Abstract
Background:
Coronavirus disease-19 (COVID-19) is associated with acute kidney injury (AKI) and acute respiratory distress syndrome (ARDS) with high mortality rates. In African American (AA) populations, COVID-19 presentations and outcomes are more severe. NIH and Interim WHO guidelines had suggested against the use of corticosteroids unless in clinical trials until the recent publication of the RECOVERY trial. Here, we analyzed the treatment effect of methylprednisolone on patients with AKI and ARDS during the initial 2 months of COVID-19 and detail the learning effect within our institution.
Methods:
Between March 1 and April 30, 2020, 75 AA patients met our inclusion criteria for ARDS and AKI, of which 37 had received corticosteroids. Twenty-eight-day mortality, improvement in PaO2/FiO2 ratio, and renal function were analyzed. The impact of methylprednisolone treatment was assessed with multivariable methods.
Results:
Survival in the methylprednisolone group reached 51% at 21 days compared to 29% in the non-corticosteroid group (P < .001). Methylprednisolone improved the likelihood of renal function improvement. PaO2/FiO2 ratio in the methylprednisolone group improved by 73% compared to 45% in the non-corticosteroid group (P = .01). Age, gender, BMI, preexisting conditions, and other treatment factors did not show any impact on renal or PaO2/FiO2 ratio improvement. The use of anticoagulants, the month of treatment, and AKI during hospitalization also influenced outcomes.
Conclusion:
In AA COVID-19 positive patients with ARDS and AKI, IV methylprednisolone lowered the incidence of mortality and improved the likelihood of renal and lung function recovery. Further investigation with a randomized control trial of corticosteroids is warranted.
Keywords: COVID-19, methylprednisolone, acute kidney injury, acute respiratory distress syndrome
Introduction
Coronavirus disease-19 (COVID-19) continues to carry high mortality with a case burden of 25 million cases worldwide and 6 million cases in the USA as of September 2nd, 2020.1 Infection with SARS-CoV-2 can range from asymptomatic carriers to mild respiratory distress to acute respiratory distress syndrome (ARDS) (2). Acute kidney injury (AKI) is often seen in association with ARDS2,3 and its presence in ARDS results in 20% mortality.4 In some cases, kidney involvement can be the sole presentation of COVID-19 with electrolyte abnormalities (both hypo and hypernatremia, extreme hyperkalemia),5-7 which can cause fatal cardiac arrhythmias and sudden death. The preferential activation of lymphocytes and macrophages within the interstitium of the kidney, disseminated intravascular coagulation, and micro thrombosis are observed in autopsy studies, similar to thrombotic microangiopathy with some features of resident lymphocyte and macrophage immune cells activation as seen in allergic interstitial nephritis (AIN).8,9 The recent RECOVERY trial also demonstrated that dexamethasone resulted in lower 28-day mortality in COVID-19 positive patients receiving invasive mechanical ventilation or oxygen.10 Equally, the CoDEX Randomized Clinical Trial demonstrated that intravenous dexamethasone reduced the number of required ventilator days for COVID-19 patients with moderate to severe ARDS.11 Equally, a recent prospective meta-analysis of critically ill patients with COVID-19 treated with systemic corticosteroids from studies throughout the world demonstrates a lower 28-day all-cause mortality.12 The effectiveness of corticosteroids is lost once the RECOVERY trial data is removed from the prospective meta-analysis.12 The CAPE COVID-19 study demonstrated no benefit of corticosteroids.13 Equally, a recent study in Brazil showed no mortality benefit of corticosteroid treatment over 28 days.14 Therefore, treating inflammation associated symptoms caused by SARS-CoV-2 infection is controversial and requires further investigation.
However, others report that the use of corticosteroids to treat SARS-CoV-2 infection may not be beneficial.15 The administration of corticosteroids had no benefit with Middle East respiratory syndrome coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS) and potentially delayed MERS coronavirus RNA clearance.16 Hence, the NIH and Interim WHO applied similar guidelines to COVID-19 treatment and suggested restricted use of corticosteroids.17-19 However, corticosteroids continue to be used under expert recommendations for patients with ARDS based on the Meduri protocol, which showed a survival benefit in ARDS.20 Clinical observations from several local institutions across New York City at the beginning of the pandemic suggested steroids could be beneficial and protective to patients with ARDS and AKI. The dose and duration of corticosteroids use in AIN21 AKI along with ARDS is unexplored.
