Abstract
Background:
Graves’ orbitopathy (GO) is the most frequent extrathyroidal manifestation of the autoimmune Graves’ disease. GO significantly impacts quality of life and has a psycho-social morbidity. Inflammation and swelling of the orbital tissue often leads to proptosis, diplopia, and decrease of visual acuity. Due to the inflammatory background of the disease, glucocorticoids (GC) have been used as a first-line treatment for decades.
Methods:
PubMed and MeSH database were searched for original articles, clinical trials, reviews, and meta-analyses published between 1 January 2000 and 31 March 2020 and pertaining to both the mechanism of action and immunological effects of GC as well as to the treatment of GO by GC. The publications were evaluated according to their setting and study design.
Results:
GC act through genomic (trans-activation and trans-repression) and rapid non-genomic mechanisms. GC in general, and the intravenous (IV) administration of GC in particular, markedly decrease the activity and number of the most potent antigen-presenting dendritic cells. According to the internationally acknowledged European Thyroid Association Guidelines for the management of GO, weekly IVGC application over 12 weeks is recommended as first-line treatment for patients with active and severe GO. The daily and cumulative dose should be tailored according to clinical severity, for example, 4.5 g of IV methylprednisolone for the inflammatory component versus 7.5 g in the presence of diplopia and severe proptosis. Fast and significant improvements in orbital symptoms and signs are noted in 65–70% of patients. Long-term experience over decades, and worldwide availability at low cost, underline the clinical and therapeutic relevance of GC. Adverse events are rarely severe, dose-dependent, and usually reversible, hence easy to handle by medical investigators. Oral GC application on a daily basis is characterized by high bioavailability but reduced efficacy and increased toxicity.
Conclusion:
IVGC still represents the standard of care in active/severe GO. Innovative biologicals, like monoclonal antibodies targeting the thyrotropin/Insulin-like growth factor-1 receptors or pro-inflammatory cytokines (e.g., Interleukin-6) should be compared with standard GC treatment with respect to short- and long-term efficacy, safety, costs, and global availability.
Keywords: Graves’ orbitopathy, glucocorticoids, intravenous methylprednisolone, mechanisms of action, pharmacology immunology, thyroid eye disease
Introduction
Graves’ orbitopathy (GO) is the most common extra-thyroidal manifestation of autoimmune Graves’ disease (GD).1,2 The prevalence of GO among patients with GD varies widely from 13% to 69% across different series. The incidence of GO is 3 cases per 100,000 males and 16 cases per 100,000 females in the United States (US).3 Most GO patients demonstrate extraocular muscle enlargement and expansion of orbital adipose/connective tissue. The increased orbital tissue volume and elevated intra-orbital pressure cause mechanical changes, which explain most of the signs and symptoms in GO.4–6 The pathological processes within the orbit include inflammatory infiltration of retro-ocular tissues within the orbit, de novo adipogenesis, and increased production of hydrophilic glycosaminoglycans (GAG) by orbital fibroblasts.7 These fibroblasts play a key role in the pathogenesis of GO. They proliferate and differentiate into myofibroblasts and adipocytes and produce excessive hydrophilic GAG, which lead to tissue edema. Orbital fibroblasts express the thyrotropin receptor (TSH-R) and are stimulated by the circulating TSH-R autoantibodies (Ab).8,9 Functional stimulatory TSH-R-Ab are the specific biomarker of GO closely correlating with disease activity and severity.10–18 Active interaction of orbital fibroblasts with mononuclear cells and production of different chemo attractants and cytokines lead to perpetuation of orbital inflammation.19 The two key autoantigens TSH-R and insulin growth factor 1 receptor (IGF-1R) are expressed on the surface of target orbital cells of GO patients. They form a physical and functional signaling complex that is potentially relevant in the pathogenesis of GO.20–23
For decades, systemic administration of glucocorticoids (GC) has been the acknowledged first-line anti-inflammatory and immunosuppressive treatment for several inflammatory diseases, for example, asthma, Crohn’s disease, psoriasis, and, more specifically, for the active and severe stages of GO.1,24–28 Recently, with the introduction of novel drugs targeting the autoantigens in GD/GO and/or the receptors of the involved pro-inflammatory cytokines, questions and doubts have emerged pertaining to the benefit–risk ratio of this drug in patients with GO. To answer these questions and offer concrete recommendations regarding the clinical relevance and utility of GC, foremost intravenous (IV) GC, in GO, this short review aims to describe the mechanism of action and immunological effects of GC, summarize the results of GC trials performed in the last 20 years in patients with GO, and evaluate the response rates and safety profile of this classic drug. More specifically, this review looks carefully at a convincing rationale for the further management of GO with GC.
Methodology
A literature search of the NCBI PubMed database (National Library of Medicine, Bethesda, MD) was performed with the key words “GC”, “GC mechanism of action”; “immunology of GC”, “safety and efficiency of GC”; “Grave’s orbitopathy”; “Grave’s eye disease”; “endocrine orbitopathy”; “thyroid eye disease” and “thyroid associated eye disease”.
As the result of the literature search, original articles, reviews, meta-analyses, and 26 studies published between 2000 and 2020 and reporting a treatment procedure for GO as well as including a minimum of one form of GC administration were selected.27–34 All studies were checked and evaluated for their quality and study design. In total, 1689 patients were included. An overview of the study design, administration form and dose of GC, response rate and adverse events are listed in Table 1.
Table 1.
Overview of performed trials with GC in GO.
| Total number of clinical studies | 26 | |||
|---|---|---|---|---|
| • Prospective | 22 | |||
| • Retrospective | 4 | |||
| • Randomized controlled | 16 | |||
| Publication years | 2000–2020 | |||
| Total number of patients | 1689a,b | |||
| Number of patients per treatment regimen | ||||
| • Intravenous | 973 | |||
| • Oral | 166 | |||
| • Peribulbar/subconjunctival | 169 | |||
| • Combination therapy | ||||
| Glucocorticoid + mycophenolate | 83 | |||
| Glucocorticoid + radiation | 128 | |||
| Min | Max | Median | Mean | |
| Cumulative dose (g) | ||||
| • Intravenous | 0.9 | 12.0 | 4.5 | 5.74 |
| • Oral | 2.24 | 6.0 | 4.0 | 3.85 |
| • Peribulbar/subconjunctival | 0.04 | 0.16 | 0.07 | 0.09 |
| Duration of treatment (weeks) | ||||
| • Intravenous | 4 | 24 | 12 | 12.65 |
| • Oral | 12 | 22 | 16 | 16.14 |
| • Peribulbar/subconjunctival | 4 | 14 | 12 | 10 |
| • Combined treatment | 14 | 24 | 19 | 19 |
| Responder rate (%) | ||||
| • Intravenous | 28 | 88 | 74 | 67.44 |
| • Oral | 49 | 66 | 54.84 | 56.77 |
| • Peribulbar/subconjunctival | 68.6 | 95 | 76 | 78.9 |
| • Combined treatment | 28.6 | 63 | 45.8 | 45.8 |
| Number of AE (n=) | 1 | 125 | 40 | 43.58 |
| Number of dropouts (n=) | 0 | 23 | 1 | 4.39 |
A total of 51 patients were withdrawn from the studies by investigators of several reasons for example, not complying with protocol.
Patients receiving no treatment n = 119.
AE, adverse events; GC, glucocorticoids; GO, Graves’ orbitopathy.
