Abstract
Background:
MicroRNAs (miRNAs) have been demonstrated to play critical roles in tumorigenesis of non-small cell lung cancer (NSCLC), and circulating miRNAs are a valuable source of biomarkers for the clinical management of NSCLC. The aim of this study was to determine the value of serum miR-762 as a diagnostic and prognostic biomarker for NSCLC.
Methods:
We examined circulating miR-762 expression in 148 NSCLC patients and 60 healthy individuals using the quantitative real-time polymerase chain reaction (qRT-PCR). The effect of miR-762 downregulation on the proliferative capacity of NSCLC cells were also explored.
Results:
The serum miR-762 levels were significantly upregulated in NSCLC patients compared to the healthy individuals. Receiver operating characteristics (ROC) curve analysis revealed that circulating miR-762, carcinoembryonic antigen (CEA), CYFRA21-1 and a combination of these 3 biomarkers yield the areas under the curve (AUC) of 0.874, 0.826, 0.41 and 0.969, respectively. Interestingly, circulating miR-762 identified the NSCLC patients at the clinical stage I from healthy controls with an AUC value of 0.920. In addition, serum miR-762 expression was significantly correlated with clinical stage, lymph node metastasis, histological grade and gefitinib-resistance. The survival analysis showed that NSCLC patients in the high serum miR-762 group suffered worse overall survival and relapse-free survival than those in the low serum miR-762 group. The multivariate Cox proportional hazards regression analysis revealed high circulating miR-762 was an independent unfavorable prognostic factor. Downregulation of miR-762 significantly suppressed the proliferative capacity of NSCLC cells in vitro, and bioinformatic analysis of the potential downstream targets of miR-762 identified many important cancer-associated pathways.
Conclusions:
In conclusion, serum miR-762 might serve as a promising diagnostic and prognostic biomarker for the NSCLC.
Keywords: serum miR-762, non-small cell lung cancer, diagnosis, prognosis, biomarker
Introduction
Lung cancer is one of the leading causes of cancer-related death worldwide.1 Non-small cell lung cancer (NSCLC) consists of 75-85% of all lung cancers, and there are 3 main histologic subtypes of NSCLC: adenocarcinoma, squamous cell carcinoma and large cell carcinoma.2 Despite the rapid advances have been achieved in early detection and treatment strategies, the 5-year survival rate for NSCLC remains less than 15%.3 As the clinical symptoms of NSCLC are not apparent at the initial stages, the majority of patients are diagnosed at advanced stages. Thus, there is an urgent need to identify novel prognostic biomarkers to improve the clinical management of NSCLC.
MicroRNAs (miRNAs) are a class of highly conserved, small, and endogenous non-coding RNAs that regulate gene expression post-transcriptionally by base-pairing with 3’-untranslated region of the target genes.4 These small molecules are widely distributed in tissues and cells, and play critical roles in many biological processes. Abnormal expression of miRNAs has been widely reported in various types of cancer including NSCLC, as they are closely involved in tumor progression by acting as either oncomiRs or tumor suppressors.5-7 Additionally, miRNAs are easily detectable in body fluids such as serum and saliva, and circulating miRNAs are extremely stable. These features enable them to become promising biomarkers for the diagnosis and prognosis prediction of NSCLC.8,9 For instance, the expression level of serum miR-494 was significantly increased in patients with NSCLC. In addition, upregulation of serum miR-484 was associated with unfavorable prognosis of NSCLC.10
It is increasingly being recognized that miR-762 plays a crucial role in regulating several types of cancer such as NSCLC, ovarian cancer and breast cancer.11-13 In addition, our preliminary RNA-seq data showed that miR-762 was one of the top upregulated miRNAs in NSCLC tissues compared to the adjacent normal tissues. However, the potential clinical significance of serum miR-762 in NSCLC was unclear. In the present study, firstly, we investigated whether the circulating miR-762 was aberrantly expressed in NSCLC. Then the associations between serum miR-762 levels and the clinicopathological parameters of NSCLC were further explore. Finally, the prognostic value of serum miR-762 and the biological function of miR-762 in NSCLC were further determined. To the best of our knowledge, this is the first study to determine whether serum miR-762 is deregulated in NSCLC and its potential clinical significance.