Of the COVID-19 associated deaths within the USA, 27% were African American (AA) patients while AA accounts for only 12.5% of the population in the USA.22 Similar disparities are reflected in the number of hospitalizations among AA compared to disease prevalence in the community. These disparities might be partially due to the biological differences in inflammation between races. AA and Hispanic children at baseline seem to have elevated C reactive protein compared to Caucasians.23 Our hospital, was a COVID-19 designated hospital for NY State and served a predominantly AA and Afro Caribbean population. Considering the cytokine storm, higher baseline inflammation markers in AAs, previous evidence of the benefit of corticosteroids to treat ARDS, and possibly AIN20,24 our institution initiated a protocol for use of corticosteroids (methylprednisolone) during the height of the COVID-19 pandemic. Not all providers agreed to this protocol. Those that did not get methylprednisolone during the same time-period served as our comparator group. Therefore, the goal of this study was to examine the effectiveness of methylprednisolone administration in COVID-19 AA subjects. We utilized 2 doses of methylprednisolone, at the discretion of the treating physician, as we were desperately trying to treat our patient population during this pandemic and at this time no dosing strategy was established for COVID-19. Here, we retrospectively examined the association between methylprednisolone use, respiratory failure, and acute kidney injury at a large academic medical center providing care to a marginalized group of COVID-19 patients in New York City.
Methods
Study population
We conducted a retrospective observational study on all patients of AA/Afro Caribbean race between March 1 and April 30, 2020, who fulfilled the inclusion criteria. The study was granted an exemption under rapid IRB approval by our Institutional Review Board and adherence to the Declaration of Helsinki. All patients included in the study had a confirmed positive Nasopharyngeal or Oropharyngeal PCR test for COVID-19, had AKI as defined by Kidney Disease Improving Global Outcomes (KDIGO) criteria either on admission or developed AKI during the study period of hospitalization, and had similarly varying degrees of respiratory failure from requiring high flow oxygen to mechanical ventilation. Based on the onset of symptoms, an arbitrary cut off time point was decided upon to group patients either as early presenters of AKI and ARDS (within 6 days of initial symptoms) or late presenters of AKI and ARDS (after 6 days of initial symptoms). As the administration and dose of steroids were left up to the discretion of the primary care provider, patients received either no methylprednisolone or received 2 different doses of methylprednisolone (1 mg/kg/day or low dose for the early presenters and 2 mg/kg/day or high dose for the late presenters). The dosage and duration were intended to follow the “Meduri protocol.”20 To be included in our analysis, patients had to receive steroids for a minimum of 3 days or longer. 37 patients each who had AKI with varying degrees of respiratory failure received methylprednisolone at 2 different doses and 38 patients each who had similar degrees of AKI and respiratory failure did not receive any steroids.
Outcomes
The primary outcome was patient survival during the first 21 days after admission. Secondary outcomes were improvement in lung function and kidney function. Improvement in lung function was defined as either successful extubation or improvement of PaO2/FiO2 ratio of more than 200, PEEP of less than 10, and FiO2 of less than 60% at any time point up to day 28 in intubated patients or up to the time point of discharge for non-ventilated patients. Similarly, improvement of renal function was defined as a 50% increase in glomerular filtration rate (GFR) up to day 28 or freedom from dialysis at any time point up to day 28 and discharge.
Data collection and statistical analysis
In this exploratory analysis, characteristics, risk factors, and information about the progression of the COVID-19 disease, AKI and ARDS were collected for all patients. Comparative descriptions of patient’s characteristics were quantified and reported as means with standard deviation or medians with ranges and for qualitative factors reported as frequencies and percentages. Comparisons between groups were performed using the Wilcoxon or Kruskal-Wallis test or Chi-Square test. All factors were tested for possible associations. Patient survival was calculated from the date of admission to the date of death or date of discharge. Survival time was truncated at 28 days after admission and censored. Patient survival was assessed using the Kaplan-Meier methods.