Molecular pathways of inflammation
Pro inflammatory mediators, for example, cytokines, chemokines, or adhesion structures are not present in healthy human cells. In contrast, during inflammation episodes, an increase of these mediators caused by pro-inflammatory interleukins or tumor necrosis factor-alpha (TNF-α) is observed.55 A key role in the inflammatory process belongs to the nuclear factor “kappa-light-chain-enhancer” of activated B-cells (NF-κB) and the activator protein 1 (AP-1). NF-κB is found under physiological conditions in all cells.56,57 Due to the association with NF-κB inhibitor (IκB), the pro-inflammatory transcription factor stays in an inactivated form and is not able to translocate into the nucleus. After binding of TNF-α to its membrane-associated receptor (which belongs to the group of tyrosine kinase), the receptor activates IκB kinase (IKK), which phosphorylates IκB.48 Due to its phosphorylation, IκB can be ubiquitinated and degraded. Without the IκB structure, NF-κB translocates into the nucleus and binds to its response element. After binding, the transcription factor associates with coactivators, i.e., the steroid receptor coactivator-1 (SCR-1) and the cAMP response element-binding protein (CREB) binding protein (CBP).58
Usually, human DNA is stored in a coiled form around histones, which have a positive charged surface area. The negatively charged DNA is coiled tightly around the histones and the transcription complex is not able to start transcription. Coactivators like CBP and SCR-1 have an action similar to that of histone acetyltransferase (HAT), hence they decrease the positively charged histone surface. After its acetylation, the DNA is able to unwind and transcription can be performed.59
NF-κB-induced transcription increases levels of pro-inflammatory (IL-2, IL-3 and IL-6) and T-cell stimulating interleukins (IL-4, IL-5 and IL-13), TNF-α, and adhesion molecules.60–62 Further, the transcription factor AP-1, a Jun enzyme family member, forms a homodimer with other Jun-proteins or a heterodimer with Fos-proteins. The dimer translocates into the nucleus and binds to its response element, causing the transcription of several pro-inflammatory mediators and enzymes, for example, collagenases.
Glucocorticoid receptor
The glucocorticoid receptor (GR) belongs to the group of intracellular, ligand regulated transcription factors and is located primarily in the cytosol.58 The GR appears in the cytosol as a monomer, and is associated with several chaperones, such as the heat shock protein 90 (HSP) and the FK506-binding protein (FKBP).59,63 These proteins prevent GR degradation or translocation into the nucleus. There is only one gene in the human DNA that codes for the receptor structure, but splicing variation causes the formation of different GR isoforms. The most common isoforms are GRα and GRβ (with a higher intracellular concentration of GRα than GRβ).60,63
The GR consists of three different functional domains linked together by a hinge region. The constitutive N-terminal domain is essential for the activation of the receptor and has a very variable structure. Next to the N-terminal domain, a very highly conserved DNA binding region with two zinc fingers (which are necessary for the interaction of the receptor with the DNA) is located. This domain also enables dimerization of the receptor. Glucocorticoids and the coactivators of the GR can bind to the C-terminal domain of the receptor.58,64
Interactions of GC with the GR
GC like cortisone and synthetic derivatives (e.g., methylprednisolone) are small molecules, which diffuse easily through the cellular membrane. After intracellular binding to GR, the chaperones dissociate from the receptor monomer and two monomeric structures form a homodimer. Importins start to translocate the homodimer into the nucleus, where it binds to its response elements. Binding to the positive or negative response element of GR induces different genomic activities.55,58,60,62,63 The positive response element of GR has a palindromic structure. The structure of the GC receptor and the mechanisms of action of GC have been studied over decades by a large number of research groups.65–73 The genomic and non-genomic activities of GC will be discussed further below in detail.
Genomic trans-activation
After binding of the GR homodimer to its positive response element, the coactivators SCR-1 and CBP associate with the receptor–ligand complex and transcription of the DNA is initiated. Transcription of the anti-inflammatory genes increases production of anti-inflammatory proteins, for example, annexin-1 (or lipocortin-1), anti-inflammatory cytokines (IL-10, IL-12, IL-1 receptor antagonist), IκB, and mitogen-activated protein kinase phosphatase-1 (MAPKP-1).63
Genomic trans-repression
For trans-repression, two very similar mechanisms with different effects on the human body are known. The anti-inflammatory effects of GC are best explained by the mechanism of trans-repression. However, this genomic effect is also responsible for a few side effects (SE) of GC. The anti-inflammatory effect of the trans-repression activity occurs when GR interacts with mediators, that is, NF-κB or AP-1, in a protein–protein interaction. The GR dimer interacts with NF-κB, even when the mediator is already bound to its response element and human DNA. When the GR binds to the NF-κB-DNA complex instead of the coactivators, transcription is inhibited because the DNA cannot unwind from the histones. NF- κB itself has no HAT-like function, and is therefore dependent on the same cofactors as GR for acetylation of the amino acids on the histone surface. A similar protein–protein interaction among GR and AP-1 can be found. This fact explains why GC affects the transcription of pro-inflammatory genes only.58 The amount of SRC-1 and CBP and the concentration of GC are important for the anti-inflammatory effect of GC. If the concentration of the coactivators is very high, and only a low amount of GC is available in the cell, the anti-inflammatory effect can be negated by the high concentration of NF-κB/AP-1 and its coactivators.58 Unlike GRα, GRβ does not bind steroids as a ligand but may interact with human DNA. The beta isoform of the GR is translocated into the nucleus by importins and binds to the negative GR response element. Through this spontaneous and unplanned inactivation of genes, transcription of several physiological important proteins, for example, osteocalcin, CRF-1, and keratin POMC is decreased.
Furthermore, the GR recruits histone deacetylase 2 (HDAC-2) to the CBP-HAT complex of NF-κB. Through this pathway, GR is able to interrupt the unwinding of the DNA by splitting the acetyl structures on the histone surface.58 Because of the tightly coiled DNA, the transcription complex cannot accumulate to the DNA and transcription will not start.
Non-genomic activity
The anti-inflammatory and immunosuppressive activity of GC arises within minutes after intravenous administration, emphasizing its non-genomic mechanism of action.61,74,75 GC inhibit T-cell activating cytokines and adhesion molecules, hence lowering both proliferation and infiltration of the immune cells. The same holds true for the anti-inflammatory protein IκB.
GC impact the production of prostaglandins (PG) via three different mechanisms.59 First, suppression of NF-κB-induced transcription decreases cyclooxigenase-2 (COX-2) concentration, one of the key enzymes in PG synthesis. Second, the high amount of lipocortin-1 inhibits the cytosolic phospholipase A2α (cPLA2α) responsible for releasing the arachidonic acid (AA) from the cell membrane of the inflamed cell; AA is transformed to PG and leukotrienes. Third, the increase in MAPKP-1 dephosphorylates several mitogen-activated protein kinases (MAPK). These kinases stimulate the activity of cPLA2α and increase free AA.62,66,76
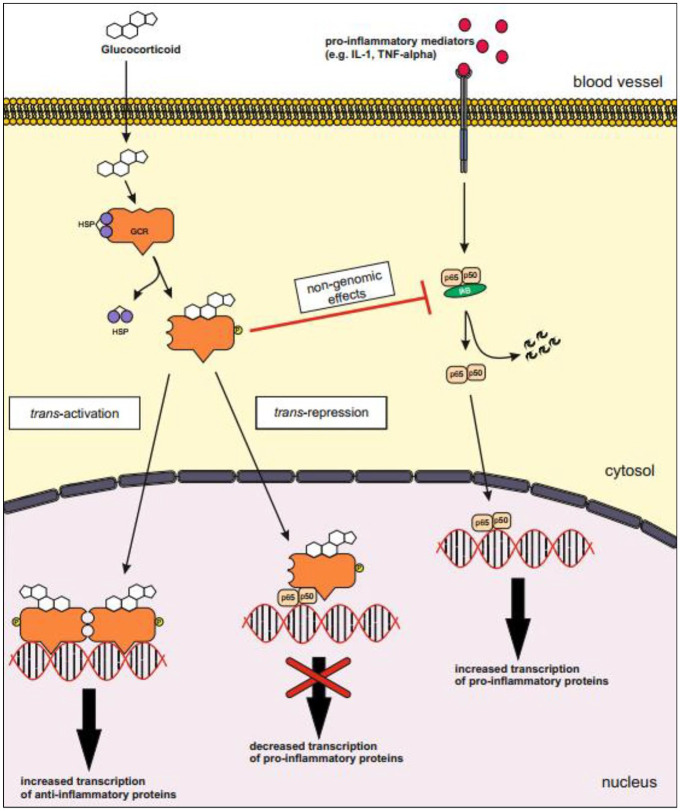
The mRNA generated by the transcription of pro-inflammatory mediators is very fragile under physiological conditions. In an inflamed cell, several enzymes become activated to avoid premature degeneration of the mRNA. Activated GR inhibits these enzymes, thus the mRNA is degraded and translation of pro-inflammatory proteins is stopped. An overview of the genomic and non-genomic effects of GC is shown in Figure 1.
