Abstract
Objective
The relationship between psychosocial stress and chronic pain is bidirectional. An improved understanding regarding the relationships among chronic pain, life stress, and ethnicity/race will inform identification of factors contributing to health disparities in chronic pain and improve health outcomes. This study aims to assess relationships between measures of clinical pain, life stress, sociodemographics, and salivary cortisol levels.
Methods
A cross-sectional analysis involving data from 105 non-Hispanic White (NHW) and non-Hispanic Black (NHB) participants aged 45–85 years old with or at risk for knee osteoarthritis. Data included sociodemographics, clinical pain, psychosocial stress, and salivary cortisol across five time points over an approximate 12-hour period. Non-parametric correlation analysis, sociodemographic group comparisons, and regression analyses were performed.
Results
Clinical pain and psychosocial stress were associated with salivary cortisol levels, particularly morning waking and the evening to morning awakening slope. With the inclusion of recognized explanatory variables, the Graded Chronic Pain Scale characteristic pain intensity and financial satisfaction were identified as the primary pain and psychosocial measures associated with cortisol levels. Sociodemographic group differences were indicated such that NHB participants reported higher pain-related disability, higher levels of discrimination, lower financial and material satisfaction, and showed higher evening salivary cortisol levels compared to NHW participants. In combined pain and psychosocial stress analyses, greater financial satisfaction, lower pain intensity, and lower depression were associated with higher morning waking saliva cortisol levels while greater financial satisfaction was the only variable associated with greater evening to morning awakening slope.
Conclusion
Our findings show relationships among clinical pain, psychosocial stress, sociodemographic factors, and salivary cortisol levels. Importantly, with inclusion of recognized explanatory variables, financial satisfaction remained the primary factor accounting for differences in morning waking cortisol and evening to morning awakening cortisol slope in an ethnic/racially diverse group of middle aged and older adults with or at risk for knee osteoarthritis.
Keywords: chronic pain, health disparities, psychosocial stress, salivary cortisol, stress system functioning
Introduction
The relationship between chronic pain and psychosocial stress is bidirectional. Individuals who experience adverse life events are at greater risk for the development of chronic musculoskeletal pain.1,2 Living with chronic pain is associated with increased psychosocial stress.3–5 Persistent physiological and psychosocial stress drive functional and structural remodeling of biologic processes.3 Importantly, perception and behavioral responses can modify or exacerbate the experiences associated with various challenging life events. For example, higher levels of perceived stress are associated with greater reports of pain intensity in older adults.6,7 In contrast, active coping and other protective factors such as trait optimism are associated with lower levels of clinical pain.8,9
Cortisol is one of the many “instruments” in a broad and diverse biological “orchestra” involved in stress system functioning. A corticosteroid hormone produced in the cortex of the adrenal glands, cortisol regulates the stress response, metabolism, and immune system functioning.10 Cortisol levels vary diurnally, increasing upon waking and gradually declining across the day.11–13 Additionally, cortisol is released in response to physiological or psychological arousal. Short term stress or the anticipation of stress stimulates a spike in cortisol levels.12,14,15 Initially, chronic stress may intensify cortisol secretion, which leads to higher morning cortisol.12,15–17 However, prolonged chronic stress results in a dysregulation or dampening of the morning waking response.15,18,19 Additionally, cortisol diurnal slopes are flatter with increased and persisting chronic psychosocial stress, which has also been correlated with poorer health outcomes.15,20,21 The dysregulation of cortisol functioning can contribute to widespread inflammation and pain.11,22
Ethnic/race minority groups are at a higher risk for experiencing environmental stressors such as economic hardships, discrimination, and limited access to healthcare and health promoting experiences, which can contribute to increased vulnerabilities for poor health outcomes.23–27 It has been shown that non-Hispanic Black (NHB) individuals report greater discrimination, have higher perceived stress, and report higher rates of osteoarthritis-related pain than non-Hispanic White (NHW) individuals.28–30 Research has also shown NHB individuals demonstrate lower waking cortisol levels, slower afternoon decline in cortisol, and flatter diurnal cortisol slopes compared to their NHW peers.13,15,19,20,31,32 Additionally, experiences of early life discrimination during adolescence not only affects cortisol during the adolescent period but also predict a flatter diurnal cortisol slope in NHB compared to NHW adults.33 Less is known regarding the similarities and differences by ethnic/race groups in chronic pain, stress-related life experiences, and stress system functioning. As self-reported ethnicity/race is a surrogate measure for a complex array of cultural, socioeconomic, and environmental factors, it is considered a sociodemographic variable in the current study.
The purpose of this study is to: 1) examine the relationship between clinical pain, prior/current psychosocial stress, and salivary cortisol levels; 2) evaluate possible sociodemographic group differences in measures of clinical pain, psychosocial stress, and salivary cortisol levels; and 3) investigate the combined contributions of prior/current psychosocial stress, pain, and sociodemographic variables to salivary cortisol levels. We hypothesized that: 1) prior/current psychosocial stress will be positively associated with clinical pain and salivary cortisol levels; 2) sociodemographic group differences will be demonstrated with greater clinical pain, psychosocial stress, and cortisol dysregulation in NHB compared to NHW participants as indicated by higher evening cortisol levels, lower morning waking cortisol, and flatter cortisol slopes; and 3) combined factors of pain, psychosocial stress, and sociodemographic variables will predict lower morning waking salivary cortisol levels and flatter evening to morning awakening slope.
Methods
Participants
Adults between 45 and 85 years of age with and without knee pain who self-identified as either NHB or NHW were recruited and enrolled to the Understanding Pain and Limitations in Osteoarthritic Disease (UPLOAD) study at the University of Florida between 2010 and 2014. Participants were recruited from the general community via fliers, newspaper and radio ads and word of mouth. Inclusion criteria for those with knee pain was based on a knee osteoarthritis screening instrument.34 Individuals were excluded from the study if they were taking opioids on a daily basis. Additional information on the UPLOAD study including inclusion and exclusion criteria have been previous published.35,36 Participants who completed the UPLOAD protocol were eligible to participate in the prospective study, UPLOAD Follow-Up targeted within a 24 to 48-month timeframe from the baseline study. Study visits were conducted between 2013 and 2017. Participants with salivary cortisol measures who completed a visit within the 24 to 48-month timeframe were included in the analysis. The UPLOAD study was approved by the University of Florida Institution Review Board (IRB201500906). All participants provided verbal and written informed consent.
Procedures
The current investigation is a cross-sectional analysis of the UPLOAD Follow-Up Study. The procedures described are limited to the current investigation. We used the STROBE cross-sectional checklist when writing our report.37 Individuals participated in two study sessions. The first session was a health assessment update in which participants completed questionnaires specific to sociodemographics, health status, pain history, and life stress. The second session involved quantitative sensory testing (QST). Salivary cortisol samples were collected the night preceding, the morning of, and during the second study session. Questionnaires and cortisol collection procedures were reviewed with participants to help reduce collection errors and missing information.