To assess the overall impact of methylprednisolone on outcomes, different multivariable models were developed for the administration of methylprednisolone compared to no methylprednisolone treatment adjusted for possible risk factors. In addition, secondary analyses were performed in which the methylprednisolone group was divided into the low and high dosage of methylprednisolone. Cox-regression models were used for determining risk factors for patient survival. To measure the impact of the potential start of dialysis and/or mechanical ventilation, those factors were included as time-dependent variables into the model. Due to the differences in start dates for steroids (<6 days vs >6 days from symptom onset) and due to different doses used at those start dates (low dose for <6 days post symptom onset and >6 days post symptom onset for high dose methylprednisolone), the overall analysis of treatment effect was performed for the combined versus no methylprednisolone treatment first and then further analyzed whether there was an impact of time of the start of steroids or an impact from the 2 different doses of methylprednisolone separately. All proportional hazard assumptions for the Cox regression were checked using weighted Schoenfeld residuals.
The multivariable test for the outcome of kidney and lung function was performed using logistic regression models. For the Cox as well as for the logistic regression models, clinically relevant factors with a P-value <.2 in the univariate model were entered into the multivariable model. Comprehensive tests for interactions between the time of admission, patient (age, gender), treatment (HCQ, AZI, anticoagulation, vasopressors), and risk (DM, Hypertension, MAP < 70) factors were also performed.
Results
Methylprednisolone treatment was primarily administered in the second month of the COVID-19 pandemic
The 2 treatment groups were comparable for available clinically relevant risk factors (see Table 1 for patient characteristics). The patients in both groups were elderly, with similar preexisting risk factors. The number of patients treated with methylprednisolone in April was higher than in March. Out of the 37 patients who received methylprednisolone, 28 patients were treated with a low dose, while 9 patients were treated with high dose methylprednisolone. This higher dose was only administered in April. Additional medication use (anticoagulants, Hydroxychloroquine, and Azithromycin) was distributed similarly between both groups.
Table 1.
Demographics and patient characteristics.
| Characteristics | Methylprednisolone | P | |
|---|---|---|---|
| No (N = 38) | Yes (N = 37) | ||
| Age | 72.5 [34-92] | 73 [48-93] | .78 |
| Male | 21 (55%) | 23 (62%) | .54 |
| BMI | 28 [14.7-50.1] | 26.6 [20.1-50.2] | .42 |
| Diabetes | 20 (53%) | 23 (62%) | .40 |
| Hypertension | 26 (68%) | 33 (89%) | .03 |
| CAD | 6 (16%) | 4 (11%) | .52 |
| Previous stroke | 4 (11%) | 3 (8%) | .72 |
| MAP < 70 at admission | 17 (45%) | 12 (32%) | .27 |
| GCS | 14 [3-15] | 15 [5-15] | .05 |
| SCR at admission | 1.7 [0.6-15.7] | 1.5 [0.9-10.0] | .19 |
| PaO2 at admission | 67.6 [30.9-227.0] | 72.0 [23.0-275.0] | .37 |
| PaO2/FiO2 ratio | 97.5 [41.0-568.0] | 95.9 [41.6-575.0] | .81 |
| Admission month | |||
| March | 19 (79%) | 5 (21 %) | .0007 |
| April | 19 (37%) | 32 (63%) | |
| Treatment | |||
| HCQ & AZI | 25 (65%) | 27 (73%) | .21 |
| HCQ&AZI&TOC | 3 (8%) | 6 (16%) | |
| Other | 10 (17%) | 4 (11%) | |
| Anticoagulation | 6 (16%) | 19 (51%) | .001 |
| Vassopressors | 10 (26%) | 8 (22%) | .63 |
| Mechanical ventilated | 16 (42%) | 15 (41%) | .89 |
| Hemodialysis | 7 (18%) | 6 (16%) | .80 |
| AKI during hospitalization | 14 (37%) | 14 (38%) | .92 |
Source: Results are reported as number (percentage or range).