Figure 1.
Genomic and non-genomic effects of glucocorticoids.
Trans-activation: The genomic effect of GC after binding of the GR to its positive response element causes increased transcription of anti-inflammatory proteins, for example, lipocortin-1, IL-10, IL-12, MAPK phosphatase I and IκB.
Trans-repression: The molecule–molecule interaction between activated GR and pro-inflammatory transcription factors for example, AP-1 or NF-κB causes decreased transcription of pro-inflammatory mediators, for example, Il-2, IL-3, IL-4, IL-5, IL-6, IL-13, IL-15, TNF-α and VCAM-a.
Most of the non-genomic, anti-inflammatory effect of glucocorticoids are based on interactions of pro and anti-inflammatory proteins, for example, dephosphorylating of MAP kinases by the MAPK phosphatase I, inhibition of cPLA2α by lipocortin-1 or the inactivation of NF-κB due to the increased level of IκB.
AP-1, activator protein-1; cPLA2α, cytosolic phospholipase A2α; GC, glucocorticoids; GR, glucocorticoid receptor; IκB, inhibitor of nuclear factor κB; IL, interleukin; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; TNF-α, tumor necrosis factor-alpha; VCAM-a, vascular cell adhesion molecule-a.
Immunosuppressive effect of GC
GC impact the immune system in a multifunctional manner. GC directly modulate pro-inflammatory mediators as well as number and functionality of immune system cells.66,69
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) in the unspecific human immune system. A key role of APC belongs to the identification of pathogens and presenting structures of antigens to cells of the specific immune system.77–79 Two different morphologic types of DC are found in the peripheral blood: myeloid (mDC) and plasmacytoid DC (pDC). PDC produce interferons (e.g., interferon-α) to protect the human body against blood-borne pathogens, i.e., viruses, while mDC are the essential source for DC in peripheral tissues. High doses of IVGC (0.5–1 g methylprednisolone) decrease markedly the number and activity of dendritic and plasma cells.61,77 DC of patients treated with IV prednisolone (cumulative dose 3.0 g over 3 days) disappeared rapidly within 1 day; 8 days after the last GC application, the number of mDC nearly normalized; however, the number of pDC was still significantly decreased. Three different mechanisms were discussed by the authors61,77: (i) homing of DC to peripheral tissues or lymphoid organs; (ii) decrease of production and differentiation of progenitor cells; and (iii) initiation of apoptosis.
IV pulse GC application impacts T cells. Already during application, the number of suppressor-inducer T-cells (CD4+CD45RA+) and cytotoxic T-cells (CD11–CD8+) increased. The number of suppressor T-cells (CD8++CD11+) decreased during the GC treatment but normalized within days after the last application.61,80 Proliferation and activation of immature T-cells depends on IL-2 and co-stimulatory receptor interactions, that is, cluster of differentiation 28 (CD28) interactions with CD80. Recently, numerous mechanisms effecting T-cell proliferation have been published: (i) blocked cell cycle entry of native T-cells; (ii) increased proliferation of cytotoxic T-lymphocyte-associated protein 4 (CLTA-4) on the T-cell surface, CLTA-4-induced negative feedback on proliferation after CD28-CD80 interactions to avoid overreactions; (iii) decreased differentiation in different T-cell phenotypes; and IV induced apoptosis of T-cells.81,82 Adhesion molecules, that is, CD2, lymphocyte function-associated antigen-1 (LFA-1), or LFA-3 are required for migration of immune cells. GC reduce the amount of anti-inflammatory cells in the target tissue by lowering the LFA-1 and CD2 level on the lymphocyte surface, and inhibiting their ability to migrate by decreasing the expression of adhesion molecules on the surface of endothelial cells and fibroblasts.80,83 This induces lymphocytosis and a marked reduction of cell–cell interactions. Subsequently, GC-induced, lymphocytes interact less with other immune cells, i.e., B-cells, APC, and natural killer cells, hence markedly impairing the ability of an effective immune response to pathogens.
Clinical application of GC in GO
Detailed treatment recommendations for GO have been published and updated in recent years.41,54 The European Thyroid Association (ETA) recommends an IV GC pulse therapy, as first line treatment for active and moderate-to-severe GO. The treatment scheme of the ETA specifies a cumulative dose of 4.5 g methylprednisolone over 12 weeks, starting with a single dose of 500 mg IV once weekly for the next 6 weeks, due to the inflammatory background of GO.27,28,84 This moderate regimen is very well tolerated, as recently shown in an extensive and careful Medical Dictionary of Regulatory Activities (MedDRA) analysis.85 However, the European Guidelines also strongly recommend tailoring both single and cumulative dose according to clinical disease severity. Hence, in the presence of motility disturbances, diplopia, severe proptosis, and severe lid retraction, a starting dose of 750 mg IV methylprednisolone per week (instead of 500 mg) and a cumulative dose of 7.5 g of IVCG should be applied. Response rates for both schedules approximate 65–70%, with the opportunity to start a second cycle in case of partial response and good tolerability. If the response to the standard intravenous administration of GC is either partial or poor (~20% of cases) at least 12 weeks after starting the IVGC regimen, novel strategies and second-line treatments, for example, monoclonal antibodies targeting the TSH and IGF-1 receptors and/or the IL-6 receptor should be discussed individually with the patient. Add-on treatments, e.g., mycophenolate36,85,86 or retrobulbar irradiation (in case of diplopia or disturbances of eye muscle motility) to a second course of IVGC are also indicated.28,87 Furthermore, in the worst case of sight-threatening compression of the optic nerve, urgent application of high dose IVGC treatment with alternate doses of 750 mg methylprednisolone every second day for 2 weeks is very helpful, secures patients vision, and avoids orbital decompression surgery in more than 50% of the cases.87 Other therapy options, for example, oral GC, are less recommended due to higher toxicity and lower efficacy.38,43,49,50,52 In contrast to oral administration, IVGC neither induce adrenal failure,88 nor decrease bone mineral density.49 Finally, peribulbar injections of GC were performed in only a small number of cases,35,37,40,42,44–46,51,89 as they can cause intra-orbital bleeding, myopathy, and other local SE.
Summary of performed trials
A “proof-of-concept”, double blind, placebo-controlled, randomized study performed in 2008 confirmed the efficacy and the disease-modifying potency of IVGC. The study included 16 patients showing a response rate of >80% for IVGC versus 11% only for placebo. The study was stopped prematurely for ethical reasons because IVGC did so well in contrast to the placebo-treated subjects. Rate of SE was dose dependent.47 Further, two randomized controlled studies demonstrated rapid improvement, significantly higher efficacy, and lower morbidity with the weekly IVGC regime in contrast to daily oral administration of GC.41,90 Previous treatment with IVGC significantly reduces the number of required rehabilitative surgeries thereafter.28,49,87 The difference between IV versus oral therapy, using high doses of methylprednisolone or prednisone was tested within a controlled trial. The authors recommended both application forms for GO treatment; however, they underlined that IVGC are more effective, and should be recommended for GO treatment.54 Finally, a large, multicenter, double-blind, randomized trial evaluated efficiency and safety of three different cumulative dosages of IVGC in 159 patients.41 Each of the three cumulative doses, i.e., 2.25, 4.5, and 7.47 g methylprednisolone had a certain efficacy in active severe GO, though patients randomized to the highest dose showed earlier response and improvement with slightly more SE compared with patients with a lower dose. Average costs of a 10- to 12-week treatment course with oral GC vary between 415 and 1360 Euros (€). Advantages of the oral application are easier handling and worldwide availability. In comparison, average costs of IV administration of “pulse” GC treatment course vary between 204 and 364 €.91 IVGC treatment requires a logistic infrastructure and trained personnel, and should optimally follow in specialized centers. At our institution (Johannes Gutenberg University Medical Center), more than 2000 patients with active, severe GO have been treated with IVGC; more than 80% of those have received the recommended 4.5 g regimen. Over a period of more than 20 years, not a single major SE was observed in Table 2.
Table 2.