Measures
Sociodemographic and Health Status
Baseline Characteristics and Health Status
Sociodemographic characteristics included age, sex, self-reported ethnicity/race, highest education completed, and income range. Participants completed a health assessment and health history questionnaire assessing current comorbidities including high blood pressure, heart disease, cancer, diabetes, asthma/breathing problems, kidney disease, thyroid problem, stroke, seizure, chronic pain, neurological disorder, depression, and other health conditions. Waist and hip measurements were also collected.
Clinical Pain Measures
Graded Chronic Pain Scale (GCPS).38 The GCPS was used to assess the severity of knee pain and its impact on activities. The measure is scored using two sub-scales, characteristic pain intensity (CPI) (0 to 100 score) and disability score (0 to 100 score) over a 6-month period. The higher the score, the higher the pain intensity and greater physical disability.
Total Pain Sites. Participants were asked to select pain sites from a preselected list if they had pain “more days than not over the past three months.” Bilateral body sites included hands, arms, shoulders, neck, head/face, chest, stomach, upper back, lower back, knees, legs (other than knees), or feet/ankles (0–24 sites).
Chronic Pain Burden Index. Participants reporting pain for more than six months “on more days than not” were provided with the below description and then asked to circle a percent that represents how much their life is affected by chronic pain from 0–100% with higher numbers indicating greater chronic pain burden. “Chronic pain can be experienced in many areas (physical functioning, employment, relationships) of a person's life. Imagine the figure below as representing your life. How much of your daily life is influenced by chronic pain? Please circle the % that represents how much of your life is affected by chronic pain.”
Psychosocial Stress Measures
Experiences of Discrimination (EOD).39 The EOD is a reliable and valid measure of incidences of discrimination over the individual’s lifetime. Participants were asked about the frequency of each event, how much did they worry about each event, the reason certain events occurred, and how they responded to certain situations. The responses have assigned values being 0 to “never”, 1 to “once”, 2.5 to “2 to 3 times” and 5 to “4 or more times.” These values are summed with higher scores signifying more experiences of discrimination.
The Schedule of Recent Experiences (SRE).40 The SRE is a 43-item instrument that evaluates whether you have experienced specific life events such as the death of a spouse, divorce, or loss of employment over the past two years. Each event is weighted and summed for a total score 0 to 1466. Scores higher than 300 indicate high stress.
The Perceived Stress Scale (PSS).41 The PSS consists of 10 items that assesses self-reported perception of stress over the last month. Scores range from 0 to 40 (0 to 13 low stress, 14 to 26 moderate stress, 27 to 40 high stress) with higher scores indicating higher perceived stress.
Financial and Material Satisfaction. Current level of satisfaction with financial situation and material (living) standards was assessed by two questions on a self-report questionnaire:
- “Overall, how satisfied are you with your current financial situation?”
- “Overall, how satisfied are you with the material (living) standards of your life?”
Responses ranged from 0 – Totally dissatisfied to 10 - Totally satisfied.
Sleep Measure
The Pittsburgh Sleep Quality Index (PSQI).42 The PSQI measures sleep quality and patterns across seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficacy, sleep disturbances, sleep medication usage, and daytime dysfunction over the previous month. Scores range from 0 to 21 with a higher score indicating worse quality sleep.
Depressive Symptoms Measure
Center for Epidemiologic Studies Depression Scale (CES-D).43 The CES-D measures depressive symptoms within the past week. Scores range from 0 to 60 with higher scores indicating more depressive symptoms.
Biological Functioning Measure
Salivary Cortisol. Participants were instructed during the first session how to collect saliva samples prior to their second session (the QST visit). Saliva samples were collected in salivettes (Sarstedt, Inc.) at five separate time points: T1) in the evening after 9 pm or prior to going to bed, T2) immediately upon waking prior to getting out of bed, T3) 45 minutes after waking in the morning, T4) in the laboratory prior to QST testing between 8:30 and 10:30 AM, and T5) immediately following QST testing. In order to standardize testing, all the visits were scheduled between 8:00 and 10:00 am. Participants were asked to refrain from brushing their teeth or drinking anything for 30 minutes and to refrain from eating or exercise one hour prior to collecting samples. Participants were instructed to place the swab directly in their mouth for approximately one minute and return to the container without touching. Collection time was documented and samples collected at home were stored at room temperature until returned to the lab. Salivary cortisol samples were spun down and transferred into 1 aliquot per tube, and frozen at −80°C until analysis. De-identified salivary cortisol was processed and analyzed at the University of Alabama at Birmingham Diabetes Research Center Human Physiology Core Laboratory (Dr. Barbara Gower) in duplicate using the Salimetrics (State College, PA) Salivary Cortisol EIA kit according to the manufacturer’s instructions.
Statistical Analysis
Sociodemographic characteristics by ethnic/race group differences were assessed using χ2 for dichotomous variables and Mann-Whitney U test for ordinal and continuous variables. Mann-Whitney U test was completed for all clinical pain, psychosocial stress, sleep and depression measures. For primary outcome variables, evening to morning awakening slope was treated as missing for participants who had one missing variable at any of the three time points (T1-T3) (n = 5 excluded). AUC was treated as missing for participants who were missing evening (T1), morning waking (T2) or both pre-QST (T4) and post-QST (T5) salivary cortisol levels (n = 3 excluded).
Non-parametric correlation analyses were completed between clinical pain measures, stress-related psychosocial measures, and salivary cortisol levels using Spearman’s correlation. Differences in salivary cortisol levels were assessed comparing the five separate time points (T1-T5); slope (T1-T3) which included evening (T1), immediately upon wakening (T2), and 45 minutes after waking (T3); and AUC for T1-T5 [including prior to QST testing (T4) and immediately following QST testing (T5)]. As our AUC was not comprised of a standard 24-hour capture, and individuals may have different responses to QST session which could influence the T5 sample, the morning waking cortisol and the evening to morning awakening slope were used as the primary outcome measures to address our research questions. Additionally, previous research supported the use of the morning waking (T2) cortisol11,12,18,44 and the evening to morning awakening slope (T1-T3).20,21,31,45
To address Question 1, examine the relationship between clinical pain, prior/current psychosocial stress, and saliva cortisol levels, unadjusted and adjusted analyses were completed. To reduce the number of analyses completed and to determine the most predictive variables of interest, least absolute shrinkage and selection operator (LASSO) specifying the elastic net option were used. LASSO modeling was completed for clinical pain (GCPS CPI, GCPS disability, chronic pain burden index, total pain sites), and psychosocial stress (EOD, SRE, PSS, financial satisfaction, and material satisfaction) for the two primary outcome variables (morning waking cortisol and evening to morning awakening slope). Variable selections were based on LASSO models with primary (age, sex, ethnicity/race, and total number of comorbidities) and secondary explanatory variables (PSQI, CESD, annual income, and education) in the model. Following the selection of the clinical pain and psychosocial stress measures, regression analyses with primary explanatory variables were completed.