Abbreviations: AKI, acute kidney injury; AZI, azithromycin; BMI, body mass index; CAD, coronary artery disease; FiO2, fraction of inspired oxygen; HCQ, hydroxychloroquine; GCS, glasgow coma scale; MAP, mean arterial pressure; PaO2, partial pressure of oxygen in arterial blood; SCR, serum creatinine; TOC, tocilizumab.
Low dose methylprednisolone improved kidney function outcomes
Overall, 55% of patients who did not receive methylprednisolone and 76% of patients who did receive it showed an improvement in the composite endpoint of improved kidney function (defined by improvement in their GFR and being off dialysis, P = .06; Figure 1). In the methylprednisolone group, 82% of patients improved as compared to 56% in the high dose methylprednisolone group (Supplemental Table E1). Patients that developed AKI during hospitalization had the worst outcomes (Figure 1). For those patients, the OR (odds ratio) for improvement was 0.118 (P = .001; Figure 1). MAP < 70 at admission and the need for hemodialysis negatively impacted on patient improvement (Figure 1). Age, gender, BMI, the month of admission, preexisting conditions, and other treatment factors did not significantly impact on GFR improvement in the univariate models (data not shown). When the improvement of GFR was analyzed by the different methylprednisolone dosages, the improvement with low dose versus no methylprednisolone showed a clear advantage with an OR of 4.6 (P = .04) compared to high dose versus none OR 1.65 (P = .61; Supplemental Table E1). The prediction in both models reached a c-statistic of over 0.85.
Figure 1.
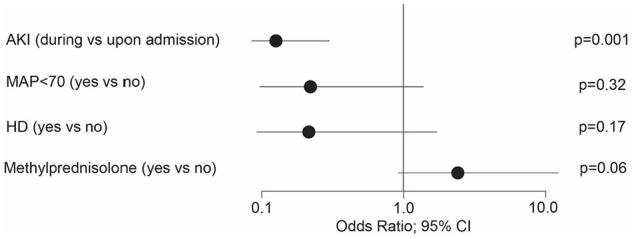
Risk factors for improvement of kidney function. Age, Gender, BMI, preexisting conditions, and other treatment factors did not show any significant impact of improvement of kidney function.
Abbreviations: AKI, acute kidney injury; HD, Hemodialysis; MAP, mean arterial pressure.
Methylprednisolone treatment improved the PaO2/FiO2 ratio
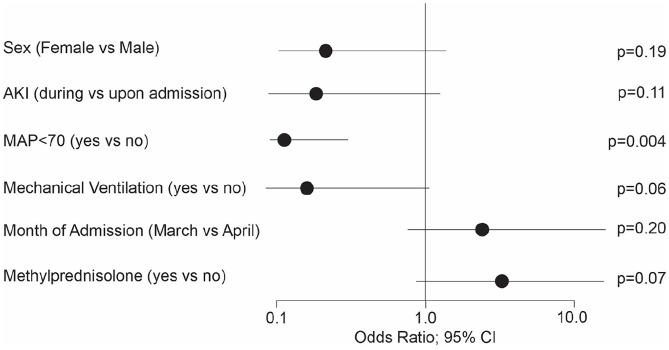
Overall, ARDS (PaO2/FiO2 ratio) improved in the methylprednisolone group by 73% compared to 45% in the patients without steroid treatment (P = .01; Figure 2). The low dose methylprednisolone group improved by 79% compared to 56% in the high dose methylprednisolone group (P = .02, Supplemental Table E2). Multivariable risk factor analysis for methylprednisolone versus no methylprednisolone demonstrated MAP < 70 and AKI developed during hospitalization and required mechanical ventilation intervention, resulting in a decreased likelihood of ARDS improvement (Figure 2). The OR for female patients showed a trend to a less favorable outcome (Figure 2). Patients admitted during the month of April showed an improved outcome compared to patients admitted before April. The treatment with methylprednisolone increased the likelihood of ARDS improvement by a factor of 4.5 (P = .07). When taking the methylprednisolone dosage into account, the low dose group achieved an OR for ARDS improvement of 4.43 (P = .06, Supplemental Table E2) compared to no methylprednisolone treatment. The high dose methylprednisolone group compared to no steroid treatment achieved an OR for ARDS improvement of 3.28 (P = .36). All other potentially influential factors as age, BMI, preexisting conditions, and other treatment factors did not show any impact of ARDS improvement. The c-statistic for both models reached values over 0.9.