GC treatment of GO – trials performed 2000–2020.
| Author Publication year (reference) |
Study design | Patients enrolled (n) | Application form | patients per treatment form | Treatment protocol | Duration (weeks) | Cumulative GC dose [g] |
Response rate [%]*D |
Dropout (n) | SE (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bagheri et al.35 | Prospective | 22 | intraorbital | 22 | - one injection per month - 20 mg triamcinolone and 5 mg dexamethasone per application |
12–16 | triamcinolone 0.06 – 0.08 dexamethasone 0.015 – 0.02 |
n/a*A | 5/22 | 10 |
| Kahaly et al.36 | Randomized Prospective Observer blind |
164 | iv | 81 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction to 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
53 | 23/164 | total: 201 drug related: 68 |
| iv & oral | 83 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction to 0.25 g, 6-wk interval - additional 0.36 g mycophenolate twice a day (oral application) |
1224 | methylprednisolone 4.5mycophenolate 120 |
71 | |||||
| He et al.29 | Randomized Prospective |
40 | iv weeklyiv monthly |
1517 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction to 0.25 g, 6-wk interval- three infusions per week (consecutive days, once a month) - initial dose 0.5 g methylprednisolone per infusion |
1612 | methylprednisolone 4.5methylprednisolone 6.0 |
71.9*E | 8/40 | 40 |
| Ueda-Sakane et al.30 | Retrospective | 43 | iv low dose |
18 | - three infusions per week (consecutive days) - initial dose 0.5 g methylprednisolone per infusion, 3-wk interval - after 3rd week oral follow up with prednisolone, initial dose depending on body weight (weight < 50 kg ,dose 30 mg/day; weight 50-70 kg, dose 35; weight > 70 kg, 40 mg/day) - decrease of dosage 5 mg/week to 20 mg/day - bilateral orbital irradiation during oral treatment, 10 fractions |
32 – 42 | methylprednisolone 4.5prednisolone 0.14 – 1.105radiation 20 Gy |
72.2*E | 8/38 | 54 |
| iv high dose |
20 | - three infusions per week (consecutive days) - initial dose 1.0 g methylprednisolone per infusion, 3-wk interval - after 3rd week oral follow up with prednisolone, initial dose depending on body weight (see scheme above) - bilateral orbital irradiation (see scheme above) |
32 – 42 | methylprednisolone 9.0prednisolone 0.485 – 0.93radiation 20 Gy |
||||||
| Hamed-Azzam et al.37 | Prospective | 7 | subconjunctival | 7 | - 20 mg triamcinolone per application - monthly application |
12 | triamcinolone 0.06 |
77 | 0/7 | 1 |
| Roy et al.38 | Randomized Prospective |
65 | iv | 32 | - three infusions per week (constitutive days, once a month) - 0.5 g methylprednisolone per infusion |
16 | methylprednisolone 6 |
87.1 | 3/65 | 42 |
| oral | 33 | - initial 1 mg/kg body weight per day prednisolone, 6-wk interval - continuous dose reduction until withdraw |
n/a | prednisolone *B |
54.8 | |||||
| Sisti et al.39 | Retrospective | 376 | iv | 353 | - one infusion per week - initial dose 15 mg/kg body weight methylprednisolone per infusion, 4-wk interval - dose reduction 7.5 mg/kg body weight, 8-wk interval - followed by oral prednisone treatment (40 mg/day, tapered every ten days, withdrawn after 50 days) |
approx. 19 |
methylprednisolone 6.3 – 8.7 |
n/a | n/a | n/a |
| Beleslin et al.32 | Retrospective | 50 | iv | 50 | - application duration 4 h - infusion volume 500 mL - two infusions per week - initial dose 0.5 g methylprednisolone per infusion - followed by oral steroid treatment (40 mg/day, 1 week; 30 mg/day, 1 week; 20 mg/day, 1 week, 10 mg/day, 1 week) |
24 | methylprednisolone 10.2 |
74 | n/a | 125 |
| Zhu et al.31 | Randomized Prospective Single blind |
80 | iv short term |
41 | - three infusions per week (consecutive days) - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
4 | methylprednisolone 4.5 |
41 | n/a | 81 |
| iv long term |
39 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
76.9 | |||||
| Lee et al.40 | Randomized Prospective |
105 | subconjunctival | 55 | - 20 mg triamcinolone per application - 1-3 injections, 3-wk interval |
3–9 | triamcinolone 0.02 – 0.06 |
75 | 10/105 | 3 |
| Single blind | no treatment | 40 | - control group | 3–9 | n/a | 57 | ||||
| Philip et al.33 | Randomized Prospective |
21 | iv | 10 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
n/a*A | 0/21 | total: 15 dexamethasone: 8 methylprednisolone:7 |
| 11 | - one infusion per week - initial dose 0.1 g dexamethasone per infusion, 6-wk interval - dose reduction 0.05 g, 6-wk interval |
12 | dexamethasone 0.9 |
|||||||
| Bartalena et al.41 | Randomized Prospective Double blind |
159 | iv low dose |
53 | - one infusion per week - initial dose 0.25 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.125 g, 6-wk interval |
12 |
methylprednisolone 2.25 |
52 | 12/159 | 44 |
| iv moderate dose |
54 | - one infusion per week - initial dose 0.54 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 |
methylprednisolone 4.98 |
35 | |||||
| iv high dose |
52 | - one infusion per week - initial dose 0.83 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.415 g, 6-wk interval |
12 | methylprednisolone 7.47 |
28 | |||||
| Wichary and Gasińska34 | Prospective | 30 | iv | 30 | No details of scheme were reported | 4 | methylprednisolone 8.0 |
n/a*A | n/a | n/a |
| Xu et al.42 | Retrospective | 36 | subconjunctival | 21 | - one injection per month - 20 mg triamcinolone per application |
12 | triamcinolone 0.06 |
68.6 | 0/36 | 12 |
| no treatment | 15 | - control group | 12 | n/a | 17.4 | |||||
| Akarsu et al.43 | Prospective | 68 | iv | 18 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
88 | 0/68 | n/a |
| oral | 15 | - initial dose 72 mg/day, 2-wk interval - dose reduction 8 mg/2 weeks |
12 | methylprednisolone 4.0 |
66 | |||||
| no treatment | 35 | - control group | 12 | n/a | n/a | |||||
| Alkawas et al.44 | Randomized Prospective Observer blind |
29 | Peribulbar | 14 | - one injection per week - 20 mg triamcinolone per application (each eye) |
4 | triamcinolone 0.16 |
n/a*A | 17/29 | total: 29 oral group: 27 peribulbar group: 2 |
| oral | 15 | - 60 – 100 mg/day prednisolone | 4 | prednisolone 1.68 – 2.8 |
||||||
| Bordaberry et al.45 | Prospective | 21 | peribulbar | 21 | - one injection per week - 40 mg triamcinolone per application (each eye) |
8 | triamcinolone 0.160 |
95 | 0/21 | n/a |
| Chee and Chee46 | Prospective | 4 | subconjunctival | 4 | - 20 mg triamcinolone per application | * B | * B | n/a | 0/4 | 0 |
| van Geest et al.47 | Randomized Prospective Double blind Placebo controlled |
15 | iv | 6 | - application duration of 60 min - infusion volume 500 mL - three infusions per week (consecutive days) - initial dose 0.5 g methylprednisolone per infusion |
12 | methylprednisolone 6.0 |
83 | 6/15 | 19 |
| placebo | 9 | - application duration of 60 min - infusion volume 500 mL - three infusions per week (consecutive days) |
12 | n/a | 11 | |||||
| Aktaran et al.48 | Randomized Prospective Single blind |
52 | iv | 25 | - application duration 30 min - infusion volume 100 mL - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
72 | 0/52 | 36 |
| oral | 27 | - initial dose of 72 mg/day methylprednisolone for 2 weeks, followed by 64 mg/day for 2 weeks, 56 mg/day for 2 weeks - thereafter decrease of dosage 8 mg/day in 6-wk interval |
12 | methylprednisolone 4.0 |
49 | |||||
| Kahaly et al.49 | Randomized Prospective Single blind |
70 | iv | 35 | - one infusion per week - initial dose 0.5 g methylprednisolone per infusion, 6-wk interval - dose reduction 0.25 g, 6-wk interval |
12 | methylprednisolone 4.5 |
77 | 0/70 | 37 |
| oral | 35 | - initial dose 0.1 g/day prednisolone, 1-wk - dose reduction 0.01 g/week |
12 | prednisolone 4.0 |
51 | |||||
| Ng et al.50 | Randomized Prospective Single blind |