Materials and Methods
Patient and Control Subjects
A total of 148 NSCLC patients were recruited between April 2013 and October 2014, and 60 healthy volunteers were enrolled as the controls. All the NSCLC cases were pathologically confirmed and staged based on the eighth edition of the tumor, node, and metastasis (TNM) staging system. The clinicopathological information of the NSCLC patients were summarized in Table 1. The exclusion criteria were as follows: (1) NSCLC patients who received any therapy prior to serum sample collection; (2) NSCLC patients who had other severe systemic diseases or secondary malignancy. Overall survival (OS) was defined as the period from diagnosis to death or the last follow-up. Relapse-free survival (RFS) was defined as the period from diagnosis until the date of the first recurrence. The patients received the standardized treatments according to the American Society of Clinical Oncology (ASCO) guideline. The median follow-up time of the patient cohort was 42.4 months, and no patient lost to follow up. The follow up was performed mainly through phone call, texting and outpatient service etc. This study was approved by the Institutional Review Board of the Central Hospital Affiliated to Shenyang Medical College (Approval number: 20180047). The written informed consent was obtained from all participants.
Table 1.
The Expression Level of Serum miR-762 and the Clinicopathological Parameters of NSCLC.
| Characteristics | Total (n = 148) | Serum miR-762 | P | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.739 | |||
| <60 | 62 | 32 | 30 | |
| ≥60 | 86 | 42 | 44 | |
| Gender | 0.434 | |||
| Male | 114 | 59 | 55 | |
| Female | 34 | 15 | 19 | |
| Smoking history | 0.250 | |||
| No | 36 | 21 | 15 | |
| Yes | 112 | 53 | 59 | |
| Histological type | 0.740 | |||
| Adenocarcinoma | 84 | 41 | 43 | |
| Squamous cell carcinoma | 64 | 33 | 31 | |
| Distant metastasis | 0.290 | |||
| Negative | 132 | 68 | 64 | |
| Positive | 16 | 6 | 10 | |
| Lymph node metastasis | 0.001 | |||
| Negative | 78 | 49 | 29 | |
| Yes | 70 | 25 | 45 | |
| Histological grade | <0.001 | |||
| Well/moderate | 101 | 61 | 40 | |
| Poor | 47 | 13 | 34 | |
| Clinical stage | 0.021 | |||
| I-II | 72 | 43 | 29 | |
| III-IV | 76 | 31 | 45 | |
Serum Preparation
In general, approximate 5 mL of peripheral blood was collected from each subject. All samples were processed with 1 h after blood-draw. After centrifuging at 1600 g for 10 min at room temperature, serum was transferred into new microcentrifuge tubes and then stored at −80°C until further analysis.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from the serum samples and cells using the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) and miRNeasy Mini Kit (Qiagen) as per the manufacturer’s instructions. Cel-miRNA-39 was used as a spike-in control and added into each serum sample. Complementary DNAs (cDNAs) were synthesized with Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). The cDNAs were amplified with the SYBR Premix Ex TaqII (Takara, Dalian, China) on the CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The qRT-PCR conditions were: 95°C for 15 min, followed by 40 cycles of 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. The PCR primers are as follows: miR-762: forward 5′-ATTATGGGGCTGGGGCCGGG-3′ and reverse 5′-GTGCAGGGTCCGAGGT-3′; cel-miR-39: forward 5′-TCACCGGGTGTAAATCAGCTTG-3′ and reverse 5′-GTGCAGGGTCCGAGGT-3′; U6: forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The relative expression of serum miR-762 was normalized to that of cel-miRNA-39 or U6 using the 2−ΔΔCt method.
Serum Detection of Carcinoembryonic Antigen (CEA) and CYFRA21-1
CEA Human ELISA Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) and Human CYFRA21-1 ELISA Kit (MyBioSource, San Diego, CA, USA) were used to quantify the levels of CEA and CYFRA21-1 in the serum samples following manufacturer’s instructions.
Cell Culture and siRNA Transfection
The NSCLC cell line A549 was cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified atmosphere of 5% CO2 at 37°C. The cells were transfected with siCTRL, miR-762 inhibitor #1, and miR-762 inhibitor #2 with Lipofectamine RNAiMAX Transfection Reagent (Invitrogen).
Cell Counting Kit-8 (CCK-8) Analysis
The cells were seeded into a 96-well plate at the density of 3 × 103 cells/ well. At the indicated time points, 10 µL of CCK-8 was added to each well and the cells were incubated for 2 h at 37°C. The absorbance was measured at the wavelength of 450 nm using a microplate reader.
Cell Cycle Analysis
The transfected cells were collected by trypsinization and washed with PBS twice. Follow by fixing in 75% cold ethanol at 4°C overnight. the cells were stained with FxCycle™ Violet stain (Invitrogen) in the dark at room temperature for 30 min. Cell cycle was evaluated using a FACS Calibur flow cytometry (BD Biosciences, Bedford, MA, USA), and the data were analyzed using Flowjo 10.0 software (FlowJo LLC, Ashland, OR, USA).
Bioinformatic Analysis
The downstream target genes of miR-762 were predicted using miRDB (http://mirdb.org/). Gene Ontology and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analyses of the downstream target genes were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics resource (https://david-d.ncifcrf.gov/).
Statistical Analysis
The Mann-Whitney U test was used to compare the differences in serum miR-762 level between NSCLC patients and healthy controls. The receiver operating characteristic (ROC) curves were built up to evaluate the diagnostic value of serum miR-762. The associations between serum miR-762 level and the clinicopathological features of NSCLC were confirmed by Chi-square test. The survival analyses were performed with the Kaplan-Meier method the log-rank test. The univariate and multivariate Cox hazard regression analysis were used to determine the independent prognostic factors for NSCLC. One-way ANOVA was performed to analyze the data of the in vitro experiments. Statistical analyses were performed with GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA), and P < 0.05 was considered to represent a statistically significant difference.
Results
Serum miR-762 Was Upregulated in Patients With NSCLC
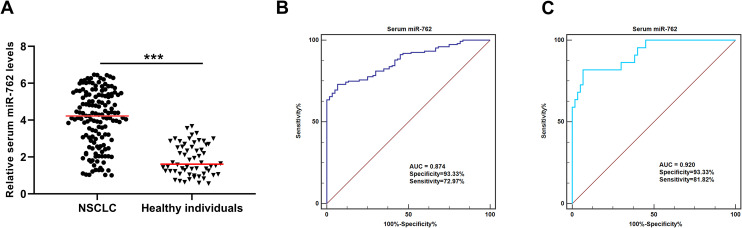
We have profiled the abnormally expressed miRNAs in NSCLC tissues, and miR-762 was found to be overexpressed in NSCLC tissues (Supplementary Table 1). The expression levels of miR-762 were significantly higher in the serum samples of NSCLC patients compared to those of the heathy individuals (***P < 0.001) (Figure 1A). In addition, the diagnostic performance of the serum miR-762 was evaluated. As shown in Figure 1B, the AUC value of circulating miR-762 for discriminating patients with NSCLC and healthy controls was 0.874, with a sensitivity of 72.97% and a specificity of 93.33%. To further evaluate the potential of serum miR-762 for early detection of NSCLC, ROC analysis was used to assess the diagnostic accuracy of circulating miR-762 for distinguishing NSCLC patients at the clinical stage I and healthy controls. The results showed that the AUC value was 0.920, with a sensitivity of 81.82% and a specificity of 93.33% (Figure 1C).
Figure 1.
Serum miR-762 was upregulated in patients with NSCLC. (A) The expression level of serum miR-762 was higher in NSCLC patients than in healthy controls. (B) Serum miR-762 effectively discriminated NSCLC patients from healthy controls. (C) Serum miR-762 identified NSCLC patients at the clinical stage I from healthy individuals with high accuracy.
Combination of Serum miR-762, CEA and CYFRA21-1 Identified NSCLC With High Accuracy
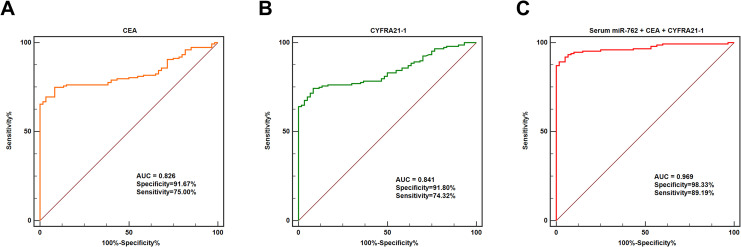
The AUC value of CEA for discriminating NSCLC patients and healthy controls was 0.826, with a sensitivity of 75.00% and a specificity of 91.67% (Figure 2A). The AUC value of CYFRA21-1 for differentiating NSCLC patients and healthy controls was 0.841, with a sensitivity of 74.32% and a specificity of 91.80% (Figure 2B). Surprisingly, combination of serum miR-762, CEA and CYFRA21-1 resulted in an AUC value of 0.969, with a sensitivity of 89.19% and a specificity of 98.33% (Figure 2C).
Figure 2.
Combination of CEA and CYFRA21-1 enhanced the diagnostic accuracy of serum miR-762. (A) The diagnostic value of CEA for NSCLC. (B) The diagnostic potential of CYFRA21-1 for NSCLC. (C) Combination of serum miR-762, CEA and CYFRA21-1 identified NSCLC patients from healthy controls with extremely high accuracy.
High Circulating miR-762 Was Associated With Unfavorable Clinical Characteristics of NSCLC
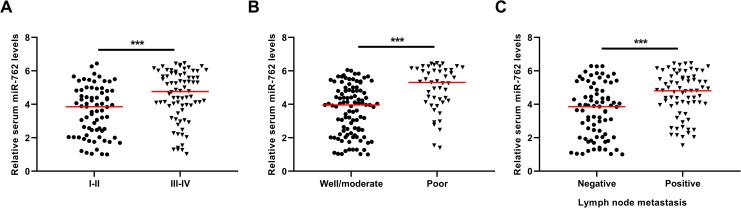
We first compared the expression levels of serum miR-762 in NSCLC patients with favorable or unfavorable clinical features. Our results showed that the circulating miR-762 levels were markedly higher in patients at the advanced clinical stage (III-IV), or with poorly differentiated tumors, or with positive lymph node metastasis than in patients at the early clinical stage (I-II) (***P < 0.001) (Figure 3A), or with well/moderately differentiated tumors(***P < 0.001) (Figure 3B), or with negative lymph node metastasis (***P < 0.001) (Figure 3C).
Figure 3.
The association between serum miR-762 and clinical characteristics of NSCLC. (A) The NSCLC patients at the advanced stages had higher expression of serum miR-762. (B) The NSCLC patients with poor tumor grade had higher expression of serum miR-762. (C) The NSCLC patients with positive lymph node metastasis had higher expression of serum miR-762.
Then the associations between serum miR-762 and clinical characteristics of NSCLC were evaluated. The median value of serum miR-762 was used to divide the NSCLC patients into high serum miR-762 group and low serum miR-762 group. Our results showed that serum miR-762 was significantly associated with histological grade (P < 0.001), clinical stage (P = 0.021) and lymph node metastasis (P = 0.001). However, it was not correlated with other parameters including age, gender, smoking history, histological type, and distant metastasis (all P > 0.05) (Table 1).
High Circulating miR-762 Was Associated With Treatment Response and Worse Survivals of NSCLC
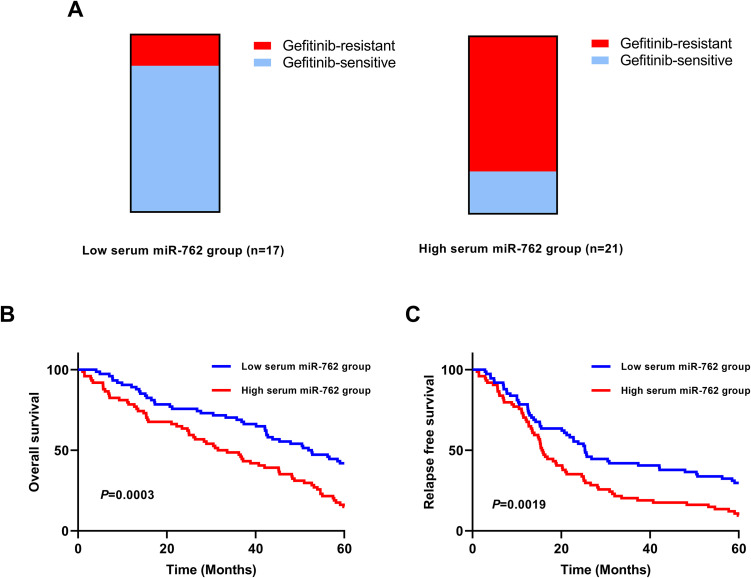
A total of 38 NSCLC patients received gefitinib treatment. Our results showed that a higher percentage of gefitinib-resistance cases was observed in the high serum miR-762 group (16/21) compared to that in the low serum miR-762 group (3/17) (Figure 4A). The survival analysis showed that NSCLC patients in the high serum miR-762 group had significantly shorter OS than those in the low serum miR-762 group (P = 0.0003) (Figure 4B). The patients with high serum miR-762 expression had dramatically reduced RFS compared with those with low serum miR-762 expression (P = 0.0019) (Figure 4C).
Figure 4.
The association between serum miR-762 and gefitinib-resistance as well as OS/RFS of NSCLC. (A) A higher percentage of gefitinib-resistance NSCLC cases was found in the high serum miR-762 group. (B) The NSCLC patients in the high serum miR-762 group had shorter OS than the patients in the low serum miR-762 group. (C) The NSCLC patients in the high serum miR-762 group had worse RFS than those in the low serum miR-762 group.
Serum miR-762 Was an Independent Prognostic Factor for NSCLC
The univariate Cox hazard regression analysis showed that lymph node metastasis, clinical stage, histological grade and serum miR-762 were significantly associated with the OS of NSCLC (Table 2). The multivariate Cox hazard regression analysis revealed that the clinical stage (RR = 3.454, 95%CI = 1.410-6.193, P = 0.005), histological grade (RR = 2.313, 95%CI = 1.095-3.956, P = 0.036) and serum miR-762 (RR = 2.802, 95%CI = 1.216-4.794, P = 0.018) were independent prognostic factors for NSCLC (Table 3).
Table 2.
Univariate Analysis of the Risk Factor Associated With NSCLC.
| Characteristics | Risk ratio | 95% CI | P |
|---|---|---|---|
| Age (≥60 vs <60) | 1.426 | 0.802-1.752 | 0.530 |
| Gender (male vs female) | 1.523 | 0.774-2.820 | 0.482 |
| Smoking history (yes vs no) | 1.305 | 0.715-2.117 | 0.512 |
| Histological type (AC vs SCC) | 1.025 | 0.675-1.509 | 0.758 |
| Distant metastasis (negative vs positive) | 1.071 | 0.848-2.354 | 0.627 |
| Lymph node metastasis (negative vs positive) | 2.407 | 1.214-3.845 | 0.028 |
| Clinical stage (III/IV vs I/II) | 5.129 | 2.014-10.367 | <0.001 |
| Histological grade (poor vs well/moderate) | 2.816 | 1.350-5.129 | 0.008 |
| Serum miR-762 (high vs low) | 3.418 | 1.541-7.208 | 0.002 |
Table 3.
The Independent Prognostic Factors for NSCLC Revealed by Multivariate Analysis.
| Characteristics | Risk ratio | 95% CI | P |
|---|---|---|---|
| Age (≥60 vs <60) | 1.166 | 0.741-1.625 | 0.471 |
| Gender (male vs female) | 1.360 | 0.849-2.054 | 0.368 |
| Smoking history (yes vs no) | 1.489 | 0.814-2.523 | 0.324 |
| Histological type (AC vs SCC) | 0.893 | 0.571-1.329 | 0.874 |
| Distant metastasis (negative vs positive) | 1.104 | 0.714-1.750 | 0.506 |
| Lymph node metastasis (negative vs positive) | 1.894 | 0.974-3.017 | 0.113 |
| Clinical stage (III/IV vs I/II) | 3.454 | 1.410-6.193 | 0.005 |
| Histological grade (poor vs well/moderate) | 2.313 | 1.095-3.956 | 0.036 |
| Serum miR-762 (high vs low) | 2.802 | 1.216-4.794 | 0.018 |
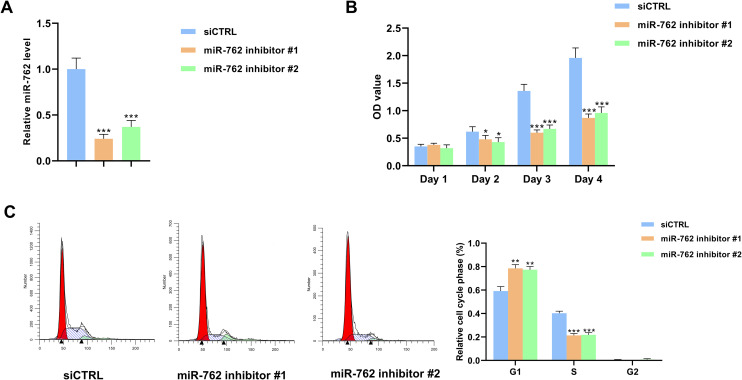
Knockdown of miR-762 Suppressed the Proliferation Capacity of NSCLC Cells
The expression level of miR-762 was significantly lower in miR-762 knockdown groups compared to the control group (***P < 0.001) (Figure 5A). The CCK-8 assay showed that the OD values were markedly lower in the miR-762 knockdown groups than the control group (*P < 0.05, ***P < 0.001) (Figure 5B). In addition, the cell cycle analysis showed that the percentage of cells at the S phase was lower in miR-762 knockdown groups compared to the control group, while the percentage of cells at the G1 phase was higher in the miR-762 knockdown groups (**P < 0.01, ***P < 0.001) (Figure 5C).
Figure 5.
Downregulation of miR-762 inhibited the proliferation of NSCLC cells. (A) The expression level of miR-762 was significantly lower in miR-762 inhibitors transfected cells compared to the control cells. (B) The OD values were lower in miR-762 inhibitors transfected cells than in the control cells. (C) The percentage of S phase cells was lower in miR-762 inhibitor groups compared to the control group.
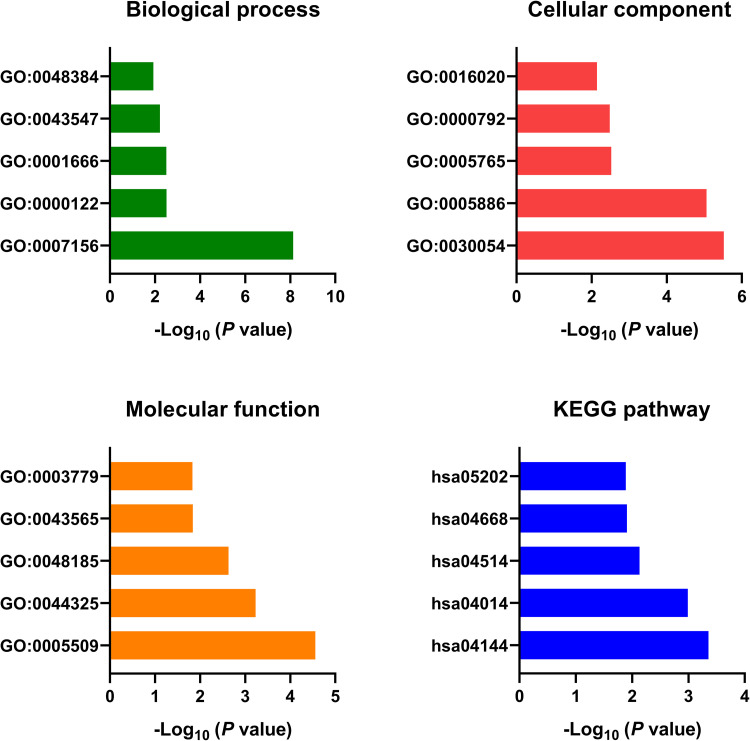
Bioinformatic Analysis of the Downstream Targets of miR-762
The downstream targets of miR-762 were predicted and identified using miRDB (Supplementary Table 2). The bioinformatic analysis showed that GO:0007156∼homophilic cell adhesion via plasma membrane adhesion molecules, GO:0000122∼negative regulation of transcription from RNA polymerase II promoter, GO:0001666∼response to hypoxia, GO:0043547∼positive regulation of GTPase activity and GO:0048384∼retinoic acid receptor signaling pathway were the top enriched biological processes. GO:0030054∼cell junction, GO:0005886∼plasma membrane, GO:0005765∼lysosomal membrane, GO:0000792∼heterochromatin and GO:0016020∼membrane were the top enriched cellular components. GO:0005509∼calcium ion binding, GO:0044325∼ion channel binding, GO:0048185∼activin binding, GO:0043565∼sequence-specific DNA binding and GO:0003779∼actin binding were the top enriched molecular functions. The pathway analysis revealed that hsa04144: Endocytosis, hsa04014: Ras signaling pathway, hsa04514: Cell adhesion molecules (CAMs), hsa04668: TNF signaling pathway and hsa05202: Transcriptional misregulation in cancer were top enriched pathways (Figure 6).
Figure 6.
GO and KEGG analysis of the downstream target genes of miR-762.
Discussion
In this study, we were the first to demonstrate that the expression level of serum miR-762 was significantly increased in NSCLC patients compared to healthy individuals. Circulating miR-762 distinguished NSCLC patients from healthy controls with high accuracy. In addition, the integration of serum miR-762 with CEA and CYFRA21-1 improved the diagnostic efficiency for NSCLC. Overexpression of serum miR-762 was significantly associated with worse clinicopathological parameters of NSCLC. Moreover, NSCLC patients in the high serum miR-762 expression group had significantly shorter OS and RFS than those in the low serum miR-762 expression group. Serum miR-762 was demonstrated to be an independent prognostic factor for NSCLC. Mechanistically, knockdown of miR-762 significantly suppressed the proliferative capacity of NSCLC cells in vitro, and bioinformatic analysis of the downstream targets of miR-762 identified many important cancer-associated pathways. Taken together, serum miR-762 might be a promising biomarker for detecting and prognosis prediction of NSCLC. Quantification of serum miR-762 level might contribute to stratify the high-risk NSCLC patients with unfavorable prognosis and therefore improve the clinical outcome for NSCLC. Consistent with our results, upregulation of miR-762 in NSCLC tissues and cells was strongly associated with the chemoresistance. Ectopic expression of miR-762 enhanced cell survival and suppressed the cell death induced by gefitinib, indicating that miR-762 might exert an oncogenic role in NSCLC tumorigenesis.11
Similarly, miR-762 has been reported to play an oncogenic role in other types of cancers. The expression level of miR-762 was significantly upregulated in breast cancer tissues and cell lines. In addition, enforced expression of miR-762 promoted the proliferation and invasion capabilities of breast cancer cells through regulating interferon regulatory factor 7, suggesting that miR-762 acts as an oncomiR in breast cancer.13 MiR-762 was also highly expressed in ovarian cancer (OC) tissues, and upregulation of miR-762 predicted unfavorable survival in OC. In addition, overexpression of miR-762 increased the proliferation and invasion as well as inhibited the apoptosis of OC cells, and vice versa.12 The expression level of miR-762 was highly expressed in head and neck squamous cell carcinoma (HNSCC) tissues, and upregulation of miR-762 was associated with unfavorable prognosis. Ectopic expression of miR-762 promoted the malignant behaviors of HNSCC cells both in vitro and in vivo by targeting PH domain and leucine-rich repeat protein phosphatase 2 and forkhead box O4.14 MiR-762 was one of the most upregulated miRNAs in radiation-induced thymic lymphomas in the mouse model, suggesting that miR-762 might involve in the initiation and progression of radiation-induced thymic lymphoma.15
MiR-762 might also serve as the downstream target of circular RNAs (circRNAs) to regulate tumorigenesis. For instance, the expression level of circFAM114A2 was significantly reduced in bladder cancer tissues and cell lines. Overexpression of circFAM114A2 suppressed the proliferation, migration and invasion of bladder cancer cells in vitro and inhibited tumor growth in vivo, indicating that circFAM114A2 might act as a tumor suppressor in bladder cancer. Interestingly, circFAM114A2 and miR-762 were demonstrated to competitively regulate the levels of ΔNP63 which is a known oncoprotein, suggesting miR-762 played an oncogenic role in bladder cancer.16 Likewise, circLPAR1 suppressed the tumorigenesis of bladder cancer, and served as a competing endogenous RNA of miR-762 in regulating the invasion and metastasis of bladder cancer cells.17
To the best of our knowledge, currently no study has reported that miR-762 might functioned as a tumor suppressor in cancer. However, as the biological functions of miRNAs depend on the downstream genes that they target, it is common to observe that a miRNA might either act as either an oncomiR or a tumor suppressor in different types of cancers or even at different stages of the same cancer.18,19 Therefore, further studies are warranted to determine the role of miR-762 in various types of cancers including NSCLC. In addition to NSCLC, circulating miR-762 might also be deregulated in other human diseases. For instance, the expression level of plasma miR-762 was significantly increased in patients with Graves’ disease.20 Combination of circulating miR-762 and other known biomarkers for NSCLC as well as clinical features might improve the early detection and prognosis prediction of NSCLC.
Conclusion
In conclusion, our present study reveals that the levels of serum miR-762 are significantly increased in NSCLC patients. Upregulation of serum miR-762 is strongly correlated with the poor prognosis of NSCLC. There findings indicate that circulating miR-762 might serve as a promising prognostic biomarker in NSCLC. However, further validation with a larger cohort is warranted to confirm our results.
Supplemental Material
Supplemental Material, Supplementary_Table_1_(3) for Identification of Circulating miR-762 as a Novel Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer by Lei Chen, Yunxia Li and Jingshu Lu in Technology in Cancer Research & Treatment
Supplemental Material, Supplementary_Table_2 for Identification of Circulating miR-762 as a Novel Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer by Lei Chen, Yunxia Li and Jingshu Lu in Technology in Cancer Research & Treatment
Acknowledgments
Thanks to all the authors.
Authors’ Note: This study was approved by the Institutional Review Board of the Central Hospital Affiliated to Shenyang Medical College (Approval number: 20180047). The written informed consent was obtained from all participants.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lei Chen  https://orcid.org/0000-0002-0977-5633
https://orcid.org/0000-0002-0977-5633
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;59(3):288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd ed.). Chest. 2007;132(3 Suppl):29S–55S. [DOI] [PubMed] [Google Scholar]
- 4. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469. [DOI] [PubMed] [Google Scholar]
- 5. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H, Liu X, Jin Z, Gou C, Liang M, Cui L, Zhao X. A three miRNAs signature for predicting the transformation of oral leukoplakia to oral squamous cell carcinoma. Am J Cancer Res. 2018;8(8):1403–1413. [PMC free article] [PubMed] [Google Scholar]
- 7. Zarredar H, Ansarin K, Baradaran B, et al. Critical microRNAs in lung cancer: recent advances and potential applications. Anticancer Agents Med Chem. 2018;18(14):1991–2005. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao W, Liu L, Lu X, Shu Y. Circulating microRNAs: possible prediction biomarkers for personalized therapy of non-small-cell lung carcinoma. Clin Lung Cancer. 2011;12(1):14–17. [DOI] [PubMed] [Google Scholar]
- 10. Zhang J, Wang T, Zhang Y, et al. Upregulation of serum miR-494 predicts poor prognosis in non-small cell lung cancer patients. Cancer Biomark. 2018;21(14):763–768. [DOI] [PubMed] [Google Scholar]
- 11. Ge P, Cao L, Chen X, Jing R, Yue W. miR-762 activation confers acquired resistance to gefitinib in non-small cell lung cancer. BMC Cancer. 2019;19(1):1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou R, Yang Z, Wang S, et al. miR-762 can negatively regulate menin in ovarian cancer. Onco Targets Ther. 2017;10:2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Huang R, Wang L, et al. microRNA-762 promotes breast cancer cell proliferation and invasion by targeting IRF7 expression. Cell Prolif. 2015;48(6):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S, Zhang JY, Sun LS, et al. miR-762 promotes malignant development of head and neck squamous cell carcinoma by targeting PHLPP2 and FOXO4. Onco Targets Ther. 2019;12:11425–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao F, Chen S, Sun M, et al. MiR-467a is upregulated in radiation-induced mouse thymic lymphomas and regulates apoptosis by targeting Fas and Bax. Int J Biol Sci. 2015;11(1):109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu T, Lu Q, Liu J, et al. Circular RNA FAM114A2 suppresses progression of bladder cancer via regulating ΔNP63 by sponging miR-762. Cell Death Dis. 2020;11(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin G, Sheng H, Xie H, et al. circLPAR1 is a novel biomarker of prognosis for muscle-invasive bladder cancer with invasion and metastasis by miR-762. Oncol Lett. 2019;17(3):3537–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76(13):3666–36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353(17):1768–17671. [DOI] [PubMed] [Google Scholar]
- 20. Yao Q, Wang X, He W, et al. Circulating microRNA-144-3p and miR-762 are novel biomarkers of Graves’ disease. Endocrine. 2019; 65(1):102–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_Table_1_(3) for Identification of Circulating miR-762 as a Novel Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer by Lei Chen, Yunxia Li and Jingshu Lu in Technology in Cancer Research & Treatment
Supplemental Material, Supplementary_Table_2 for Identification of Circulating miR-762 as a Novel Diagnostic and Prognostic Biomarker for Non-Small Cell Lung Cancer by Lei Chen, Yunxia Li and Jingshu Lu in Technology in Cancer Research & Treatment