Figure 2.
Factors for improvement of ARDS. Age, BMI, preexisting conditions, and other treatment factors did not show any significant impact of ARDS improvement.
Abbreviations: AKI, acute kidney injury; MAP, mean arterial pressure.
Improved survival in AKI/ARDS patients treated with methylprednisolone
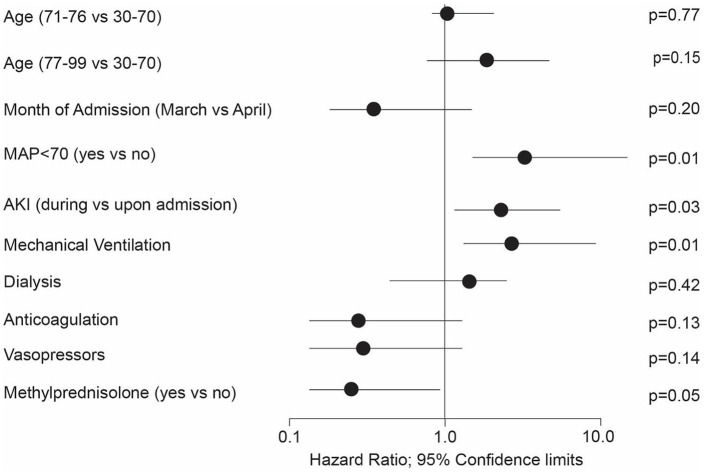
The Kaplan-Meier survival curve showed greater survival in patients treated with methylprednisolone compared with patients without methylprednisolone during the first 28 days (Figure 3). The survival in the methylprednisolone group reached 73% (51%) at day 14 (21) compared to 36% (29%) in patients without methylprednisolone. The median survival from hospital admission was 9 [7-17] days in patients without methylprednisolone. Patients with methylprednisolone reached the study endpoint in more than 50% of the cases. The impact of high dose methylprednisolone is shown in Supplemental Figure E1. The multivariable Cox regression for the risk factor analysis for patient death found a trend for a slightly higher risk to die for patients over the age of 76 as compared to the younger 30-70-year-old patients (Figure 4). There was a trend for patients admitted in April for a slightly decreased relative risk of death compared to earlier admitted patients. AKI during hospitalization and a MAP < 70 were significant risk factors as well as the need for mechanical ventilation during the hospital stay. The impact of hemodialysis during the hospital stay did not reach significance. The use of anticoagulation and vasopressor treatment had a positive impact on patient survival. Methylprednisolone overall reduced the relative risk to die by 77% (P = .047). The subsequent Cox regression by methylprednisolone dosage showed that the low dose methylprednisolone group gained a reduction in the relative risk of death of 70% (P = .05) while the high dose methylprednisolone group showed a reduction in 53% (P = .30, Supplemental Table E3).
Figure 3.
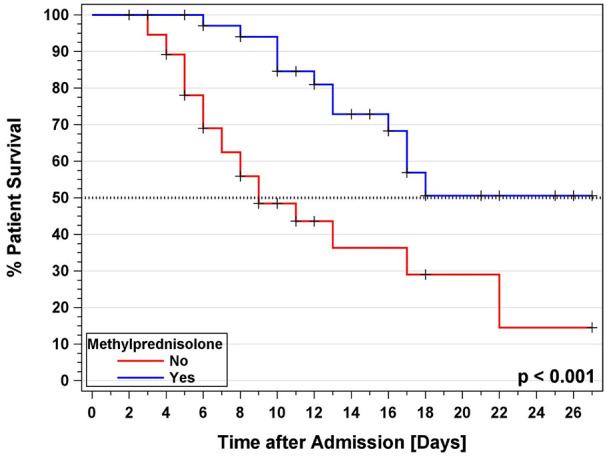
Kaplan-Meier survival analysis for patient survival after hospital admission for COVID-19 infection with and without Methylprednisolone treatment.