16 | iv | 8 | - application duration approx. 60 min - infusion volume 100 mL - three infusions per week (consecutive days) - initial dose 0.5 methylprednisolone per infusion - followed by oral prednisolone treatment 0.7 mg/kg per day, 4-wk - dose reduction 5 mg/week (until 5mg/day was reached) - followed by 2.5 mg, 1-wk |
1212 | methylprednisolone 4.46prednisolone *B |
87.5 | 1/16 | 50 |
| iv & orbital irradiation |
8 | - steroid application scheme see above - bilateral orbital irradiation,10 fractions |
122 | methylprednisolone 4.24radiation 20 Gy |
28.6 | |||||
| Ebner et al.51 | Randomized Prospective |
50 | peribulbar | 25 | - one injection per week - 20 mg triamcinolone per injection |
4 | triamcinolone 0.08 |
* C | 4/45 | n/a |
| no treatment | 20 | n/a | 4 | n/a | n/a | |||||
| Kauppinen-Mäkelin et al.52 | Randomized Prospective |
33 | iv | 18 | - application duration of 30 min - infusion volume 250 mL - two infusions per week - 0.5 g methylprednisolone per infusion - followed by oral treatment - scheme was repeated once (modified oral treatment scheme) |
14 | methylprednisolone 3.66 |
n/a*A | 0/33 | n/a |
| oral | 15 | - 60 mg/day prednisone, 2-wk - 40 mg/day, 2-wk - 30 mg/day, 4-wk - 20 mg/day,4-wk - 10 mg/day, 2-wk - 5 mg/day, 1-wk - 5 mg every 2nd day, 1-wk |
16 | prednisone 2.99 |
n/a*A | |||||
| Marcocci et al.53 | Randomized Prospective Single blind |
82 | iv | 41 | - application duration of 60-90 min - infusion volume 500 mL - two infusions in two week - initial dose of 15 mg/kg methylprednisolone per Infusion, 4 times - dose reduction 7.5 mg/kg, 4 times - bilateral orbital irradiation, 10 fractions |
142 | methylprednisolone 9.0 – 12.0radiation 20 Gy |
83 | 0/82 | 88 |
| oral | 41 | - initial dose of 100 mg/day prednisone, 1-wk - weekly dose reduction until 0.25 mg - followed by reduction of 5 mg/day, 2-wk - bilateral orbital irradiation, 10 fractions |
222 | prednisone 6.0radiation 20 Gy |
63 | |||||
| Macchia et al.54 | Randomized Prospective |
51 | iv | 25 | - application duration approx.120 min - infusion volume 250-500 mL - two infusions per week (constitutive days) - initial dose 1.0 g methylprednisolone per infusion |
6 | methylprednisolone 12.0 | 84 | 4/51 | 72 |
| oral | 26 | - initial dose 60-80 mg/day prednisone - gradual reduction up to withdrawal |
16 – 24 | prednisone *B |
57 |
*A = Improvement of CAS ⩾ 2
*B = Patient individual duration or dose
*C = Improvement of visual symptoms and decrease of diplopia
*D = Response rate as defined by authors
*E = Total response rate for all treatment arms
GC = Glucocorticoids, n/a = not available, SE = Side effects
Toxicity
The first randomized controlled study with GC in GO following the ICH Guidelines is described extensively in Table 3.36,85 Adverse events (AE) were analyzed according to the highly acknowledged and well-structured MedDRA. Of the 164 patients included, 79 (48%) reported at least one AE; 68 AE were classified as drug related and analyzed according to MedDRA system organ classes (SOC). AE occurred mainly as gastrointestinal disorders (15/68; 22%), infections and infestations (10/68: 15%), vascular disorders (7/68; 10%), and general disorders and administration site reactions (6/68; 9%). The overall-rate of GC-induced AE affected 60–70% of the participating patients. Most AEs occurred within the first few days of the therapy. Frequency and severity of SE (i.e., weight gain, insomnia, hypertension, hyperglycemia, and hepatotoxicity) depend on dosage, duration of treatment, route of administration (oral, IV, peribulbar), and previous morbidity of the patients receiving GC.61,74,92 The ETA conducted a survey aiming to compare AE induced by oral versus IVGC therapy.93 Severity and frequency of AE and severe AE (SAE) differed between both dosage forms. AE and SAE are less likely to occur during IVGC therapy. Morbidity and mortality were 6.5% and 0.6% for IVGC, respectively while morbidity was similar with 6.25% for the oral application. Mortality was not reported. Of note, a few cases of IVGC-induced lethal hepatoxicity were reported 20 years ago and were, in most cases, dose-dependent. The first case of fatal liver damage under IVGC therapy was reported in 2000.94 A 71-year-old woman died after receiving a cumulative dose of 15.0 g IV methylprednisolone. Other authors also reported cases of acute liver damage at doses much higher than what is now recommended.39,95 Three patients died within a short timespan (two because of liver failure and one after liver transplantation because of kidney failure). Liver damage can arise from different mechanisms: GC may have direct damage potential for liver cells, which substantiates the dose-dependent increase in liver enzymes reported in several cases.30,32,34,93–95 Due to the immunosuppressive effect of GC, a previous infection of hepatitis B and/or hepatitis C could be reactivated. Moreover, the immunosuppressive effect of GC could precipitate an autoimmune hepatitis.39,95 Cardiovascular complications (pulmonary edema, pulmonary embolism, myocardial infarction and coronary thrombosis, occlusion of the cerebral-medial artery) have been also reported following IVGC.41,93,96–98 Under physiological conditions, GC cause sodium retention due to activation of the renin-angiotensin-aldosterone-system (RAAS). This increases blood pressure, body volume, and restlessness. With one exception, all severe cardiovascular and hepatic SE occurred after high cumulative doses (>8–10 g of GC) or daily (not weekly) administrations of IVGC. Therefore, the European Guidelines for the management of GO recommend weekly IV applications only, and a maximum cumulative dose of 8 g only per treatment cycle.28 Even for the worst case of sight-threatening GO and optic neuropathy, alternate (every second day) and not successive daily very high doses (750–1000 mg) are recommended. Furthermore, prior to starting an IVGC therapy, screening of liver function, blood sugar profile, blood pressure, and cardiovascular status are mandatory.74 Therefore, when following the guideline recommendations for careful laboratory screening and thorough tailored dosage, IVGC are, in the vast majority of managed patients with clinically active and severe GO, a well-tolerated and effective therapy.
Table 3.
| MedDRA SOC | Number of patients with AR | Number of patients with AR in the methylprednisolone-group | Number of patients with AR in the methylprednisolone-mycophenolate-group |
|---|---|---|---|
| cardiac disorders | 1 | 0 | 1 |
| - palpitations | 0 | 1 | |
| ear and labyrinth disorders | 2 | 1 | 1 |
| - vertigo | 1 | 1 | |
| gastrointestinal disorders | 15 | 5 | 10 |
| - abdominal discomfort | 2 | 5 | |
| - nausea | 1 | 2 | |
| - dyspepsia | 1 | 2 | |
| - gastritis | 1 | 0 | |
| - diarrhea | 0 | 1 | |
| general disorder & administration site reactions | 6 | 2 | 4 |
| - fatigue | 2 | 2 | |
| - feeling cold/hot | 0 | 2 | |
| infections & infestations | 10 | 5 | 5 |
| - cystitis | 2 | 2 | |
| - oral fungal infections | 2 | 0 | |
| - herpes simplex | 0 | 1 | |
| - herpes zoster | 0 | 1 | |
| - sinusitis | 0 | 1 | |
| - bronchitis | 1 | 0 | |
| injury, poisoning & procedural complications | 1 | 0 | 1 |
| - scratch | 0 | 1 | |
| investigations | 4 | 1 | 3 |
| - increase in serum liver enzyme concentrations | 1 | 2 | |
| - weight increase | 0 | 1 | |
| metabolism & nutrition disorders | 4 | 2 | 2 |
| - hyperglycemia | 2 | 1 | |
| - decreased appetite | 0 | 1 | |
| musculoskeletal & connective tissue disorders | 2 | 0 | 2 |
| - myalgia | 0 | 2 | |
| nervous system disorders | 4 | 2 | 2 |
| - headache | 2 | 1 | |
| - dizziness | 0 | 1 | |
| psychiatric disorders | 7 | 3 | 4 |
| - sleeping disorders | 2 | 3 | |
| - depressive mood | 1 | 1 | |
| reproductive system & breast disorders | 1 | 1 | 0 |
| - metrorrhagia | 1 | 0 | |
| skin & subcutaneous tissue disorders | 4 | 2 | 2 |
| 1 | 0 | 0 | 1 |
| 0 | 1 | 2 | 1 |
| 1 | 1 | 2 | 0 |
AR, Adverse reactions; MedDRA, Medical Dictionary of Regulatory Activities; SE, side effects; SOC, system organ classes.
