver biopsy.7 8
Currently, European Association for the Study of the Liver guidelines have evaluated the use of non-invasive monitoring in various liver diseases, including alcoholic liver disease, non-alcoholic fatty liver disease and infective forms of hepatitis. However, as of the 2015 guidelines, no evaluation has been published about non-invasive assessment of A1ATD liver disease.9 Further research is required regarding streamlining surveillance of liver disease in A1ATD. This highlights an unmet area of need, especially given the central involvement of respiratory physicians in A1ATD care for patients who present with respiratory manifestations or who are detected while asymptomatic through screening family members.
The most common diagnosis established in A1ATD patients is emphysema/COPD,10 such that patients are more likely to interact with a respiratory physician than a hepatologist, even if a significant contributor to A1ATD morbidity and mortality are complications arising from liver fibrosis.11 The aim of this work was to identify opportunities to streamline disease monitoring for A1ATD patients where specialist hepatology input is not immediately available. At the London A1ATD service, based at the Royal Free Hospital, disease progression is monitored at an outpatient clinic with both respiratory and hepatology input12 yet even here the need to have two teams providing input in a single clinic is resource-intensive. Current UK National Institute for Health and Care Excellence guidance recommends offering referral of A1ATD patients to a specialist centre,13 which can further delay provision of care. Hence, the aim of this work was to assess whether it may be possible for clinicians other than hepatologists to play a greater part in the effective assessment and monitoring of liver pathology in A1ATD, and to identify those patients who require specialist hepatology input.
Methods
Patients attending the London A1ATD service provided consent for data to be collated into a database comprising clinical information including liver and respiratory evaluations.
Patients were recruited at the London A1ATD clinic. All patients were PiZZ (based on low serum concentration, phenotyping and gene probing when phenotyping was unclear). There were no other specific inclusion/exclusion criteria for inclusion of data in this analysis.
The FIB-4 score was calculated using age, platelet count, alanine aminotransferase (ALT) and AST as follows: FIB-4=age (years) x AST (iU/L)/(platelets (109/L) x (ALT (iU/L))).9 Blood tests were measured on standard hospital analysers. Liver ultrasound and transient elastography (Fibroscan) were performed on the same day. Liver biopsy was offered to patients who had liver stiffness >6 kPa or had high transaminase values with an abnormal liver ultrasound scan. The Fibroscan cut-off was selected by consultant Hepatologists aiming to ensure that all patients with possible liver fibrosis were captured for consideration of definitive investigation. Liver biopsies were assessed for the presence of A1AT (periodic acid-schiff with diastase (DPAS) for tissue staining), steatosis, fibrosis and inflammation by a consultant histopathologist.
Using these data, we evaluated the relationships between blood tests (FIB-4 score and APRI) and other tests in patients with A1ATD. Fibroscan scores of >6 kPa or more were locally accepted as the cut-off for liver pathology for A1ATD.
Data were assessed for normality using histograms and are reported as n (%), mean (SD) or median (IQR) as appropriate. Data were analysed using SPSS; p≤0.05 was defined as statistically significant.
Results
75 patients with A1ATD had both serum blood tests and Fibroscan results available for analysis. Of this population, 56% were female and demographic data are presented in table 1. The age ranged from 16 to 82 years. 12 patients had biopsy data. 11 of these were suitable for analysis. Fibroscan scores ranged from 2.8 to 15.3 kPa. FIB-4 scores ranged from 0.29 to 4.37. APRI scores ranged from 0.08 to 1.00.
Table 1.
Demographics of patients in the London A1AT cohort
| Total patients, n | 75 |
| Male | 33 (44%) |
| Biopsies | 12 (16%) |
| Age (years) | 54 (15.6) |
| FIB-4 score | 1.20 (0.75) |
| Fibroscan (kPa) | 5.57 (2.22) |
| AST (iu/L) | 27.56 (12.64) |
| ALT (iu/L) | 29.74 (13.72) |
| Platelets (x10∧9/L) | 258.9 (76.8) |
Data presented as n (%) or mean and SD as appropriate.
A1AT, alpha-1 antitrypsin deficiency; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
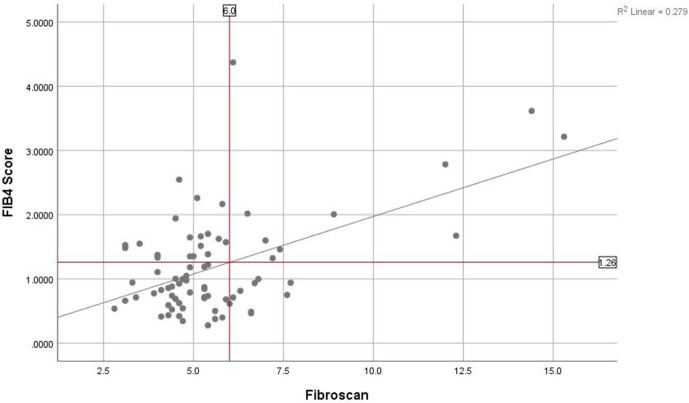
There was a weak but statistically significant correlation between FIB-4 and liver stiffness (r=0.244, p<0.035). Using the established Fibroscan cut-off at 6 kPa, the corresponding FIB-4 score was established to be 1.26 and this was used as the basis for assessing the performance of liver assessment without access to Fibroscan (figure 1). Using a FIB-4 cut-off of 1.26, there was poor sensitivity (53%), specificity (68%) and PPV (36%) to identify a Fibroscan >6 kPa (table 2). The NPV was higher at 81%. Evaluation using a receiver-operating characteristic curve showed that this approach had an area under the curve (AUC) of 0.646.
Figure 1.
Scatter plot of FIB-4 score against Fibroscan. Vertical line denotes the cut-off of 6 kPa used on Fibroscan to recommend liver biopsy. Horizontal line denotes the FIB-4 score which corresponds to the Fibroscan result, established along the line of best fit.
Table 2.
Calculations for evaluation of FIB-4 as a sole marker of fibrosis in the entire cohort
| FIB-4 ≥1.26 | FIB-4 <1.26 | Total | |
| Fibroscan ≥6 kPa | 10 (13%) | 9 (12%) | 19 (25%) |
| Fibroscan <6 kPa | 18 (24%) | 38 (51%) | 56 (75%) |
| Total | 28 (37%) | 47 (63%) | 75 (100%) |
When comparing male and females, a statistically significant correlation between FIB-4 and Fibroscan was seen in the female group (r=0.314, p<0.046). The males had a coefficient of 0.112 p=0.528. Using the correlation in the female group, the corresponding FIB-4 score for 6 kPa on Fibroscan was 1.32. Using this FIB-4 score for female patients, the sensitivity was 75%, specificity 70%, PPV 38% and NPV 92% (table 3). Evaluated in the same manner, the ROC curve in females had an AUC of 0.811.
Table 3.
Calculations for evaluation of FIB-4 as a sole marker of fibrosis in females
| FIB-4 ≥1.32 | FIB-4 <1.32 | Total | |
| Fibroscan ≥6 kPa | 6 (15%) | 2 (5%) | 8 (20%) |
| Fibroscan <6 kPa | 10 (24%) | 23 (56%) | 33 (80%) |
| Total | 16 (39%) | 25 (61%) | 41 (100%) |
Using APRI, a cut-off at 0.308 corresponded to a Fibroscan score of 6.0 kPa. This value resulted in high specificity (77%), but a low sensitivity (50%), PPV (41%) and NPV (52%). APRI data are reported in table 4. Evaluation using a ROC curve showed that an approach using APRI had an AUC of 0.72.
Table 4.
Calculations for evaluation of APRI as a sole marker of fibrosis in the entire cohort
| APRI≥0.308 | APRI≤0.308 | Total | |
| Fibroscan ≥6 kPa | 9 (12%) | 9 (12%) | 18 (24%) |
| Fibroscan <6 kPa | 13 (18%) | 43 (58%) | 56 (76%) |
| Total | 22 (30%) | 52 (70%) | 74 (100%) |
Of the 12 patients who had liver biopsy data available, 11 were suitable for analysis. Their mean alcohol consumption was 11.7 units week, and Body Mass Index was 26.1 kg/m-2. Biopsies were performed in patients with either a Fibroscan score of 6.0 kPa or higher, or high liver function tests with an abnormal ultrasound scan. Six had FIB-4 scores <1.26 and 5 had a Fibroscan of <6 kPa. 92% showed pathology in the biopsy specimen (only a single biopsy specimen showed no pathology). The Fibroscan scores in patients with moderate or severe fibrosis were >8 kPa. This suggested that current cut-off values for Fibroscan results for liver biopsy were sensitive.
Discussion
We report on the utility of simple blood-based scoring systems to better inform the need for liver biopsy in A1ATD in the absence of access to transient elastography (Fibroscan) which is not available in many respiratory clinics.
Currently, assessment and monitoring for early stage liver pathology in our specialist A1ATD service is performed with APRI and FIB-4 score, Fibroscan and ultrasound to guide the need for liver biopsy. However, Fibroscans to monitor liver disease require equipment and trained personnel. This can prove problematic for patients distant from the nearest specialist centre and an approach using routine blood tests would be valuable. Additionally, involvement of specialised investigations for liver disease assessment typically require the input of hepatology specialists for a condition where diagnosis and monitoring more often take place in respiratory clinics. All patients with potentially hepatotoxic A1ATD mutations warrant liver disease assessment, and thus it is important to streamline care in order to save time and resources. Furthermore, the procurement and maintenance of Fibroscanners is costly, which would further complicate having infrastructure to perform the scans in a cost-limited setting.14
Other studies relating to non-invasive disease monitoring of liver disease in patients with A1ATD have focused on tests to demonstrate structural pathology, rather than to evaluate use as a marker of fibrosis.15 This approach warrants additional scrutiny, given the lack of reliability in using just one liver test.
Research involving non-invasive markers of disease to monitor A1ATD is limited, but Guillard et al discuss the use of Fibroscans for the monitoring of liver pathology in A1ATD.16 While our focus was on concordance between APRI, FIB-4 scores and Fibroscan results, Guillard presents a case for the use of Fibroscans for screening, further reinforced by the finding that 2 of 2 patients with abnormal liver function tests (LFTs) also had abnormal Fibroscan results, but 3 of 29 patients without abnormal LFTs presented with abnormal Fibroscan results. Our identification of a relatively high NPV between FIB-4 scores and Fibroscans supports using the former as a screening tool, although Guillard used different Fibroscan thresholds to determine pathology (6.0 kPa against 7.2 kPa).
A further paper, by Tanash reported that serial liver enzyme tests can act as a marker for the development of liver disease.10 This work differs from ours by using the test as a predictor of future liver disease, as opposed to a marker of current liver disease. Additionally, the Tanash study used repeated blood markers as a predictor, while we only focused on a single measurement at the time of the Fibroscan. Further work is required to understand the value of repeated assessment of liver function tests to act as an independent marker of liver disease.
Our data are similar to Mostafavi who reported no statistically significant difference in blood liver function tests when categorising patients by elastography results.15 However, they did note a statistically significant correlation between liver stiffness and ALP. We did not have ALP data available for our cohort. Mostafavi did not attempt to use liver function as an independent marker of liver fibrosis, either with a combined FIB-4 score or ALP alone. Similarly, Hamesch17 suggested GGT may be a better individual marker for liver fibrosis than ALP. This work suggested that blood tests were inadequate for the purposes of determining risk in men, but showed promise for women, and was the motivation for our analysis by sex which had similar results.
The APRI (AST to platelet ratio) has been recognised as a useful tool to identify liver fibrosis in resource limited settings for both hepatitis B18 and C.19 Previous work considering the use of APRI in has also been completed in A1ATD by Hamesch et al,17 Clark et al20 and Kumpers et al.21 Hamesch reported that APRI was higher in A1ATD patients with liver disease. However, this work offers no sensitivity and specificity to inform the use of APRI as a diagnostic test. Clark et al20 used an APRI cut-off of 0.43, reporting a sensitivity of 0.87 (AUC 0.7). Our AUC for APRI was similar at 0.72, however this was using a lower APRI threshold of 0.308 and a Fibroscan cut-off of 6.0. Mindful of this, our work suggests that more research is required before APRI can be used to monitor liver fibrosis in A1ATD. Currently, both Clark and Kumpers conclude that liver elasticity testing is superior to APRI. This is reinforced with our work, given a serum test using local cut-offs did not have sufficient AUC to justify not measuring liver elasticity.
Since the majority of patients present to A1ATD services with lung pathology, or through screening, it would be of value to derive a simple algorithm for respiratory clinicians to assess and monitor liver disease without the need for hepatology input until a certain test ‘cut-off’ arises. However, of those tests routinely performed to monitor this, our work shows that FIB-4 score requires further investigation before it can be used in this way. Until then, the need to monitor liver disease non-invasively with a combination of blood tests, Fibroscans and ultrasound remains. Deriving a single test to screen for liver disease may also be of use in the primary care setting, particularly with patients who may already be aware of their genetic phenotype for A1ATD, but do not present with any symptoms.
Acknowledgments
We would like to like to thank the patients of the London A1ATD service.
Footnotes
Contributors: SHA, JRH and AM conceived the analysis with input from DAL, DT and BG. Data collection was supported by EP. The manuscript was drafted by SHA and then revised for important intellectual content by all other authors. All authors have approved the final version for submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. DAL is supported by the NIHR UCLH Biomedical Research Centre and is an NIHR Senior Investigator.
Competing interests: No other authors have any conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Ethics permission was given by the London (Hampstead) Committee, reference: 13/LO/1085.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request to the corresponding author.
References
- 1.Lomas DA, Mahadeva R. Alpha1-Antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest 2002;110:1585–90. 10.1172/JCI0216782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest 1986;89:370–3. 10.1378/chest.89.3.370 [DOI] [PubMed] [Google Scholar]
- 3.Roohani S, Tacke F. Non-Invasive assessment for alpha-1 antitrypsin deficiency-associated liver disease: new insights on steatosis and fibrosis in Pi*ZZ carriers. Transl Gastroenterol Hepatol 2019;4:82–3. 10.21037/tgh.2019.11.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 5.Lurie Y, Webb M, Cytter-Kuint R, et al. Non-Invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol 2015;21:11567. 10.3748/wjg.v21.i41.11567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandrin L, Fourquet B, Hasquenoph J-M, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–13. 10.1016/j.ultrasmedbio.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Kennedy P, Wagner M, Castéra L, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology 2018;286:738–63. 10.1148/radiol.2018170601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005;42:1373–81. 10.1002/hep.20935 [DOI] [PubMed] [Google Scholar]
- 9.European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado . EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–64. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Tanash HA, Piitulainen E. Liver disease in adults with severe alpha-1-antitrypsin deficiency. J Gastroenterol 2019;54:541–8. 10.1007/s00535-019-01548-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp HL, Bridges RA, Krivit W, et al. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med 1969;73:934–9. [PubMed] [Google Scholar]
- 12.Alpha-1 Awareness London alpha-1-antitrypsin clinic, 2020. Available: https://www.alpha1.uk/portfolio/london-alpha-1-antitrypsin-clinic/ [Accessed 28 Feb 2020].
- 13.National Institute for Health and Care Excellence Chronic obstructive pulmonary disease in over 16s: diagnosis and management. (Clinical guideline [NG115]), 2018. Available: https://www.nice.org.uk/guidance/ng115/ [PubMed]
- 14.WHO Guidelines for the prevention care and treatment of persons with chronic hepatitis B virus infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 15.Mostafavi B, Diaz S, Tanash HA, et al. Liver function in alpha-1-antitrypsin deficient individuals at 37 to 40 years of age. Medicine 2017;96:e6180. 10.1097/MD.0000000000006180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillaud O, Dumortier J, Traclet J, et al. Assessment of liver fibrosis by transient elastography (Fibroscan®) in patients with A1AT deficiency. Clin Res Hepatol Gastroenterol 2019;43:77–81. 10.1016/j.clinre.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 17.Hamesch K, Mandorfer M, Pereira VM, et al. Liver fibrosis and metabolic alterations in adults with alpha-1-antitrypsin deficiency caused by the Pi*ZZ mutation. Gastroenterology 2019;157:705–19. 10.1053/j.gastro.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Ding Y, Rao S, et al. Staging liver fibrosis in chronic hepatitis B with T1 relaxation time index on gadoxetic acid-enhanced MRI: Comparison with aspartate aminotransferase-to-platelet ratio index and FIB-4. J Magn Reson Imaging 2017;45:1186–94. 10.1002/jmri.25440 [DOI] [PubMed] [Google Scholar]
- 19.El Serafy MA, Kassem AM, Omar H, et al. APRI test and hyaluronic acid as non-invasive diagnostic tools for post HCV liver fibrosis: systematic review and meta-analysis. Arab J Gastroenterol 2017;18:51–7. 10.1016/j.ajg.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 20.Clark VC, Marek G, Liu C, et al. Clinical and histologic features of adults with alpha-1 antitrypsin deficiency in a non-cirrhotic cohort. J Hepatol 2018;69:1357–64. 10.1016/j.jhep.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 21.Kümpers J, Fromme M, Schneider CV, et al. Assessment of liver phenotype in adults with severe alpha-1 antitrypsin deficiency (Pi*ZZ genotype). J Hepatol 2019;71:1272–4. 10.1016/j.jhep.2019.08.011 [DOI] [PubMed] [Google Scholar]