Figure 4.
Risk Factor analysis for patient death. Gender, BMI, other treatment factors, and preexisting risk factors did not show any significant impact on patient death.
Abbreviations: AKI, acute kidney injury; MAP, mean arterial pressure.
Discussion
During the first 2 months of the COVID-19 pandemic, no validated treatment protocol was available and many physicians employed several treatment regiments. We hypothesized that IV treatment with methylprednisolone would improve pulmonary, renal and survival outcomes of COVID-19 AA patients. Here, we sought to investigate first whether IV treatment of methylprednisolone to COVID-19 positive AA patients exhibiting AKI and ARDS could improve survival and reduce complications. Equally, we applied the Meduri-protocol that advocates 2 different methylprednisolone doses (1 mg/kg/day or low dose methylprednisolone versus high dose methylprednisolone or 2 mg/kg/day) administered during the course of ARDS illness with AKI.20 We demonstrate that there is a survival advantage for both ARDS and AKI patients administered a low dose of methylprednisolone. The methylprednisolone group survived 51% at 21 days compared to 29% in the non-methylprednisolone group. The use of methylprednisolone also improved the likelihood of renal and PaO2/FiO2 ratio. The PaO2/FiO2 is an integral part of the assessment of patients with ARDS and our data suggests that there are improved ARDS outcomes in COVID-19 patients following methylprednisolone treatment. Use of anticoagulants, the month of treatment, and AKI during hospitalization influenced outcomes. Therefore, the experience gained during the first months of the COVID-19 pandemic gave us important knowledge on how to treat patients who are severely sick with COVID-19, ARDS, and AKI and the dosing strategy for methylprednisolone treatment.
In critically ill COVID-19 patients, a cytokine storm, along with unmanageable hypoxemia, can lead to multi-organ failure.25 The cytokine profile with COVID-19 consists of increased IL-2, IL-7, G-CSF, CXCL10, CCL2, CCL3, and TNF-α,26 ferritin, IL-1β, S100 proteins,27 and IL-6.28 A clinical trial in the United Kingdom published their results while our study was ongoing on the beneficial effect of using of low dose dexamethasone treatment in reducing the 28-day mortality rate by 17%.10 The CoDEX Randomized Clinical Trial in Brazil also recently showed that intravenous dexamethasone reduced the number of required ventilator days for COVID-19 patients with moderate to severe ARDS.11 A recent Spanish study confirmed that the lower dose of methylprednisolone is favorable to higher doses in COVID-19 patients and the higher dose increased mortality in elderly patients.29 Therefore, a low dose of methylprednisolone appears beneficial to modulate the immune response but utilizing high doses may blunt immune responses to dangers levels. Severe COVID-19 pneumonia cases respond well to early administration of prolonged methylprednisolone treatment by lowering hazard of death (71%) and decreased ventilator dependence.30 Our study now further validates that the low dose IV methylprednisolone significantly improves survival not only in COVID-19 patients, but also in those individuals with ARDS and AKI as well. Based on our results, we find that a low dose of methylprednisolone (1 mg/kg/day in 2 divided doses) for 3 days followed by conversion to oral dose if feasible or to continue the dose IV when not feasible over 2-weeks does result in improvement of both ARDS and AKI. This may be due to the regulation of the severity of the cytokine storm. The relative risk for improvement of AKI by the use of methylprednisolone increased by the factor 3.4, a result that indicates to us that the consequences of modulating cytokine storms towards quiescence helps mitigate their detrimental effect on many organs like the lungs and the kidney.