Novel treatments and perspectives
Several novel strategies targeting the key antigens in Graves’ hyperthyroidism and associated GO, for example, TSH-R and IgF-1R, have been developed.99 Monoclonal antibodies and/or small molecules targeting the TSH-R are being tested in phase I trials, while phase II and III trials have been performed showing impressive results of a novel anti-IGF-1R monoclonal antibody in GO.100,101 Also, monoclonal antibodies targeting pro-inflammatory cytokines, i.e., IL6-R,102 or the CD40 molecule within the immunological synapse,103 are interesting alternatives. However, these potentially more specific drugs have to be compared first in daily use with the standard IVGC treatment with respect to short- and long-term efficiency, safety, costs, and global availability.
Conclusion
GC treatment and IVGC in particular offer numerous advantages. Indeed, long-term experience with several administration forms, thousands of patients treated worldwide over the years, and numerous clinical randomized controlled trials and meta-analyses demonstrate the beneficial effect of GC in general and IVGC in particular.
In detail, GC treatment of subjects with active-severe thyroid eye disease or GO has both a proven anti-inflammatory benefit and inactivated orbital disease in at least two-thirds of cases, as well as having beneficial impacts on diplopia and proptosis. Present official guidelines clearly define indications and maximal single and cumulative doses of IVGC to avoid AE and SE, which can be managed in most cases. Hence, the high efficiency and low risks of AE are convincing arguments in favor of GC, foremost IVGC therapy. In addition, global availability at low to very low costs, and oral tablets with various dosages facilitating dose adaptation are further major advantages of this standard, internationally acknowledged and successful treatment (Table 4).
Table 4.
Advantages and disadvantages of GC treatment in GO.
| Advantages | Disadvantages |
|---|---|
| • experience over more than 70 years • global availability • low to very low drug costs • various application forms • oral application in various dosages • rapid onset of anti-inflammatory effect • rapid improvement of clinical symptoms • strong effects on inflammatory components of GO • beneficial effect on tissue swelling, visual acuity and ocular motility • positively impacts quality of life |
• only symptomatic and not causal treatment of GO • neither targets TSHR nor IGF1R • low-moderate effect on proptosis • low-moderate effect on diplopia |
GC, glucocorticoids; GO, Graves’ orbitopathy; IGF1R, insulin growth factor 1 receptor; TSHR, thyrotropin receptor.
Furthermore, both the response rate to immunosuppressive treatment and the beneficial effect of GC as well as the associated health-related quality of life can be increased significantly when combining IVGC with the well-tolerated and efficacious drug mycophenolate,36,86 with oral GC to cyclosporine,104 IV immunoglobulins,105 or orbital irradiation.53,106
Acknowledgments
The editorial assistance of M. Schmittbetz and T. Diana, Johannes Gutenberg University Thyroid Laboratory, is acknowledged.
Footnotes
Authors contribution(s): Jan Längericht: Formal analysis; Investigation; Methodology; Visualization; Writing-original draft.
Irene Krämer: Conceptualization; Writing-review & editing.
George Kahaly: Conceptualization; Formal analysis; Methodology; Project administration; Supervision; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: George J. Kahaly  https://orcid.org/0000-0003-0441-430X
https://orcid.org/0000-0003-0441-430X
Contributor Information
Jan Längericht, Department of Medicine I., Johannes Gutenberg University (JGU) Medical Center, Mainz, Rheinland-Pfalz, Germany.
Irene Krämer, Department of Pharmacy, Johannes Gutenberg University (JGU) Medical Center, Mainz, Rheinland-Pfalz, Germany.
George J. Kahaly, Department of Medicine I., Johannes Gutenberg University (JGU) Medical Center, Langenbeckstraße 1, Mainz, 55131, Germany.
References
- 1. Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 2014; 37: 691–700. [DOI] [PubMed] [Google Scholar]
- 2. Ponto KA, Binder H, Diana T, et al. Prevalence, phenotype, and psychosocial well-being in euthyroid/hypothyroid thyroid-associated orbitopathy. Thyroid 2015; 25: 942–948. [DOI] [PubMed] [Google Scholar]
- 3. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc 1994; 92: 477–588. [PMC free article] [PubMed] [Google Scholar]
- 4. Wiersinga WM, Prummel MF. Pathogenesis of Graves’ ophthalmopathy–current understanding. J Clin Endocrinol Metab 2001; 86: 501–503. [DOI] [PubMed] [Google Scholar]
- 5. Dickinson AJ, Hintschich C. Clinical manifestations. In: Wiersinga WM, Kahaly GJ. (eds) Graves’ orbitopathy: a multidisciplinary approach - questions and answers. Basel: Karger, 2017, pp.1–25. [Google Scholar]
- 6. Ponto KA, Osten-Sacken SVD, Elflein H, et al. [Healthcare relevant data from an interdisciplinary consultation for endocrine orbitopathy]. Ophthalmologe. Epub ahead of print 7 February 2020. DOI: 10.1007/s00347-020-01050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hai YP, Lee ACH, Frommer L, et al. Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J Endocrinol Invest 2020; 43: 123–137. [DOI] [PubMed] [Google Scholar]
- 8. Salvi M. Pathogenesis of Graves’ orbitopathy. In: Wiersinga WM, Kahaly GJ. (eds) Graves’ orbitopathy: a multidisciplinary approach - questions and answers. Basel: Karger, 2017, pp.41–60. [Google Scholar]
- 9. Ponto KA, Diana T, Binder H, et al. Thyroid-Stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J Endocrinol Invest 2015; 38: 769–777. [DOI] [PubMed] [Google Scholar]
- 10. Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease-methodology and clinical applications. Ophthalmic Plast Reconstr Surg 2018; 34(4S Suppl. 1): S13–S19. [DOI] [PubMed] [Google Scholar]
- 11. Lytton SD, Ponto KA, Kanitz M, et al. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab 2010; 95: 2123–2131. [DOI] [PubMed] [Google Scholar]
- 12. Ponto KA, Kanitz M, Olivo PD, et al. Clinical relevance of thyroid–stimulating immunoglobulins in Graves’ ophthalmopathy. Ophthalmology 2011; 118: 2279–2285. [DOI] [PubMed] [Google Scholar]
- 13. Diana T, Brown RS, Bossowski A, et al. Clinical relevance of thyroid-stimulating autoantibodies in pediatric Graves’ disease-a multicenter study. J Clin Endocrinol Metab 2014; 99: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 14. Kampmann E, Diana T, Kanitz M, et al. Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: a prospective study. Int J Endocrinol 2015; 2015: 678194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahaly GJ, Diana T, Olivo PD. TSH receptor antibodies: relevance & utility. Endocr Pract 2020; 26: 97–106. [DOI] [PubMed] [Google Scholar]
- 16. Kahaly GJ, Wuster C, Olivo PD, et al. High titers of thyrotropin receptor antibodies are associated with orbitopathy in patients with Graves disease. J Clin Endocrinol Metab 2019; 104: 2561–2568. [DOI] [PubMed] [Google Scholar]
- 17. Kahaly GJ, Diana T, Glang J, et al. Thyroid stimulating antibodies are highly prevalent in hashimoto’s thyroiditis and associated orbitopathy. J Clin Endocrinol Metab 2016; 101: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 18. Kahaly GJ, Diana T, Kanitz M, et al. Prospective trial of functional thyrotropin receptor antibodies in Graves disease. J Clin Endocrinol Metab 2020; 105: e1006–e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol 2016; 100: 142–150. [DOI] [PubMed] [Google Scholar]
- 20. Krieger CC, Boutin A, Jang D, et al. Arrestin-β-1 physically scaffolds TSH and IGF1 receptors to enable crosstalk. Endocrinology 2019; 160: 1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krieger CC, Perry JD, Morgan SJ, et al. TSH/IGF-1 receptor cross-talk rapidly activates extracellular signal-regulated kinases in multiple cell types. Endocrinology 2017; 158: 3676–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krieger CC, Place RF, Bevilacqua C, et al. TSH/IGF-1 receptor cross talk in Graves’ ophthalmopathy pathogenesis. J Clin Endocrinol Metab 2016; 101: 2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcus-Samuels B, Krieger CC, Boutin A, et al. Evidence that Graves’ ophthalmopathy immunoglobulins do not directly activate IGF-1 receptors. Thyroid 2018; 28: 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finsterer J, Frank M. [Glucocorticoids in neurology: mechanism of action, applications and side effects]. Fortschr Neurol Psychiatr 2014; 82: 311–322. [DOI] [PubMed] [Google Scholar]
- 25. Jaffuel D, Mathieu M, Godard P, et al. [Mechanism of action of glucocorticoids in asthma]. Rev Mal Respir 1999; 16: 431–442. [PubMed] [Google Scholar]
- 26. Vandewalle J, Luypaert A, De Bosscher K, et al. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab 2018; 29: 42–54. [DOI] [PubMed] [Google Scholar]
- 27. Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 2008; 158: 273–285. [DOI] [PubMed] [Google Scholar]
- 28. Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 2016; 5: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He Y, Mu K, Liu R, et al. Comparison of two different regimens of intravenous methylprednisolone for patients with moderate to severe and active Graves’ ophthalmopathy: a prospective, randomized controlled trial. Endocr J 2017; 64: 141–149. [DOI] [PubMed] [Google Scholar]
- 30. Ueda-Sakane Y, Kanamoto N, Fushimi Y, et al. Overall safety and efficacy of high-dose and low-dose intravenous glucocorticoid therapy in patients with moderate-to-severe active Graves’ ophthalmopathy. Endocr J 2016; 63: 703–714. [DOI] [PubMed] [Google Scholar]
- 31. Zhu W, Ye L, Shen L, et al. A prospective, randomized trial of intravenous glucocorticoids therapy with different protocols for patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 2014; 99: 1999–2007. [DOI] [PubMed] [Google Scholar]
- 32. Beleslin BN, Ciric J, Zarkovic M, et al. Efficacy and safety of combined parenteral and oral steroid therapy in Graves’ orbitopathy. Hormones (Athens) 2014; 13: 222–228. [DOI] [PubMed] [Google Scholar]
- 33. Philip R, Saran S, Gutch M, et al. Pulse dexamethasone therapy versus pulse methylprednisolone therapy for treatment of Graves’s ophthalmopathy. Indian J Endocrinol Metab 2013; 17(Suppl. 1): S157–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wichary H, Gasińska T. Methylprednisolone and hepatotoxicity in Graves’ ophthalmopathy. Thyroid 2012; 22: 64–69. [DOI] [PubMed] [Google Scholar]
- 35. Bagheri A, Abbaszadeh M, Yazdani S. Intraorbital steroid injection for active thyroid ophthalmopathy. J Ophthalmic Vis Res 2020; 15: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kahaly GJ, Riedl M, König J, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 2018; 6: 287–298. [DOI] [PubMed] [Google Scholar]
- 37. Hamed-Azzam S, Mukari A, Feldman I, et al. Fornix triamcinolone injection for thyroid orbitopathy. Graefes Arch Clin Exp Ophthalmol 2015; 253: 811–816. [DOI] [PubMed] [Google Scholar]
- 38. Roy A, Dutta D, Ghosh S, et al. Efficacy and safety of low dose oral prednisolone as compared to pulse intravenous methylprednisolone in managing moderate severe Graves’ orbitopathy: a randomized controlled trial. Indian J Endocrinol Metab 2015; 19: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sisti E, Coco B, Menconi F, et al. Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endocrinol 2015; 172: 269–276. [DOI] [PubMed] [Google Scholar]
- 40. Lee SJ, Rim THT, Jang SY, et al. Treatment of upper eyelid retraction related to thyroid–associated ophthalmopathy using subconjunctival triamcinolone injections. Graefes Arch Clin Exp Ophthalmol 2013; 251: 261–270. [DOI] [PubMed] [Google Scholar]
- 41. Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 2012; 97: 4454–4463. [DOI] [PubMed] [Google Scholar]
- 42. Xu D, Liu Y, Xu H, et al. Repeated triamcinolone acetonide injection in the treatment of upper-lid retraction in patients with thyroid-associated ophthalmopathy. Can J Ophthalmol 2012; 47: 34–41. [DOI] [PubMed] [Google Scholar]
- 43. Akarsu E, Buyukhatipoglu H, Aktaran S, et al. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2011; 74: 118–124. [DOI] [PubMed] [Google Scholar]
- 44. Alkawas AA, Hussein AM, Shahien EA. Orbital steroid injection versus oral steroid therapy in management of thyroid-related ophthalmopathy. Clin Exp Ophthalmol 2010; 38: 692–697. [DOI] [PubMed] [Google Scholar]
- 45. Bordaberry M, Marques DL, Pereira-Lima JC, et al. Repeated peribulbar injections of triamcinolone acetonide: a successful and safe treatment for moderate to severe Graves’ ophthalmopathy. Acta Ophthalmol 2009; 87: 58–64. [DOI] [PubMed] [Google Scholar]
- 46. Chee E, Chee S-P. Subconjunctival injection of triamcinolone in the treatment of lid retraction of patients with thyroid eye disease: a case series. Eye (Lond) 2008; 22: 311–315. [DOI] [PubMed] [Google Scholar]
- 47. van Geest RJ, Sasim IV, Koppeschaar HPF, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol 2008; 158: 229–237. [DOI] [PubMed] [Google Scholar]
- 48. Aktaran S, Akarsu E, Erbağci I, et al. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract 2007; 61: 45–51. [DOI] [PubMed] [Google Scholar]
- 49. Kahaly GJ, Pitz S, Hommel G, et al. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 2005; 90: 5234–5240. [DOI] [PubMed] [Google Scholar]
- 50. Ng CM, Yuen HKL, Choi KL, et al. Combined orbital irradiation and systemic steroids compared with systemic steroids alone in the management of moderate-to-severe Graves’ ophthalmopathy: a preliminary study. Hong Kong Med J 2005; 11: 322–330. [PubMed] [Google Scholar]
- 51. Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol 2004; 88: 1380–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kauppinen-Mäkelin R, Karma A, Leinonen E, et al. High dose intravenous methylprednisolone pulse therapy versus oral prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand 2002; 80: 316–321. [DOI] [PubMed] [Google Scholar]
- 53. Marcocci C, Bartalena L, Tanda ML, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 2001; 86: 3562–3567. [DOI] [PubMed] [Google Scholar]
- 54. Macchia PE, Bagattini M, Lupoli G, et al. High–Dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 2001; 24: 152–158. [DOI] [PubMed] [Google Scholar]
- 55. Hayashi R, Wada H, Ito K, et al. Effects of glucocorticoids on gene transcription. Eur J Pharmacol 2004; 500: 51–62. [DOI] [PubMed] [Google Scholar]
- 56. Guo H, Callaway JB, Ting JP-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015; 21: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yeung YT, Aziz F, Guerrero-Castilla A, et al. Signaling pathways in inflammation and anti-inflammatory therapies. Curr Pharm Des 2018; 24: 1449–1484. [DOI] [PubMed] [Google Scholar]
- 58. Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev 2004; 125: 697–706. [DOI] [PubMed] [Google Scholar]
- 59. Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J 2006; 27: 413–426. [DOI] [PubMed] [Google Scholar]
- 60. Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 2010; 120: 76–85. [DOI] [PubMed] [Google Scholar]
- 61. Zang S, Kahaly GJ. Steroids and the immune response in Graves orbitopathy. Immunol Endocr Metab Agents Med Chem 2011; 11: 90–98. [Google Scholar]
