Abstract
Mitochondrial dysfunction is thought to be a critical pathway in the development and progression of kidney diseases, but optimal methods to assess kidney mitochondrial dysfunction are not well known. Saeki and colleagues use positron emission tomography imaging with a novel probe, 2-tert-butyl-4-chloro-5-[6-(4-18F-fluorobutoxy)-pyridin-3-ylmethoxy]-2H-pyridazin-3-one (18F-BCPP-BF), to visualize and assess kidney mitochondrial status. The authors demonstrate that reduced uptake of 18F-BCPP-BF, as assessed by positron emission tomography imaging, corresponds to reduced functioning mitochondria in 3 separate animal models of kidney diseases.
The fine-tuning of the metabolic and energy demands of the cell is elegantly performed by mitochondria. To perform the remarkable task of active and selective reabsorption of solutes accompanied by water reclamation across the nephron, the kidneys consume a significant amount of energy. Although podocytes, mesangial cells, and endothelial cells have more flexibility in their glycolytic ability to generate energy, the proximal tubules primarily rely on mitochondrial oxidative phosphorylation to generate adenosine triphosphate.1 Dysfunction of mitochondrial dynamics, biogenesis, remodeling, or oxidative stress management may lead to kidney disease development and/or progression.1 Because the kidney is only second to the heart in mitochondrial abundance and energy consumption, it is plausible that mitochondrial dysfunction plays a pivotal role in acute kidney injury and chronic kidney disease progression. However, the optimal methods to specifically evaluate mitochondrial dysfunction in the kidneys remain elusive. In this issue of Kidney International, Saeki and colleagues2 tested a noninvasive imaging modality to visualize and assess kidney mitochondrial status in different animal models of kidney diseases.
Efforts to evaluate the role of mitochondrial dysfunction as a therapeutic target in kidney diseases have yielded interesting methods to assess kidney mitochondrial dysfunction. Prior studies in animals have demonstrated that imbalances in mitochondrial dynamics by excessive mitochondrial fission result in mitochondrial fragmentation, as assessed by tissue electron microscopic analysis, which may result in progressive kidney disease.1 Direct measurement of oxygen consumption in isolated mitochondria of animals with chronic kidney disease has also been used to identify mitochondrial dysfunction.3 Although these approaches may provide information about mitochondrial dysfunction, they require invasive techniques to interrogate kidney tissue, and they may not provide quantitative results or allow for a real-time assessment of mitochondrial function. In animals, still the most common method to assess mitochondrial dysfunction is through quantification of mitochondrial proteins, metabolites, or DNA. In humans, methods that use metabolites linked to mitochondrial metabolism or mitochondrial DNA from biological specimens are also promising to assess mitochondrial dysfunction and identify individuals at risk for acute kidney injury, chronic kidney disease, and adverse clinical outcomes.4 Although these methods are noninvasive, the use of blood and urine biomarkers to quantify mitochondrial dysfunction may lack specificity to kidney mitochondrial status.
Saeki et al. used a novel probe for positron emission tomography (PET) imaging, 2-tert-butyl-4-chloro-5-[6-(4-18F-fluorobutoxy)-pyridin-3-ylmethoxy]-2H-pyridazin-3-one (18F-BCPP-BF), in 3 rat models of kidney disease to assess kidney mitochondrial status. 18F-BCPP-BF has rapid uptake with long retention in kidney tissue and binds to mitochondrial complex I. Because mitochondrial complex I is the first and rate-limiting step of the electron transport chain for mitochondrial respiratory activity and oxidative phosphorylation, a probe that is able to assess its activity may provide specificity to functioning mitochondria (Figure 1).5 In the ischemia-reperfusion rat model, the investigators found decreased update of 18F-BCPP-BF in the outer portion of the kidney that corresponded with the kidney cortex and outer stripe of the outer medulla after 3 hours of reperfusion. This finding was accompanied by swollen mitochondria in the S3 segment proximal tubular cells of the outer medulla. In the 5/6 nephrectomy rats, the investigators found a graded reduction in 18F-BCPP-BF uptake in early and late kidney disease (4 and 15 weeks after nephrectomy, respectively). The imaging findings were accompanied by reduction in protein components of all mitochondrial complexes at the late phase, which was suggestive of reduced mitochondrial mass. Interestingly, treatment with angiotensin II receptor blockade seemed to protect the kidneys from mitochondrial dysfunction, as assessed by preserved 18F-BCPP-BF uptake. Similar to the 5/6 nephrectomy model, the investigators found reductions in 18F-BCPP-BF uptake in the early and late phases of the anti-glomerular basement membrane rat model accompanied by reduction in mitochondrial mass. Taken together, these findings suggest that PET imaging using the 18F-BCPP-BF probe may be able to noninvasively identify and assess mitochondrial dysfunction in the kidneys.
Figure 1 |. Kidney imaging with 2-tert-butyl-4-chloro-5-[6-(4-18F-fluorobutoxy)-pyridin-3-ylmethoxy]-2H-pyridazin-3-one (18F-BCPP-BF) probe allows visualization of mitochondrial complex (MC)-I activity.
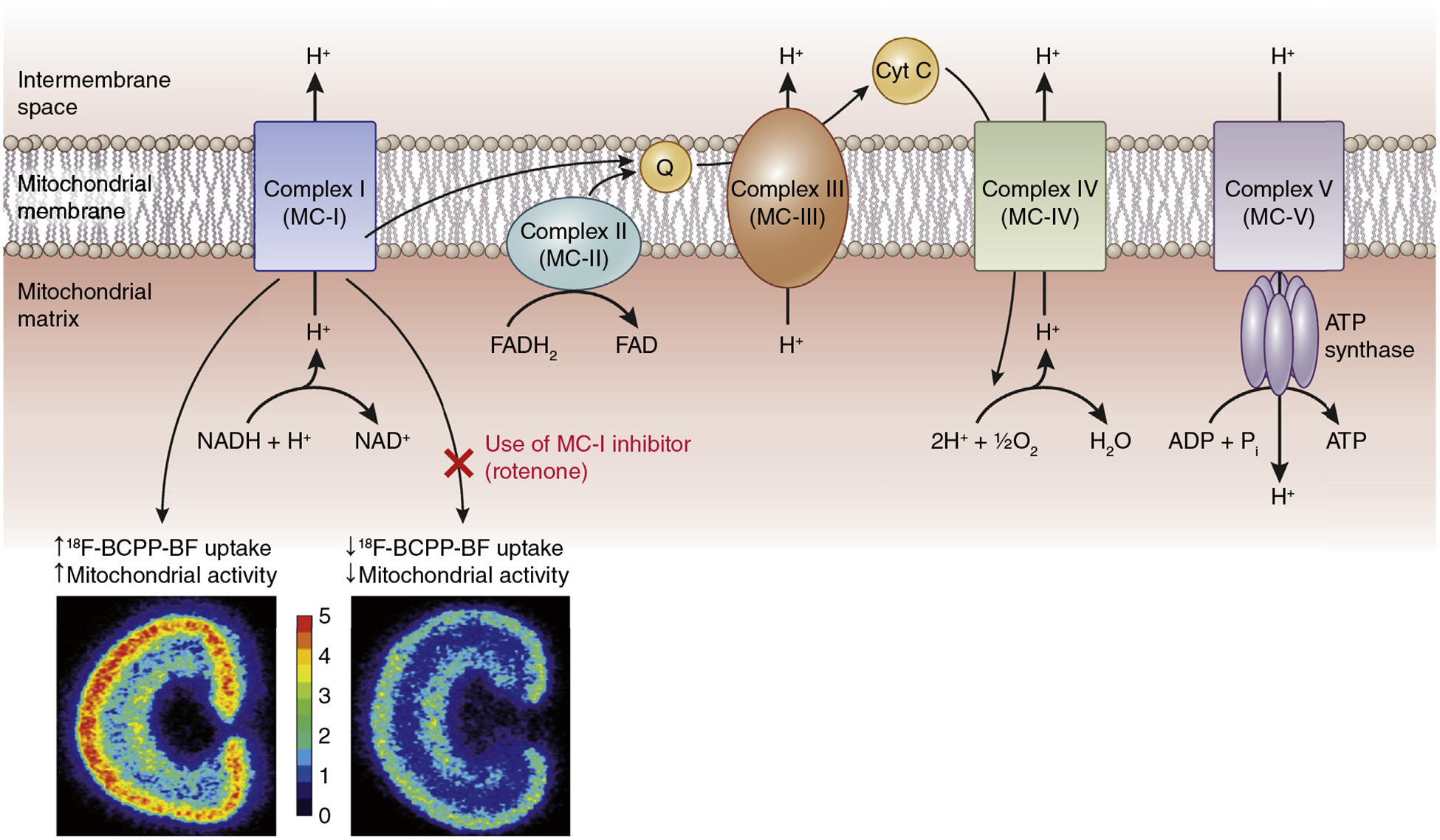
18F-BCPP-BF binds to MC-I, and its uptake is suggestive of mitochondrial activity. Schematic shows proposed 18F-BCPP-BF uptake with and without the use of rotenone, an MC-I inhibitor. Kidney images are adapted from Figure S1 from Saeki S, Ohba H, Ube Y, et al. Positron emission tomography imaging of renal mitochondria is a powerful tool in the study of acute and progressive kidney disease models. Kidney Int. 2020;98:88–99, and are just for illustrative purposes. Note that the kidney images are acquired with autoradiography and, hence, are the superior spatial resolution. ATP, adenosine triphosphate; Cyt C, cytochrome complex C; FAD, flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide; Q, coenzyme-Q.
The use of noninvasive imaging to identify patients with kidney diseases at risk of adverse clinical outcomes has gained attention recently. The use of kidney functional magnetic resonance imaging (MRI) has been on the rise.6 Of particular interest is blood oxygenation level-dependent MRI to evaluate kidney tissue oxygen availability and consumption. Because mitochondrial function is the major oxygen consumer in the kidneys, these methods may provide complementary information. PET imaging is considered the reference method for functional or metabolic imaging, probably because of the higher specificity. However, the technique is not widely applied to the kidneys, partly related to logistical issues. PET scanners are not as widely available compared with computed tomography and MRI scanners. In the United States, there were approximately 2500 PET scanners compared with approximately 12,000 MRI and 14,000 computed tomography scanners in 2017. Because PET images only provide functional or radiopharmaceutical uptake signatures, they usually need an anatomical atlas to overlay PET images. For this reason, PET is usually available as a combined PET-computed tomography or PET-MRI scanner to facilitate image fusion from the 2 modalities. Unfortunately, combination of the scanners further increases the capital expenditure on the equipment. Apart from the scanner costs, the radiopharmaceutical costs are also high. Because of the short half-life of the PET isotopes (11C [20 min], 13N [10 min], 15O [123 s], 18F [110 min], 68Ga [68 min], 82Rb [78 min]), the production and transportation of the isotopes are challenging. Further, the production of the isotope needs an expensive cyclotron in close proximity. Currently, 18F remains the most widely used PET isotope. Although the isotope provides the signal for detection, the specificity of the probe depends on the particular radiopharmaceutical used. While there are over a few thousand radiopharmaceuticals in the literature, there are only 11 agents that are approved for routine clinical use, more than half of which were approved in the last decade.7 18F-FDG remains the most widely used radiopharmaceutical and is useful to study glucose metabolism. 18F-FDG is known to be rapidly excreted into the renal tubular lumen with resultant accumulation along the collecting system, urinary tract, and bladder.8 Because of the high background signal in the kidneys, 18F-FDG lacks specificity to investigate kidney mitochondrial status. Saeki et al. maintain that 18F-BCPP-BF is rapidly eliminated from the blood compartment, possibly via the hepatobiliary tract, without passage into the kidney collecting system.5 Moreover, 18F-BCPP-BF uptake is not affected by dynamic changes in kidney blood flow in diseased animal models, as evidenced by stability in the time activity curve 40 to 60 minutes after probe injection. The investigators postulate that the high affinity of 18F-BCPP-BF for mitochondrial complex I combined with its proposed route of elimination reduce the overall background signal in the kidney and allow specificity to assess kidney mitochondrial status. If their findings are replicated, an important next step will be the translation of 18F-BCPP-BF into the clinic. Although the authors believe 18F-BCPP-BF can be safely used in humans, additional safety and radiation dosimetry assessments are required. While the vast majority (~90%) of clinical PET studies are performed in oncology, it is possible that there is sufficient financial incentive to perform clinical trials to seek approvals given the significance of mitochondrial function in all major organs. 18F-BCPP-BF requires further study, but 18F-BCPP-EF is a close analogue of 18F-BCPP-BF with a similar pharmacological profile that shows promise in human brain imaging studies to investigate normal aging and dementia.9 Future studies could test PET imaging with 18F-BCPP-EF as a novel method to investigate mitochondrial dysfunction in human kidneys.
With the worsening burden of kidney disease worldwide, the nephrology community needs to identify novel targets to test new therapies. Imaging could play an important role to identify patients at high risk of adverse clinical outcomes, perform surveillance of disease, and promote drug development. We can look to our colleagues in other fields of medicine who have significantly benefited from the advancement of imaging technology. Saeki and colleagues should be commended for their attempt to develop a method to directly visualize and quantify an important disease pathway for kidney diseases. Although PET imaging with the 18F-BCPP-BF probe to evaluate kidney mitochondrial dysfunction will require additional confirmatory studies and further translation into humans, this seems to represent a step in the direction of progress toward advancement in imaging applications in nephrology.
ACKNOWLEDGMENTS
AS is supported by National Institutes of Health grant K23DK120811, the Dixon Translational Research Grants Initiative at Northwestern Medicine and the Northwestern University Clinical and Translational Sciences Institute (UL1TR001422), and core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857. PVP is supported in part by R01DK106557.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeki S, Ohba H, Ube Y, et al. Positron emission tomography imaging of renal mitochondria is a powerful tool in the study of acute and progressive kidney disease models. Kidney Int. 2020;98: 88–99. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JL, Pham H, Li Y, et al. Hypoxia-inducible factor-1alpha activation improves renal oxygenation and mitochondrial function in early chronic kidney disease. Am J Physiol Renal Physiol. 2017;313: F282–F290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14:291–312. [DOI] [PubMed] [Google Scholar]
- 5.Tsukada H, Nishiyama S, Fukumoto D, et al. Novel PET probes 18F-BCPP-EF and 18F-BCPP-BF for mitochondrial complex I: a PET study in comparison with 18F-BMS-747158–02 in rat brain. J Nucl Med. 2014;55: 473–480. [DOI] [PubMed] [Google Scholar]
- 6.Mendichovszky I, Pullens P, Dekkers I, et al. Technical recommendations for clinical translation of renal MRI: a consensus project of the Cooperation in Science and Technology Action PARENCHIMA. MAGMA. 2020;33: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke BN. PET radiopharmaceuticals: what’s new, what’s reimbursed, and what’s next? J Nucl Med Technol. 2018;46: 12–16. [DOI] [PubMed] [Google Scholar]
- 8.Moran J, Lee H, Blaufox M. Optimization of urinary FDG excretion during PET imaging. J Nucl Med. 1999;40: 1352–1357. [PubMed] [Google Scholar]
- 9.Mansur A, Rabiner EA, Comley RA, et al. Characterization of 3 PET tracers for quantification of mitochondrial and synaptic function in healthy human brain: (18)F-BCPP-EF, (11)C-SA-4503, and (11)C-UCB-J. J Nucl Med. 2020;61: 96–103. [DOI] [PubMed] [Google Scholar]