Previous studies on glucocorticoids and lung injury demonstrated the immunosuppressive action of glucocorticoids at the cellular level (17, 27). Meduri et al using an ex vivo model of systemic inflammation reported that naïve peripheral blood leukocytes when exposed to prolonged methylprednisolone treatment of un-resolving ARDS exhibited a progressive increase in cytoplasmic binding of glucocorticoid receptor to NFκB, and a concomitant reduction in NFκB DNA binding and transcription of cytokines such as tumor necrosis factor-α and interleukin-1β.22,25 A recent unpublished study demonstrates that SARS-CoV-2 proteins interact with lung associated proteins more than other organs.31 The same study demonstrates that SARS-CoV-2 interacts with multiple innate immune pathways,31 which suggests that the lung is very susceptible to SARS-CoV-2 and targeting the cytokine storm during COVID-19 and steroid treatment may be beneficial. AKI is more prevalent in patients with ARDS,32 but we have observed AKI development independent of ARDS during this pandemic. Having AKI with ARDS carries a mortality rate of over 25%.33 However, our data on the likely benefit of administration of methylprednisolone adds to the growing body of clinical observations of the positive impact on survival in COVID-19 patients with ARDS and AKI.28
Our study has limitations in that it is retrospective and biases regarding treatment selection were observed. Renal core biopsies were unavailable for the majority of the patients. Pronounced ATN was observed in several samples but did not contribute to our clinical management of the patients. Our study, is also limited by the number of patients within our study and only sampling from 2 months. Due to the effectiveness of treatment and the lock down procedure in New York City, we have not observed sufficient COVID-19 patient numbers at our institution to expand our study. The small population size also limits our ability to analyze the patients regarding the length of treatment and clinical outcomes. Further, we did not obtain any laboratory measurements of tumor necrosis factor-α and interleukin-1β as these were considered investigational. We have learned unequivocally that the month the patients were presenting mattered most. Health care providers began utilizing methylprednisolone to increase survival, ARDS, and AKI outcomes. Whether the additive effect of introduction of many more interventions in the month of April compared to March; such as full dose anticoagulation, implementation of a dedicated “Prone Team,” mucolytic protocols for endotracheal tube (ETT) care, acted in concert to improve our outcomes were not analyzed due to small number of patients being exposed to such additive interventions. However, this study demonstrated the learning curve health care providers encountered during the COVID-19 pandemic, resulting in better outcomes in patients presenting later into the pandemic.
In conclusion, our study adds to the building literature that low dose corticosteroids are beneficial in COVID-19 and that its benefits stretch to improving outcomes in patients with ARDS and AKI in our African American population.
Supplemental Material
Supplemental material, sj-docx-1-cra-10.1177_1179548420980699 for Early Experience With Methylprednisolone on SARS-CoV-2 Infection in the African American Population, a Retrospective Analysis by Subodh J Saggi, Sridesh Nath, Roshni Culas, Seema Chittalae, Aaliya Burza, Maya Srinivasan, Rishard Abdul, Benjamin Silver, Alnardo Lora, Ishmam Ibtida, Tanuj Chokshi, Violeta Capric, Ammar Mohamed, Samrat Worah, Jie OuYang, Patrick Geraghty, Angelika Gruessner and Moro O Salifu in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The department of Medicine at SUNY Downstate funded this study.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Study design: SJS, SN, AB, AG; Data collection: SJS, SN, RC, SC, AB, MS, RA, BS, AL, II, TC, VC, AM, SW, JO, PG, AG, MS; Data analysis: AG and SJS; Manuscript preparation: SJS, SN, AB, PG, AG; All authors approved the final version of the manuscript.
ORCID iDs: Alnardo Lora  https://orcid.org/0000-0002-1995-1557
https://orcid.org/0000-0002-1995-1557
Patrick Geraghty  https://orcid.org/0000-0003-1647-5505
https://orcid.org/0000-0003-1647-5505
Supplemental Material: Supplemental material for this article is available online.
References
- 1. National Center for Immunization and Respiratory Diseases (NCIRD) DoVD. Coronavirus disease 2019 (COVID-19). Cases in the U.S. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed September 2, 2020.