- 62. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 2005; 353: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 63. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol 2011; 163: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell 1998; 93: 487–490. [DOI] [PubMed] [Google Scholar]
- 65. Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids 2002; 67: 529–534. [DOI] [PubMed] [Google Scholar]
- 66. Goulding NJ, Guyre PM. Glucocorticoids, lipocortins and the immune response. Curr Opin Immunol 1993; 5: 108–113. [DOI] [PubMed] [Google Scholar]
- 67. Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol 2000; 130: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marx J. How the glucocorticoids suppress immunity. Science 1995; 270: 232–233. [DOI] [PubMed] [Google Scholar]
- 69. Auphan N, DiDonato JA, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995; 270: 286–290. [DOI] [PubMed] [Google Scholar]
- 70. Vachier I, Chavis C, Majori M, et al. Effects of glucocorticoids on endogenous and transcellular metabolism of eicosanoids in asthma. J Allergy Clin Immunol 2001; 107: 824–831. [DOI] [PubMed] [Google Scholar]
- 71. Emilie D, Etienne S. [Glucocorticoids: mode of action and pharmacokinetics]. Rev Prat 1990; 40: 511–517. [PubMed] [Google Scholar]
- 72. Bailey JM. New mechanisms for effects of anti-inflammatory glucocorticoids. Biofactors 1991; 3: 97–102. [PubMed] [Google Scholar]
- 73. Goppelt-Struebe M. Molecular mechanisms involved in the regulation of prostaglandin biosynthesis by glucocorticoids. Biochem Pharmacol 1997; 53: 1389–1395. [DOI] [PubMed] [Google Scholar]
- 74. Zang S, Ponto KA, Kahaly GJ. Clinical review: intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 2011; 96: 320–332. [DOI] [PubMed] [Google Scholar]
- 75. Lasa M, Abraham SM, Boucheron C, et al. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 2002; 22: 7802–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Goulding NJ, Guyre PM. Lipocortin 1 binding to human leukocytes correlates with its ability to inhibit IgG interactions with Fc gamma receptors. Biochem Biophys Res Commun 1993; 192: 351–358. [DOI] [PubMed] [Google Scholar]
- 77. Suda T, Chida K, Matsuda H, et al. High-Dose intravenous glucocorticoid therapy abrogates circulating dendritic cells. J Allergy Clin Immunol 2003; 112: 1237–1239. [DOI] [PubMed] [Google Scholar]
- 78. Murphy KM, Weaver C. Janeway - Immunologie. Springer Spektrum, 2018. [Google Scholar]
- 79. Forth W, Henschler D, Rummel W, et al. Allgemeine und spezielle Pharmakologie und Toxikologie. Elsevier, 2017. [Google Scholar]
- 80. Pitzalis C, Sharrack B, Gray IA, et al. Comparison of the effects of oral versus intravenous methylprednisolone regimens on peripheral blood T lymphocyte adhesion molecule expression, T cell subsets distribution and TNF alpha concentrations in multiple sclerosis. J Neuroimmunol 1997; 74: 62–68. [DOI] [PubMed] [Google Scholar]
- 81. Giles AJ, Hutchinson M-KND, Sonnemann HM, et al. Dexamethasone-Induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 2018; 6: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marchetti MC, Di Marco B, Cifone G, et al. Dexamethasone-Induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood 2003; 101: 585–593. [DOI] [PubMed] [Google Scholar]
- 83. Pitzalis C, Pipitone N, Bajocchi G, et al. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: an additional mechanism for their anti-inflammatory and immunosuppressive effect. J Immunol 1997; 158: 5007–5016. [PubMed] [Google Scholar]
- 84. Ponto KA, Pitz S, Mann WJ, et al. [Management of Graves’ orbitopathy: evidence-based recommendations]. Dtsch Med Wochenschr 2009; 134: 2521–2524. [DOI] [PubMed] [Google Scholar]
- 85. Riedl M, Kolbe E, Kampmann E, et al. Prospectively recorded and MedDRA-Coded safety data of intravenous methylprednisolone therapy in Graves’ orbitopathy. J Endocrinol Invest 2015; 38: 177–182. [DOI] [PubMed] [Google Scholar]
- 86. Lee ACH, Riedl M, Frommer L, et al. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Invest 2020; 43: 767–777. [DOI] [PubMed] [Google Scholar]
- 87. Kahaly GJ, Bartalena L, Hegedüs L, et al. 2018 European thyroid association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J 2018; 7: 167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jespersen S, Nygaard B, Kristensen LØ. Methylprednisolone pulse treatment of Graves’ ophthalmopathy is not associated with secondary adrenocortical insufficiency. Eur Thyroid J 2015; 4: 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Poonyathalang A, Preechawat P, Charoenkul W, et al. Retrobulbar injection of triamcinolone in thyroid associated orbitopathy. J Med Assoc Thai 2005; 88: 345–349. [PubMed] [Google Scholar]
- 90. Miśkiewicz P, Kryczka A, Ambroziak U, et al. Is high dose intravenous methylprednisolone pulse therapy in patients with Graves’ orbitopathy safe? Endokrynol Pol 2014; 65: 402–413. [DOI] [PubMed] [Google Scholar]
- 91. GmbH MMI. Gelbe Liste Pharmaindex. 2019. [Google Scholar]
- 92. Zang S, Ponto KA, Pitz S, et al. Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 2011; 34: 876–880. [DOI] [PubMed] [Google Scholar]
- 93. Marcocci C, Watt T, Altea MA, et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol 2012; 166: 247–253. [DOI] [PubMed] [Google Scholar]
- 94. Weissel M, Hauff W. Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 2000; 10: 521. [DOI] [PubMed] [Google Scholar]
- 95. Marinó M, Morabito E, Brunetto MR, et al. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 2004; 14: 403–406. [DOI] [PubMed] [Google Scholar]
- 96. Gursoy A, Cesur M, Erdogan MF, et al. New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with Graves’ ophthalmopathy. Endocrine 2006; 29: 513–516. [DOI] [PubMed] [Google Scholar]
- 97. Owecki M, Sowiński J. Acute myocardial infarction during high-dose methylprednisolone therapy for Graves’ ophthalmopathy. Pharm World Sci 2006; 28: 73–75. [DOI] [PubMed] [Google Scholar]
- 98. Lendorf ME, Rasmussen AK, Fledelius HC, et al. Cardiovascular and cerebrovascular events in temporal relationship to intravenous glucocorticoid pulse therapy in patients with severe endocrine ophthalmopathy. Thyroid 2009; 19: 1431–1432. [DOI] [PubMed] [Google Scholar]
- 99. Kahaly GJ. Immunotherapies for thyroid eye disease. Curr Opin Endocrinol Diabetes Obes 2019; 26: 250–255. [DOI] [PubMed] [Google Scholar]
- 100. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020; 382: 341–352. [DOI] [PubMed] [Google Scholar]
- 101. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017; 376: 1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 2018; 195: 181–190. [DOI] [PubMed] [Google Scholar]
- 103. Kahaly GJ, Stan MN, Frommer L, et al. A novel Anti-CD40 monoclonal antibody, Iscalimab, for control of graves hyperthyroidism-a proof-of-concept trial. J Clin Endocrinol Metab 2020; 105: dgz013. [DOI] [PubMed] [Google Scholar]
- 104. Kahaly G, Schrezenmeir J, Krause U, et al. Ciclosporin and prednisone v. prednisone in treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Invest 1986; 16: 415–422. [DOI] [PubMed] [Google Scholar]
- 105. Kahaly G, Pitz S, Müller-Forell W, et al. Randomized trial of intravenous immunoglobulins versus prednisolone in Graves’ ophthalmopathy. Clin Exp Immunol 1996; 106: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bartalena L. Protocols of non-surgical therapies. In: Wiersinga WM, Kahaly GJ. (eds) Graves’ orbitopathy: a multidisciplinary approach - questions and answers. Basel: Karger, 2017, pp.330–337. [Google Scholar]