To address Question 2, evaluate possible sociodemographic group differences on measures of clinical pain, psychosocial stress, and saliva cortisol levels, adjusted regression analyses were completed. Adjusted analyses included the primary covariates in addition to income and education which differed by groups (see Descriptive Analyses).
To address Question 3, investigate the combined contributions of pain, psychosocial stress, and sociodemographic variables on saliva cortisol levels, adjusted regression analyses were completed with the clinical pain and psychosocial stress measures from LASSO in the model with the primary explanatory variables. A second analysis was conducted with both primary and secondary explanatory variables. Lastly, as evening (T1) salivary cortisol levels differed by ethnic/race groups, post hoc regression analyses were completed. Statistical analyses were completed using SAS, V.9.4 (SAS Institute, Cary, North Carolina, USA). No imputation was completed for missing variables. All tests were considered statistically significant at a 0.05 level of significance.
Results
Descriptives
A total of 105 participants (67 NHW, 38 NHB) provided salivary cortisol samples and were included in the analysis. There were a number of statistically significant differences between the NHW and NHB participants (Table 1). NHB participants were significantly younger, reported a higher number of comorbidities, lower education, and lower income compared to NHW participants. NHB participants also reported significantly higher clinical pain, higher psychosocial stress, worse sleep, and higher depressive symptoms.
Table 1.
Baseline characteristics of non-Hispanic White and non-Hispanic Black participants.
| Variable | Total sample (N = 105) | Non-Hispanic White (N = 67) | Non-Hispanic Black (N = 38) | P |
|---|---|---|---|---|
| Sociodemographics | ||||
| Gender, N (%) | 0.7910 | |||
| Male | 46 (43.8) | 30 (44.8) | 16 (42.1) | |
| Female | 59 (56.2) | 37 (55.2) | 22 (57.9) | |
| Age, M ± SD | 59.0 ± 7.6 | 60.3 ± 8.3 | 56.6 ± 5.4 | 0.0061 |
| Waist/Hip Ratio, M ± SD | 0.89 ± 0.09 | 0.89 ± 0.09 | 0.89 ± 0.09 | 0.6255 |
| No. Comorbidities (0–14), N (%) | 0.0009 | |||
| 0 | 52 (49.5) | 41 (61.2) | 11 (28.9) | |
| 1–2 | 45 (42.9) | 23 (34.3) | 22 (57.9) | |
| 3+ | 8 (7.6) | 3 (4.5) | 5 (13.2) | |
| Education, N (%) | <0.0001 | |||
| High school or less | 51 (45.6) | 22 (32.8) | 29 (76.3) | |
| Higher education | 54 (51.4) | 45 (67.2) | 9 (23.7) | |
| Income, N (%) | <0.0001 | |||
| $0–29,999 | 59 (56.2) | 26 (38.8) | 33 (86.8) | |
| $30,000–79,999 | 32 (30.5) | 27 (40.3) | 5 (13.2) | |
| $80,000+ | 12 (11.4) | 12 (17.9) | 0 (0.0) | |
| Not reported | 2 (1.9) | 2 (3.0) | 0 (0.0) | |
| Pain | ||||
| GCPS CPI, M ± SD | 32.0 ± 26.7 | 22.9 ± 23.1 | 48.0 ± 25.4 | <0.0001 |
| GCPS disability, M ± SD | 23.7 ± 28.6 | 14.1 ± 22.3 | 41.0 ± 30.9 | <0.0001 |
| Number of pain sites | 3.9 ± 4.44 | 2.9 ± 4.1 | 5.6 ± 4.6 | 0.0036 |
| Chronic pain burden Index | 60.6 ± 29.8 | 22.4 ± 28.4 | 45.0 ± 26.8 | 0.0002 |
| Psychosocial | ||||
| EOD, M ± SD | 4.0 ± 6.7 | 1.3 ± 3.1 | 8.8 ± 8.6 | <0.0001 |
| SRE, M ± SD | 259.7 ± 207.2 | 224.3 ± 178.8 | 325.5 ± 240.8 | 0.0308 |
| PSS, M ± SD | 11.7 ± 6.2 | 11.1 ± 5.8 | 12.9 ± 6.8 | 0.1787 |
| Financial satisfaction, M± SD | 7.5 ± 3.0 | 8.6 ± 2.6 | 5.6 ± 2.5 | <0.0001 |
| Material satisfaction, M ± SD | 8.5 ± 2.7 | 9.3 ± 2.0 | 6.9 ± 3.0 | <0.0001 |
| Sleep | ||||
| PSQI, M ± SD | 7.0 ± 4.1 | 6.3 ± 3.8 | 8.3 ± 4.5 | 0.0219 |
| Depression | ||||
| CES-D, M ± SD | 6.6 ± 6.5 | 5.4 ± 5.2 | 8.8 ± 8.0 | 0.0199 |
M = mean; SD = standard deviation; BMI = body mass index; GCPS CPI = Graded Chronic Pain Scale Characteristic Pain Intensity Score; GCPS Disability = Graded Chronic Pain Scale Disability Score; EOD = Experience of Discrimination; SRE=Schedule of Recent Experiences; PSS = Perceived Social Stress; PSQI = Pittsburgh Sleep Quality Index; CES-D = Center for Epidemiologic Studies Depression Scale.
Relationships Between Clinical Pain, Psychosocial Stress, and Saliva Cortisol
Unadjusted models are presented in a correlation matrix in Tables 2 and 3. The morning waking salivary cortisol level (T2) and the evening to morning awakening salivary cortisol slope (T1- T3) both showed significant correlations with the clinical pain and psychosocial stress measures.
Table 2.
Correlations between clinical pain and psychosocial stress measures.
|
Clinical pain measures |
|||||
|---|---|---|---|---|---|
| GCPS CPI | GCPS disability | Number of pain sites | Chronic pain burden index | ||
| EOD | 0.48746** | 0.41791** | 0.38061** | 0.30005* | |
| SRE | 0.20843* | 0.23507* | 0.29668* | 0.25121* | |
| PSS | 0.33165** | 0.32270** | 0.31685* | 0.33756* | |
| Financial satisfaction | −0.36553* | −0.39337** | −0.42193** | −0.39414** | |
| Material satisfaction | −0.39148** | −0.40718** | −0.37427** | −0.39356** | |
Clinical Pain Measures: GCPS CPI= Graded Chronic Pain Scale Characteristic Pain Intensity Score; GCPS disability= Graded Chronic Pain Scale Disability Score.
Psychosocial Stress Measures: EOD= Experiences of Discrimination; SRE= Schedule of Recent Experiences; PSS= Perceived Stress Scale.
*P < 0.05; **P < 0.001.
Table 3.
Correlations between salivary cortisol, clinical pain, and psychosocial stress.
|
Salivary Cortisol Measures |
||||||||
|---|---|---|---|---|---|---|---|---|
| Evening (T1) n = 102 | Waking (T2) n = 105 | 45 min After Waking (T3) n = 103 | Pre-QST (T4) n = 105 | Post-QST (T5) n = 87 | Slope (T1-T3) n = 100 | AUC (T1-T5) n = 102 | ||
| Clinical Pain MeasuresGCPS CPI | 0.06138 | −0.22268* | 0.02161 | 0.01825 | −0.03894 | −0.04206 | −0.12530 | |
| GCPS Disability | 0.06143 | −0.24494* | −0.10742 | −0.06657 | −0.07754 | −0.18734 | −0.17604 | |
| Number of Pain Sites | 0.00776 | −0.16246 | −0.00668 | −0.02931 | −0.01268 | −0.02317 | −0.09361 | |
| Chronic Pain Burden IndexPsychosocial Stress Measures | 0.04368 | −0.20540* | −0.02820 | −0.03688 | −0.05246 | −0.06650 | −0.12776 | |
| EOD | 0.13062 | −0.02968 | −0.06534 | −0.05933 | 0.11801 | −0.03382 | −0.04732 | |
| SRE | 0.10152 | 0.06937 | 0.00724 | −0.12432 | −0.14841 | 0.03211 | 0.08545 | |
| PSS | 0.00593 | 0.03209 | −0.04884 | 0.03492 | −0.06392 | −0.10376 | −0.00182 | |
| Financial Satisfaction | −0.06242 | 0.31826* | 0.33990* | 0.11903 | 0.14378 | 0.41172** | 0.30984* | |
| Material Satisfaction | −0.07460 | 0.28033* | 0.15960 | 0.03642 | 0.07164 | 0.24397* | 0.24339* | |
Clinical Pain Measures: GCPS CPI = Graded Chronic Pain Scale Characteristic Pain Intensity Score; GCPS disability = Graded Chronic Pain Scale Disability Score.
Psychosocial Stress Measures: EOD = Experiences of Discrimination; SRE = Schedule of Recent Experiences; PSS = Perceived Stress Scale.
Note: T1 = first collection; T2 = second collection; T3 = third collection; T4 = fourth collection; T5 = fifth collection; Slope (T1-T3) = slope of evening, waking and 45 min after waking; AUC (T1-T5) = Area Under the Curve over all 5 time points.
*P < 0.05; **P < 0.001.
LASSO analyses with clinical pain measures as the variables of interest with primary and secondary explanatory variables in the model identified the GCPS CPI as the most predictive clinical pain measure for morning waking cortisol (T2). LASSO modeling did not identify a pain measure for the evening to morning awakening salivary cortisol slope (T1-T3) but did identify ethnicity/race in both models.
LASSO analyses with psychosocial stress measures with explanatory variables in the model identified financial satisfaction and EOD as predictors of morning waking salivary cortisol (T2). LASSO analyses with psychosocial stress measures with explanatory variables in the model identified financial satisfaction and annual income as predictors of evening to morning awakening salivary cortisol slope (T1-T3).
In regression analyses, clinical pain was not associated with either the morning waking salivary cortisol (T2) or the evening to morning awakening salivary cortisol slope (Table 4a). Among psychosocial stress variables, financial satisfaction was significantly associated with both morning waking salivary cortisol (T2) and the evening to morning awakening cortisol slope (T1-T3) such that greater financial satisfaction associated with higher morning cortisol levels and a greater evening to morning awakening slope (Table 4b).
Table 4.
Individual adjusted regression analyses.
|
Morning Waking (T2) |
Awakening Slope (T1-T3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard Error | t Value | P | Estimate | Standard Error | t Value | P | |
| A. Regression analysis assessing clinical pain with primary explanatory variables | |||||||||
| Intercept | 0.6508517202 | 0.20143443 | 3.23 | 0.0017 | 0.0005605506 | 0.00031816 | 1.76 | 0.0813 | |
| Age | −0.0038365159 | 0.00318873 | −1.20 | 0.2318 | 0.0000021430 | 0.00000521 | 0.41 | 0.6819 | |
| Female | −0.0702454503 | 0.04337013 | −1.62 | 0.1085 | 0.0000699530 | 0.00007851 | 0.89 | 0.3752 | |
| NHB | −0.0139706427 | 0.05081243 | −0.27 | 0.7839 | −0.0001930958 | 0.00008684 | −2.22 | 0.0285 | |
| No. comorbidities | −0.0174505499 | 0.02300955 | −0.76 | 0.4500 | −0.0000235486 | 0.00003950 | −0.60 | 0.5524 | |
| GCPS CPI | −0.0017814126 | 0.00098400 | −1.81 | 0.0733 | – | – | – | – | |
| B. Regression analysis assessing psychosocial stress with primary explanatory variables | |||||||||
| Intercept | 0.2917743014 | 0.18260250 | 1.60 | 0.1133 | 0.0004106642 | 0.00031634 | 1.30 | 0.1975 | |
| Age | −0.0021277423 | 0.00302477 | −0.70 | 0.4835 | −0.0000021048 | 0.00000508 | −0.41 | 0.6797 | |
| Female | −0.0572067728 | 0.04255212 | −1.34 | 0.1820 | 0.0000840440 | 0.00007537 | 1.12 | 0.2678 | |
| NHB | −0.0285008307 | 0.05873582 | −0.49 | 0.6286 | −0.0000547617 | 0.00009498 | −0.58 | 0.5657 | |
| No. comorbidities | −0.0262819621 | 0.02153824 | −1.22 | 0.2253 | −0.0000201981 | 0.00003700 | −0.55 | 0.5864 | |
| Financial Satisfaction | 0.0237385718 | 0.00849693 | 2.79 | 0.0063 | 0.0000379614 | 0.00001553 | 2.44 | 0.0165 | |
| EOD | 0.0067156097 | 0.00379505 | 1.77 | 0.0799 | – | – | – | – | |
| Income | – | – | – | – | 0.0000122608 | 0.00001716 | 0.71 | 0.4768 | |
A. The overall models were not significant. B. Overall models: Morning Waking (p = 0.0034) and Slope (p = 0.0166) - Indicates variables were not included in the overall model. NHB = non-Hispanic Black; GCPS CPI = Graded Chronic Pain Scale Characteristic Pain Intensity, EOD = Experience of Discrimination.
Measures of Clinical Pain, Psychosocial Stress, and Saliva Cortisol by Ethnic/Race Group
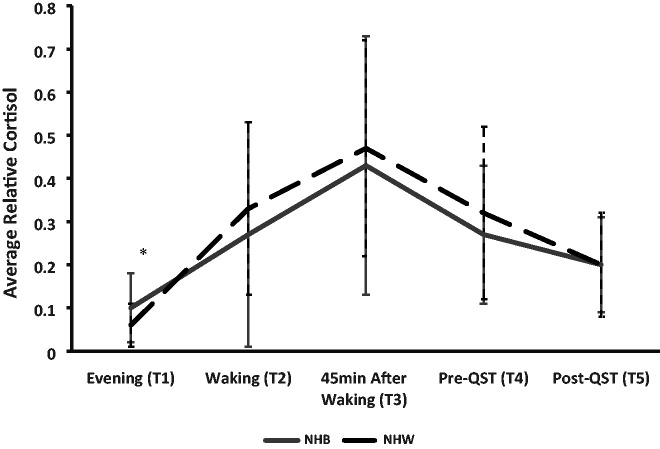
Significant ethnic/race group differences emerged for pain disability, experiences of discrimination, and financial and material satisfaction after adjusting for age, sex, and total number of comorbidities, education, and income (Table 5). Of the cortisol measures (Table 5), only evening (T1) salivary cortisol significantly differed between NHB and NHW participants after accounting for covariates with NHB participants exhibiting higher levels (Figure 1).
Table 5.
Adjusted ethnic/race group comparisons in clinical pain, psychosocial stress and salivary cortisol.
| F Value | P | |
|---|---|---|
| Clinical pain measures | ||
| GCPS CPI | 10.47 | <0.0001 |
| GCPS Disability* | 8.79 | <0.0001 |
| Number of Pain Sites | 8.14 | <0.0001 |
| Chronic Pain Burden Index | 6.69 | <0.0001 |
| Psychosocial stress measures | ||
| EOD* | 8.35 | <0.0001 |
| SRE | 4.90 | 0.0002 |
| PSS | 1.80 | 0.1071 |
| Financial Satisfaction* | 8.89 | <0.0001 |
| Material Satisfaction* | 7.58 | <0.0001 |
| Salivary cortisol measures | ||
| Evening (T1)* | 2.11 | 0.0423 |
| Morning Waking (T2) | 1.89 | 0.0709 |
| 45 Minute After Waking (T3) | 0.91 | 0.5141 |
| Pre-QST (T4) | 0.62 | 0.7612 |
| Post-QST (T5) | 1.08 | 0.3850 |
| Slope (T1-T3) | 1.87 | 0.0757 |
| AUC (T1-T5) | 1.39 | 0.2099 |
Clinical Pain Measures: GCPS CPI= Graded Chronic Pain Scale Characteristic Pain Intensity Score; GCPS disability= Graded Chronic Pain Scale Disability Score.
Psychosocial Stress Measures: EOD= Experiences of Discrimination; SRE= Schedule of Recent Experiences; PSS= Perceived Stress Scale.
Note: T1 = first collection; T2 = second collection; T3 = third collection; T4 = fourth collection; T5 = fifth collection; Slope (T1-T3)= slope of evening, waking and 45 min after waking; AUC (T1-T5)= Area Under the Curve over all 5 time points.
Explanatory variables: sex, race, age, number of comorbidities, highest education, income.
For cortisol analyses – PSQI and CESD also included.
*Ethnicity/race found to be statistically significantly different (p < 0.05).
Figure 1.
12-hour cortisol pattern by ethnicity/race group over five time points. *Adjusted p<0.05; NHB= non-Hispanic Black; NHW= non-Hispanic White; QST= Quantitative Sensory Testing. Note: T1 = first collection; T2 = second collection; T3 = third collection; T4 = fourth collection; T5=fifth collection.
Combined Contributions of Clinical Pain and Psychosocial Stress on Saliva Cortisol Levels
A regression analysis with GCPS CPI, EOD, financial satisfaction, and primary explanatory variables (age, sex, ethnicity/race, and total number of comorbidities) in the model indicated GCPS CPI, EOD, and financial satisfaction as the strongest predictors of morning waking (T2) cortisol (p = 0.001) explaining 21.3% of the variance. For evening to morning awakening cortisol slope (T1-T3), the model was significant (p = 0.017) with financial satisfaction as the only significant predictor (p = 0.016), with the overall model explaining 15.4% of the variance (Table 6a). An additional analysis including the secondary explanatory variables (PSQI, CESD, annual income and education) in the model showed financial satisfaction, GCPS CPI, and CESD as predictive of morning waking (T2) cortisol (overall model, p = 0.0017) explaining 27.4% of the variance (Table 6b) and financial satisfaction remained as the sole predictor for the evening to morning awakening (T1-T3) cortisol slope (p = 0.039), with the overall model explaining 18.6% of the variance. Overall modeling indicated decreased pain intensity, less depressive symptoms, and greater financial satisfaction were predictive of higher morning waking (T2) cortisol levels and increased financial satisfaction was predictive of greater evening to morning awakening (T1-T3) cortisol slope.
Table 6.
Combined adjusted regression analyses.
|
Morning waking (T2) |
Awakening slope (T1-T3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard error | t Value | p | Estimate | Standard error | t Value | p | |
| A. Regression analyses with combined contributions for clinical pain and psychosocial stress with primary explanatory variables | |||||||||
| Intercept | 0.4669392284 | 0.19921225 | 2.34 | 0.0211 | 0.0004106642 | 0.00031634 | 1.30 | 0.1975 | |
| Age | −0.0041690956 | 0.00314089 | −1.33 | 0.1875 | −0.0000021048 | 0.00000508 | −0.41 | 0.6797 | |
| Female | −0.0551313767 | 0.04188968 | −1.32 | 0.1913 | 0.0000840440 | 0.00007537 | 1.12 | 0.2678 | |
| NHB | −0.0125920187 | 0.05832931 | −0.22 | 0.8295 | −0.0000547617 | 0.00009498 | −0.58 | 0.5657 | |
| No. comorbidities | −0.0144896485 | 0.02197262 | −0.66 | 0.5112 | −0.0000201981 | 0.00003700 | −0.55 | 0.5864 | |
| GCPS CPI | −0.0019852505 | 0.00097436 | −2.04 | 0.0444 | – | – | – | – | |
| Financial Satisfaction | 0.0218167306 | 0.00841521 | 2.59 | 0.0110 | 0.0000379614 | 0.00001553 | 2.44 | 0.0165 | |
| EOD | 0.0081031742 | 0.00379644 | 2.13 | 0.0354 | – | – | – | – | |
| Income | – | – | – | – | 0.0000122608 | 0.00001716 | 0.71 | 0.4768 | |
| B. Regression analyses with combined contributions of clinical pain and psychosocial stress with primary and secondary explanatory variables | |||||||||
| Intercept | 0.3006345358 | 0.22386105 | 1.34 | 0.1827 | 0.0006791813 | 0.00035233 | 1.93 | 0.0572 | |
| Age | −0.0040730057 | 0.00340873 | −1.19 | 0.2353 | −0.0000045983 | 0.00000531 | −0.87 | 0.3889 | |
| Female | −0.0787574654 | 0.04350347 | −1.81 | 0.0736 | 0.0000753923 | 0.00007492 | 1.01 | 0.3170 | |
| NHB | 0.0271914016 | 0.06423854 | 0.42 | 0.6731 | −0.0000984503 | 0.00009551 | −1.03 | 0.3055 | |
| No. comorbidities | −0.0102599716 | 0.02287953 | −0.45 | 0.6549 | −0.0000364047 | 0.00003774 | −0.96 | 0.3374 | |
| GCPS CPI | −0.0022762273 | 0.00106284 | −2.14 | 0.0350 | – | – | – | – | |
| Financial Satisfaction | 0.0288438298 | 0.00933655 | 3.09 | 0.0027 | 0.0000336705 | 0.00001607 | 2.10 | 0.0391 | |
| EOD | 0.0050381823 | 0.00432110 | 1.17 | 0.2467 | – | – | – | – | |
| CES-D | 0.0100950638 | 0.00405104 | 2.49 | 0.0146 | −0.0000007808 | 0.00000684 | −0.11 | 0.9094 | |
| PSQI | 0.0011929637 | 0.00569085 | 0.21 | 0.8344 | −0.0000046965 | 0.00000933 | −0.50 | 0.6160 | |
| Education | 0.0051807795 | 0.01958240 | 0.26 | 0.7920 | −0.0000068128 | 0.00003301 | −0.21 | 0.8370 | |
| Income | 0.0077431147 | 0.01069222 | 0.72 | 0.4709 | 0.0000102155 | 0.00001758 | 0.58 | 0.5627 | |
A: Overall models: Morning Waking (p = 0.0013) and Slope (p = 0.0166).
B: Overall models: Morning Waking (p = 0.0017) and Slope (p = 0.0287).
–Variables not included in the overall model.
NHB= non-Hispanic Black; GCPS CPI= Graded Chronic Pain Scale Characteristic Pain Intensity; EOD= Experience of Discrimination; CES-D= Center for Epidemiologic Studies Depression Scale; PSQI= Pittsburgh Sleep Quality Index.
Additional analyses were completed to better understand factors contributing to sociodemographic group differences in the evening cortisol level. Post-hoc models were run with clinical pain, psychosocial stress, and explanatory variables in the models. Ethnic/race group remained the primary predictor with CESD as a secondary predictor. A regression analysis was also completed including explanatory variables. The model was significant (p = 0.042) and ethnicity/race as the only predictor (p = 0.009) with NHB participants having a significantly higher evening (T1) cortisol levels compared to the NHW participants. The model explained 15.8% of the variance observed for the evening cortisol level.
Discussion
The purpose of the study was to examine: 1) the relationship between clinical pain, prior/current psychosocial stress, and salivary cortisol levels; 2) possible sociodemographic group differences on measures of clinical pain, psychosocial stress, and saliva cortisol levels; and 3) the combined contributions of clinical pain, prior/current psychosocial stress, and sociodemographic variables on saliva cortisol levels. In an unadjusted analysis, clinical pain and psychosocial stress were most strongly correlated with the morning waking and evening to morning awakening slope cortisol levels. Adjusted analyses did not indicate a relationship for clinical pain and cortisol levels however a relationship with psychosocial stress, specifically higher financial satisfaction, was indicated for both higher morning waking cortisol and greater evening to morning awakening cortisol slope. Regarding question 2, we found significant sociodemographic group differences with NHB participants reporting greater pain disability, more experiences of discrimination, and less financial and material satisfaction compared to the NHW participants. Of the cortisol measures, only the evening (T1) prior to bed was found to differ by ethnic/race group with NHB participants having higher levels. Lastly, when evaluating the combined effects of clinical pain and psychosocial stress, increased financial satisfaction was indicated as a significant predictor for both higher morning waking, and greater evening to morning awakening slope cortisol measures while additional contributing factors including characteristic pain intensity and depressive symptoms were also relevant for morning waking cortisol.
Relationships Among Clinical Pain, Psychosocial Stress, and Saliva Cortisol
Previous studies have shown that having more stress-related experiences are related to worse clinical pain.46–48 In line with previous reports, we found significant relationships between clinical pain measures and psychosocial stress measures. Previous research examining associations between salivary cortisol and experiences of stress has revealed inconsistent findings.11,49 Although our correlation analyses revealed significant relationships between clinical pain and cortisol measures, the associations were not significant in the adjusted models. Importantly, in unadjusted and fully adjusted psychosocial stress models, higher financial satisfaction remained a significant predictor of both higher levels of morning waking cortisol and greater evening to morning awakening cortisol slope. This finding remained with inclusion of primary and secondary explanatory variables in the model including annual income. Research suggests morning waking salivary cortisol to be more strongly influenced by long term chronic stress, while evening salivary cortisol is more sensitive to current/recent social stress.45,50 Our results suggest psychosocial stress, specifically financial satisfaction, is a key factor contributing to differences in morning waking cortisol and evening to morning awakening cortisol slope measures.
Clinical Pain, Psychosocial Stress, and Saliva Cortisol by Ethnic/Race Group
Reports of ethnic/race group differences in clinical pain, psychosocial stress, and cortisol levels are ample.13,15,19,28,32,51 Findings in the present study align; NHB participants reported experiencing greater pain-related disability, more experiences of discrimination, and less financial and material satisfaction than their NHW peers. Differences in evening salivary cortisol levels (T1) also emerged, consistent with previous research with NHB participants having higher levels.45 Group differences were not found for other cortisol measures. Importantly, as noted in the introduction, ethnic/race group affiliation is a proxy for numerous cultural, environmental, and psychosocial factors. Our groups differed on key baseline descriptive variables including age, comorbidities, income, education, sleep, and depressive symptoms. Improving our understanding of disparities in health outcomes for minority groups will require consideration of a comprehensive array of social and environmental factors influencing behavior and biology.
Combined Contributions of Clinical Pain and Psychosocial Stress on Saliva Cortisol Levels
Our analysis revealed a combined contribution of financial satisfaction, characteristic pain intensity, and depressive symptoms as significant predictors of morning waking cortisol, while financial satisfaction was the only predictor of evening to morning awakening cortisol slope. Although ethnic/race groups differed in the evening cortisol level, ethnic/race group was not a significant predictor in the morning waking cortisol level and the evening to morning awakening slope. Findings suggest that “chronic” stress experiences associated with financial dissatisfaction, chronic pain, and depressive symptoms may contribute toward maladaptive shifts in cortisol regulation.3,18,52 Additionally, in the investigation of possible ethnic/race group differences in health-related outcomes, consideration of sociodemographic, environmental, and psychosocial factors is important.53,54
Additional Considerations
Several factors should be considered when evaluating the study’s findings. In regard to strengths, first, this study includes a robust sample of a community dwelling older adults with/without knee pain with a strong representation of both NHB and NHW adults. Second, we included multiple measures to capture the experience of pain and previous/current psychosocial stress. Third, we implemented statistical methods to help identify primary variables of interest for clinical pain and psychosocial stress to reduce the number of analyses completed. Fourth, we were able to collect salivary cortisol at multiple time points capturing five time points across an approximate 12-hour period. Cortisol analyses were completed with high quality processing standards by a recognized and reputable lab at the University of Alabama at Birmingham.
There are also limitations to acknowledge that can inform future efforts. First, collection of salivary cortisol across multiple time points has some inherent challenges (e.g., insufficient saliva, missed home collections). Additionally, our fifth saliva sample was collected after the QST session which could potentially influence the cortisol level. Second, as our ethnic/race groups differed on key sociodemographic variables, interpretations of group differences are limited and warrant the recognition of the influence of an array of sociodemographic factors (some measured with others remaining to be explored). Third, the GCPS questionnaire was specific to knee pain, therefore pain experiences associated with other body sites was not captured. Fourth, although number of pain sites has been indicated as a strong indicator of chronic pain severity,55,56 improved phenotyping of stage of chronic pain with consideration for overall pain frequency, intensity, and duration would be informative moving forward.57,58 Fifth, although we evaluated a number of key measures of psychosocial stress, other measures might be more effective in quantifying short and long-term stress, (e.g., including a measure of appraisal in addition to evaluation of potential stressful events and including a measure to capture childhood stress exposure). Additionally, many measures are bi-directional and inter-related, e.g., pain, functional limitations, depression, ability to work, income, and financial satisfaction. Sixth, there are inherent limitations in self-report questionnaires. Lastly, further investigations including “longer term” biological markers of stress, e.g., hair cortisol, an allostatic load composite and/or telomere length would provide valuable information.
Conclusion
Our findings indicate relationships between clinical pain, psychosocial stress, sociodemographics, and saliva cortisol. NHB participants reported experiencing greater pain disability, more experiences of discrimination, and lower financial and material satisfaction compared to NHW participants. Additionally, NHB participants reported more sleep disturbance, greater depressive symptoms, and had higher evening cortisol levels. However, ethnicity/race was not a significant predictor of morning waking cortisol or evening to morning awakening cortisol slope. Financial satisfaction was the most consistent predictor of cortisol levels, with characteristic pain intensity and depressive symptoms also contributing to morning waking cortisol. Understanding relationships among sociodemographic characteristics, psychosocial stress, clinical pain, and the biological interface will help identify targets to improve health outcomes in individuals living with chronic pain.
Acknowledgements
We would like to extend an expression of thanks to the UPLOAD participants; the University of Florida Clinical and Translational Science Institute nursing team and clinical lab; Eric Weber, Laboratory Manager, UF Pain Research and Intervention Center of Excellence; Barbara Gower, PhD; and to Burel Goodin, PhD for reviewing this manuscript. Finally, we are grateful for the support and guidance provided over the years by Dr. Bruce McEwen related to our stress and pain research efforts.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Institute of Arthritis and Musculoskeletal and Skin Diseases [K23AR062099 (KTS)], the National Institute on Aging [R01AG054370 (KTS)], the University of Florida Clinical and Translational Science Institute [UL1TR000064], the University of Alabama at Birmingham Center for Clinical and Translational Science [UL1TR003096], and the Diabetes Research Center Human Physiology Core Laboratory [P30DK079626]. The content is our responsibility and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Angela M. Mickle https://orcid.org/0000-0001-9175-5342
References
- 1.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015; 48(4): 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Generaal E, Vogelzangs N, Macfarlane GJ, et al. Biological stress systems, adverse life events and the onset of chronic multisite musculoskeletal pain: a 6-year cohort study. Ann Rheum Dis. 2016; 75(5): 847–854. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress (Thousand Oaks). 2017; 1: 247054701770476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crofford LJ. Chronic pain: where the body meets the brain. Trans Am Clin Climatol Assoc. 2015; 126: 167–183. [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng J, Liu S, Wang Y, et al. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017; 2017: 9724371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Partanen J, Chuong L, et al. Discrimination hurts: the effect of discrimination on the development of chronic pain. Soc Sci Med. 2018; 204: 1–8. [DOI] [PubMed] [Google Scholar]
- 7.White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014; 62(12): 2350–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musich S, Wang SS, Slindee L, Kraemer S, et al. Association of resilience and social networks with pain outcomes among older adults. Popul Health Manag. 2019; 22(6): 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodin BR, Bulls HW. Optimism and the experience of pain: benefits of seeing the glass as half full. Curr Pain Headache Rep. 2013; 17(5): 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thau L, Sharma S. Physiology, cortisol StatPearls. Treasure Island, FL: StatPearls Publishing StatPearls Publishing LLC; 2020. [Google Scholar]
- 11.Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014; 94(12): 1816–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Šušoliaková O, Šmejkalová J, Bičíková M, et al. Assessment of work-related stress by using salivary cortisol level examination among early morning shift workers. Cent Eur J Public Health. 2018; 26(2): 92–97. [DOI] [PubMed] [Google Scholar]
- 13.Simon CD, Adam EK, Holl JL, et al. Prenatal stress and the cortisol awakening response in African-American and Caucasian women in the third trimester of pregnancy. Matern Child Health J. 2016; 20(10): 2142–2149. [DOI] [PubMed] [Google Scholar]
- 14.Dedovic K, Duchesne A, Andrews J, et al. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage. 2009; 47(3): 864–871. [DOI] [PubMed] [Google Scholar]
- 15.Karlamangla AS, Friedman EM, Seeman TE, et al. Daytime trajectories of cortisol: demographic and socioeconomic differences–findings from the national study of daily experiences. Psychoneuroendocrinology. 2013; 38(11): 2585–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stehlik R, Ulfberg J, Zou D, et al. Morning cortisol and fasting glucose are elevated in women with chronic widespread pain independent of comorbid restless legs syndrome. Scand J Pain. 2018; 18(2): 187–194. [DOI] [PubMed] [Google Scholar]
- 17.Hernández LM, Markwald RR, Kviatkovsky SA, et al. Morning cortisol is associated with stress and sleep in elite military men: a brief report. Military Medicine. 2018; 183(9–10):e255–e259. [DOI] [PubMed] [Google Scholar]
- 18.Duan H, Yuan Y, Zhang L, et al. Chronic stress exposure decreases the cortisol awakening response in healthy young men. Stress. 2013; 16(6): 630–637. [DOI] [PubMed] [Google Scholar]
- 19.Skinner ML, Shirtcliff EA, Haggerty KP, et al. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011; 23(4): 1167–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeSantis AS, Adam EK, Hawkley LC, et al. Racial and ethnic differences in diurnal cortisol rhythms: are they consistent over time? Psychosom Med. 2015; 77(1): 6–15. [DOI] [PubMed] [Google Scholar]
- 21.Adam EK, Quinn ME, Tavernier R, et al. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017; 83: 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008; 583(2–3): 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed AT, Mohammed SA, Williams DR. Racial discrimination & health: pathways & evidence. Indian J Med Res. 2007; 126(4): 318–327. [PubMed] [Google Scholar]
- 24.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009; 32(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 25.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003; 4(3): 277–294. [DOI] [PubMed] [Google Scholar]
- 26.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012; 2(3): 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tait RC, Chibnall JT. Racial/ethnic disparities in the assessment and treatment of pain: psychosocial perspectives. Am Psychol. 2014; 69(2): 131–141. [DOI] [PubMed] [Google Scholar]
- 28.Booker S, Cardoso J, Cruz-Almeida Y, et al. Movement-evoked pain, physical function, and perceived stress: an observational study of ethnic/racial differences in aging non-Hispanic blacks and non-Hispanic whites with knee osteoarthritis. Exp Gerontol. 2019; 124: 110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vines AI, Ta M, Esserman D, et al. A comparison of the occurrence and perceived stress of major life events in black and white women. Women Health. 2009; 49(5): 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIlvane JM, Baker TA, Mingo CA. Racial differences in arthritis-related stress, chronic life stress, and depressive symptoms among women with arthritis: a contextual perspective. J Gerontol Ser B. 2008; 63(5):S320–S327. [DOI] [PubMed] [Google Scholar]
- 31.Samuel LJ, Roth DL, Schwartz BS, et al. Socioeconomic status, race/ethnicity, and diurnal cortisol trajectories in middle-aged and older adults. J Gerontol Ser B. 2016; 73(3): 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Webb Hooper M. Racial/ethnic differences in physiological stress and relapse among treatment seeking tobacco smokers. Int J Environ Res Public Health. 2019; 16(17): 3090. [DOI] [PMC free article] [PubMed]
- 33.Adam EK, Heissel JA, Zeiders KH, et al. Developmental histories of perceived racial discrimination and diurnal cortisol profiles in adulthood: a 20-year prospective study. Psychoneuroendocrinology. 2015; 62: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux CH, Saraux A, Mazieres B, et al. ; KHOALA Osteoarthritis Group. Screening for hip and knee osteoarthritis in the general population: predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis. 2008; 67(10): 1406–1411. [DOI] [PubMed] [Google Scholar]
- 35.King CD, Sibille KT, Goodin BR, et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthr Cartil. 2013; 21(9): 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glover TL, Goodin BR, Horgas AL, et al. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthr Rheum. 2012; 64(12): 3926–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Elm EA, Egger M, Pocock SJ, et al. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008; 61(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 38. Grading the severity of chronic pain. Pain. 1992; 50(2): 133–149. [DOI] [PubMed] [Google Scholar]
- 39. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005; 61(7): 1576–1596. [DOI] [PubMed] [Google Scholar]
- 40.Miller MA, Rahe RH. Life changes scaling for the 1990s. J Psychosom Res. 1997; 43(3): 279–292. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24(4): 385–396. [PubMed] [Google Scholar]
- 42.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 43.Radloff LS, The CES. D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1(3): 385–401. [Google Scholar]
- 44.Lindholm H, Ahlberg J, Sinisalo J, et al. Morning cortisol levels and perceived stress in irregular shift workers compared with regular daytime workers. Sleep Disord. 2012; 2012: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm Behav. 2012; 61(5): 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zautra AJ, Parrish BP, Van Puymbroeck CM, et al. Depression history, stress, and pain in rheumatoid arthritis patients. J Behav Med. 2007; 30(3): 187–197. [DOI] [PubMed] [Google Scholar]
- 47.Gil KM, Carson JW, Porter LS, et al. Daily mood and stress predict pain, health care use, and work activity in african american adults with sickle-cell disease. Health Psychol. 2004; 23(3): 267–274. [DOI] [PubMed] [Google Scholar]
- 48.Terry EL, Fullwood MD, Booker SQ, et al. Everyday discrimination in adults with knee pain: the role of perceived stress and pain catastrophizing. J Pain Res. 2020; 13: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogelman N, Canli T. Early life stress and cortisol: a meta-analysis. Horm Behav. 2018; 98: 63–76. [DOI] [PubMed] [Google Scholar]
- 50.Powell DJ, Schlotz W. Daily life stress and the cortisol awakening response: testing the anticipation hypothesis. PLoS One. 2012; 7(12):e52067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaughn IA, Terry EL, Bartley EJ, et al. Racial-Ethnic differences in osteoarthritis pain and disability: a meta-analysis. J Pain. 2019; 20(6): 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017; 1391(1): 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams DR, Yan Y, Jackson JS, et al. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997; 2(3): 335–351. [DOI] [PubMed] [Google Scholar]
- 54.Vina ER, Ran D, Ashbeck EL, et al. Natural history of pain and disability among African–Americans and whites with or at risk for knee osteoarthritis: a longitudinal study. Osteoarthr Cartil. 2018; 26(4): 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergman S, Herrström P, Jacobsson LT, et al. Chronic widespread pain: a three year followup of pain distribution and risk factors. J Rheumatol. 2002; 29(4): 818–825. [PubMed] [Google Scholar]
- 56.Coggon D, Ntani G, Palmer KT, et al. Patterns of multisite pain and associations with risk factors. Pain. 2013; 154(9): 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sibille KT, Chen H, Bartley EJ, et al. Accelerated aging in adults with knee osteoarthritis pain: consideration for frequency, intensity, time, and total pain sites. Pain Rep. 2017; 2(3):e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sibille KT, Steingrímsdóttir ÓA, Fillingim RB, et al. Investigating the burden of chronic pain: an inflammatory and metabolic composite. Pain Res Manag. 2016; 2016: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]