- 2. Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with Covid-19. Kidney Int. Published online May 16, 2020. doi: 10.1016/j.kint.2020.05.006 [DOI] [Google Scholar]
- 3. Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824-828. doi: 10.1016/j.kint.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christ Crain M, Hoorn EJ, Sherlock M, Thompson CJ, Wass JAH. Endocrinology in the time of COVID-19: management of hyponatraemia and diabetes insipidus. Eur J Endocrinol. Published online April 1, 2020. doi: 10.1530/EJE-20-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sigurdsson TS, Thornorvaldsson AP, Asgeirsdottir S, Sigvaldason K. Cardiac arrest in a COVID-19 patient after receiving succinylcholine for tracheal reintubation. Br J Anaesth. Published online May 1, 2020. doi: 10.1016/j.bja.2020.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol. Published online May 5, 2020. doi: 10.1681/ASN.2020040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harenberg J, Favaloro E. COVID-19: progression of disease and intravascular coagulation - present status and future perspectives. Clin Chem Lab Med. Published online May 14, 2020. doi: 10.1515/cclm-2020-0502 [DOI] [PubMed] [Google Scholar]
- 10. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. doi: 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 11. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. Published online September 2, 2020. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Group WHOREAfC-TW, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330-1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:1298-1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. Published online August 12, 2020. doi: 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. doi: 10.1093/cid/ciaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767. doi: 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 17. Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. 2020;12:e7560. doi: 10.7759/cureus.7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar K, Hinks TS, Singanayagam A. Treatment of COVID-19 exacerbated asthma: should systemic corticosteroids be used? Am J Physiol Lung Cell Mol Physiol. Published online May 13, 2020. doi: 10.1152/ajplung.00144.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Ong YK, Wang Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil Med Res. 2020;7:22. doi: 10.1186/s40779-020-00251-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meduri GU, Siemieniuk RAC, Ness RA, Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care. 2018;6:53. doi: 10.1186/s40560-018-0321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleinknecht D. Acute interstitial nephritis caused by drug hypersensitivity. Current controversies [Les nephrites interstitielles aigues par hypersensibilite medicamenteuse. Controverses actuelles]. Ann Med Interne (Paris). 1988;139:100-102. [PubMed] [Google Scholar]
- 22. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). Published online May 21, 2020. doi: 10.1377/hlthaff.2020.00598 [DOI] [PubMed] [Google Scholar]
- 23. Schmeer KK, Tarrence J. Racial-ethnic disparities in inflammation: evidence of weathering in childhood? J Health Soc Behav. 2018;59:411-428. doi: 10.1177/0022146518784592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parikh CR, Schaub JA. Acute kidney injury: steroids for prevention of AKI after cardiopulmonary bypass. Nat Rev Nephrol. 2015;11:509-510. doi: 10.1038/nrneph.2015.106 [DOI] [PubMed] [Google Scholar]
- 25. Berhes M, Fabian A, Laszlo I, et al. Organ replacement therapy and life-supporting treatment modalities in critically ill COVID-19 patients [Emelt szintu szervtamogato es eletfenntarto kezelesek kritikus allapotu COVID-19-fertozott betegeken]. Orv Hetil. 2020;161:704-709. doi: 10.1556/650.2020.31813 [DOI] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monreal E, Sainz de la Maza S, Natera-Villalba E, et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. Published online October 20, 2020. doi: 10.1007/s10096-020-04078-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020;7:ofaa421. doi: 10.1093/ofid/ofaa421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2-Human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv. Published online Mar 22, 2020. doi: 10.1101/2020.03.22.002386 [DOI] [Google Scholar]
- 32. Panitchote A, Mehkri O, Hastings A, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9:74. doi: 10.1186/s13613-019-0552-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Darmon M, Clec’h C, Adrie C, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9:1347-1353. doi: 10.2215/CJN.08300813 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cra-10.1177_1179548420980699 for Early Experience With Methylprednisolone on SARS-CoV-2 Infection in the African American Population, a Retrospective Analysis by Subodh J Saggi, Sridesh Nath, Roshni Culas, Seema Chittalae, Aaliya Burza, Maya Srinivasan, Rishard Abdul, Benjamin Silver, Alnardo Lora, Ishmam Ibtida, Tanuj Chokshi, Violeta Capric, Ammar Mohamed, Samrat Worah, Jie OuYang, Patrick Geraghty, Angelika Gruessner and Moro O Salifu in Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine