Abstract
Background:
People with neurological disorders are found to have abnormal resting-state functional connectivity (rsFC), which is associated with the persistent functional impairment found in these patients. Recently, transcranial direct current stimulation (tDCS) has been shown to improve rsFC, although the results are inconsistent.
Objective:
We hope to explore whether tDCS induces rsFC changes among patients with neurological disorders, whether rsFC is clinically relevant and how different tDCS parameters affect rsFC outcome among these individuals.
Methods:
A systematic review was conducted according to PRISMA guidelines (systematic review registration number: CRD42020168654). Randomized controlled trials that studied the tDCS effects on rsFC between the experimental and sham-controlled groups using either electrophysiological or neuroimaging methods were included.
Results:
Active tDCS can induce changes in both localized (ie, brain regions under the transcranial electrodes) and diffused (ie, brain regions not directly influenced by the transcranial electrodes) rsFC. Interestingly, fMRI studies showed that the default mode network was enhanced regardless of patients’ diagnoses, the stimulation paradigms used or the rsFC analytical methods employed. Second, stimulation intensity, but not total stimulation time, appeared to positively influence the effect of tDCS on rsFC.
Limitations and conclusion:
Due to the inherent heterogeneity in rsFC analytical methods and tDCS protocols, meta-analysis was not conducted. We recommend that future studies may investigate the effect of tDCS on rsFC for repeated cathodal stimulation. For clinicians, we suggest anodal stimulation at a higher stimulation intensity within the safety limit may maximize tDCS effects in modulating aberrant functional connectivity of patients with neurological disorders.
Keywords: Transcranial direct current stimulation, neurological disorders, resting-state functional connectivity, systematic review, functional magnetic resonance imaging, electroencephalography
Introduction
Neurological disorders encompass a wide range of diseases involving abnormalities in the central and peripheral nervous systems.1 These disorders contribute to the leading causes of disability and death, contributing to nearly 12% of global disabilities and approximately 17% of global deaths.2 Although the etiologies of different neurological disorders are inherently distinctive, which results in a great variety of physical/mental dysfunctions, aberrant network functioning in the brain is a common issue that has been associated with the persistence of impairments among these individuals.3
Neural network functioning can be quantified in multiple ways, of which functional connectivity (FC) is one of the indicators commonly used in academia. FC is defined as the statistical dependencies among remote neurophysiological events, which reflects the degree of the nondirectional synchrony between 2 brain regions.4 FC can be broadly categorized into 2 types—task-based FC and resting-state FC (rsFC). Rather than recording brain network activity during performance of a task that engages certain neural processes (eg, motor network recruited during the finger tapping task), measurements of rsFC, which results in the discoveries of resting-state networks,5 are taken when an individual’s task engagement is minimal (eg, eye-closed rest, passive fixation), that is, when the person is “at rest.”6 Due to the great variety of experimental paradigms adopted in different studies, comparing task-based FC between studies could be challenging; rsFC serves as an alternative option that makes a comparison more feasible by minimizing the impact from paradigm designs.7 In terms of measurement, rsFC can be studied with neurophysiological (eg, electroencephalography, EEG) or neuroimaging (eg, functional magnetic resonance imaging, fMRI) methods, which complement the limitations of each other,8 leading to a more holistic understanding of brain functions.9 While EEG rsFC measures the phase synchronization between 2 electrical signals measured by 2 scalp electrodes located in different positions,10 fMRI rsFC is determined by the synchrony of the low-frequency fluctuations in blood-oxygen-level-dependent (BOLD) signals detected in 2 different brain regions.11 To determine the degree of synchrony, there are a number of analytical approaches for each measurement method; while coherence, phase-locking value and graph theory approaches are commonly utilized to evaluate EEG data,12 independent component analysis (ICA), seed-based FC analysis and graph analysis are widely adopted in the fMRI research world.13
A growing body of evidence indicates that rsFC is closely associated with specific neural processes that support motor, cognitive and perceptual functions,14 which also predicts recovery from diseases as well as success in one’s daily life functioning.15 People with neurological disorders who manifest different types and levels of functional impairments are found to have abnormal rsFC when compared to their healthy counterparts, which has also been shown to correlate with the severity of signs and symptoms. For instance, from a meta-analysis studying rsFC among people with depression when compared to healthy controls, patients were found to have a hypoconnected brain network for cognitive control and attention and a hyperconnected network for self-referential thoughts.16 Another meta-analysis showed internetwork hypoconnectivity in patients with schizophrenia when compared to controls.17 Other diagnoses with individuals who manifest different kinds of intra/internetwork rsFC abnormalities include but are not limited to attention-deficit/hyperactivity disorder,18 stroke,19 Parkinson’s disease,20 and fibromyalgia.21 These studies collectively imply that rsFC abnormalities can be identified in many neurological disorders, which are found to be a problem underlying persistent cognitive/motor/perceptual dysfunctions.
In recent decades, increasing efforts have been made regarding the development of treatment techniques that potentially alleviate rsFC abnormalities among people with neurological disorders. One of the potentially promising techniques is transcranial direct current stimulation (tDCS).22 During tDCS application, a weak direct current (1-2 mA) is passed through the brain by connecting a battery with 2 electrodes that are placed over the scalp.23 The transcranial electric current has been found to modulate neuronal excitability in humans.24 Sustained stimulation has been found to result in long-lasting cortical excitability, a phenomenon that resembles the putative neurophysiological mechanism of learning and memory—long-term potentiation.25 Most of the previous electrophysiological and neuroimaging studies have focused on the regional effects of tDCS in both healthy people and those with neurological disorders (eg, epilepsy26 and depression27), showing encouraging results that indicate tDCS can indeed alter neuroplasticity, which correlates with cognitive and motor function improvements.28 With the understanding that brain regions do not act separately but rather are functionally connected, more researchers have started to investigate the effect of tDCS on network connectivity via rsFC in healthy individuals and patients with neurological disorders.29,30
Despite the fact that some studies reported significant changes in rsFC after patients received tDCS,30,31 others reported negative results.32 It has been suggested that interindividual variability in brain structures,22 study designs,33 variations in tDCS stimulation protocols used among different studies34 or even differential analytical methods for rsFC35 could result in inconsistent results between studies. To the best of our knowledge, there is no previously published paper that synthesizes the available studies that investigate tDCS effects on rsFC among people with neurological disorders, leaving these inconsistencies unexplained. Thus, we hope to address 3 questions by conducting a systematic review: (1) whether tDCS induces rsFC changes among patients with neurological disorders, (2) whether these rsFC changes correlate with clinical outcomes (representing the clinical relevance of tDCS effects on rsFC), and (3) how different tDCS parameters affect rsFC outcome among these patients.
Methods
Literature search
This systematic review was performed with guidance from the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)36 and was registered in the International Prospective Register of Systematic Reviews system (PROSPERO; register ID: CRD42020168654). A preliminary search was conducted in early February 2020 to confirm the choice of keywords and electronic databases among all authors. A main literature search for retrieving relevant records was conducted on 18-19 February 2020 with the search terms “transcranial direct current stimulation,” “tDCS,” “functional magnetic resonance imaging,” “fMRI,” “electroencephalography,” and “EEG” from the electronic databases PsycINFO, Scopus, Embase, and Web of Science. No limit was set for the publication dates. Hand searching of the reference lists of the relevant articles was performed to identify additional records.30,31
Study inclusion
Randomized parallel group/crossover trials, which administered active tDCS stimulation in the experimental group and sham tDCS stimulation in the control group, on patients with neurological disorders as defined in The International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10),37 with functional connectivity outcomes measured by either resting-state EEG/fMRI were included in this review. The article screening process could be divided into 3 parts. First, duplicated records were removed. Then, titles and abstracts of the articles were screened; non-English papers, studies without peer-reviewed empirical data (eg, review, conference proceedings, book chapters, and editorial), observational studies (eg, case series, nonrandomized studies, and studies without a sham-tDCS control group), nonhuman studies, studies that did not apply tDCS to patients with any kinds of neurological disorders and studies that did not use tDCS as the sole brain stimulation technique were excluded. Finally, the full texts of the remaining studies were examined for systematic review eligibility. A paper would be included if (1) functional connectivity measures, recorded by either resting-state EEG or fMRI, were reported, (2) the functional connectivity measures were conducted at both baseline and after the treatment, and (3) between-group (ie, active- vs sham-stimulation) comparisons that reflected tDCS effects were reported. The above screening processes were independently conducted by the first author and an experienced research assistant, and their decisions were recorded in separate Excel spreadsheets. When there were discrepancies, the second author made the final decision regarding study inclusion.
Data extraction
After final decisions regarding the paper selection issues were made, the demographic data and experimental and outcome measurement details of the included papers were extracted and entered into an Excel spreadsheet by the first author, which was checked by the second author to minimize errors. Demographic data included the diagnosis, number of participants in each group (N), age, and whether participants were on concurrent medication within the experimental period. Experimental details included the montage placement, tDCS stimulation current intensity (mA), duration of stimulation for each session (minutes), total number of treatment sessions, and concurrent task accompanied during tDCS stimulation. Outcome measures included the assessments used for measuring the clinical effect, posttreatment between-group difference of fMRI/EEG rsFC data (or between-group contrast showing changes of rsFC from pretreatment to posttreatment) and the description/statistics showing the association between the change in rsFC measures and the change in clinical outcomes. Electronic mail was sent to the corresponding authors to ask for additional information/clarification if the data to be extracted were not complete.
Data coding and synthesis
Extracted data were coded by the first author and then checked and confirmed by the second author. Participant age was recoded into 3 age groups (adolescent, adult, elderly), and montage placement was coded as an addition variable, “stimulation type,” according to a previously proposed framework.38 We coded the “total stimulation time” by multiplying the total number of sessions by the stimulation time of each session, while the current density at the electrode (A/m2), which is a widely adopted parameter in animal research representing the current applied to the participants,39 was calculated by dividing the current intensity by the electrode’s surface area. Based on the purposes of the clinical assessments used by each study, the clinical outcome would be categorized into 3 categories—symptomatic relief, enhancement of specific/global neurological function, and description/statistics showing the association between the change in rsFC and the change in clinical outcome recoded to be a dichotomous outcome (yes/no). For fMRI peak coordinates, recoding according to MNI space would be done if Talairach coordinates were given, followed by categorizing these brain regions within a priori rsFC networks (at a resolution of 400 parcels) defined by a local-global network parcellation in 1489 subjects.40
In view of the marked diversity in the subdiagnoses under the branch of neurological disorders, as well as that regarding the rsFC analytical methods, meta-analysis was not planned. To answer whether tDCS induces rsFC changes, we provided a narrative synthesis of the results, supported by a forest plot that provides readers with a visual impression of the data. The effect size calculation and generation of the forest plot were performed using Comprehensive Meta-Analysis (CMA; Biostat, Englewood, NJ) software. Effect sizes for coordinate-based fMRI rsFC studies would be calculated for all reported T-values of the peak coordinates/P-values for all reported clusters. For the remaining studies, a combination of test statistics (eg, f-values, t-values, P-values) was used.41 For studies that presented non-parametric statistics, effects sizes would be calculated manually according to the previously published formulae.41,42 For studies that did not report a pre-post correlation while reporting pre/posttreatment between-group contrast, a conservative estimation of pre-post correlation of r = .7 would be used.43,44 If test statistics were unable to be obtained after contacting the corresponding authors but the results were described in text, nonsignificant results would be assumed to have P-values of .5 (1-tailed) and significant results were assumed to have P-values of .0545; these P-values were chosen as we opted to keep our estimation as conservative as possible. The forest plot was generated with the Hedges’s g effect size with 99% confidence intervals. A follow-up narrative synthesis would summarize whether the documented rsFC changes correlate with the clinical outcomes. To explore the effects of different tDCS parameters on rsFC outcomes for continuous data (ie, stimulation time and current density), we conducted meta-regressions with CMA software by plotting these parameters against g. For rsFC changes for discrete data (ie, stimulation type), narrative syntheses would be given. The risk of bias in individual studies was assessed by the Cochrane Collaboration’s tool.46 Publication bias was evaluated by visual inspection of the funnel plot,47 with a small-study effect evaluated with Egger’s test.48
Results
Study selection
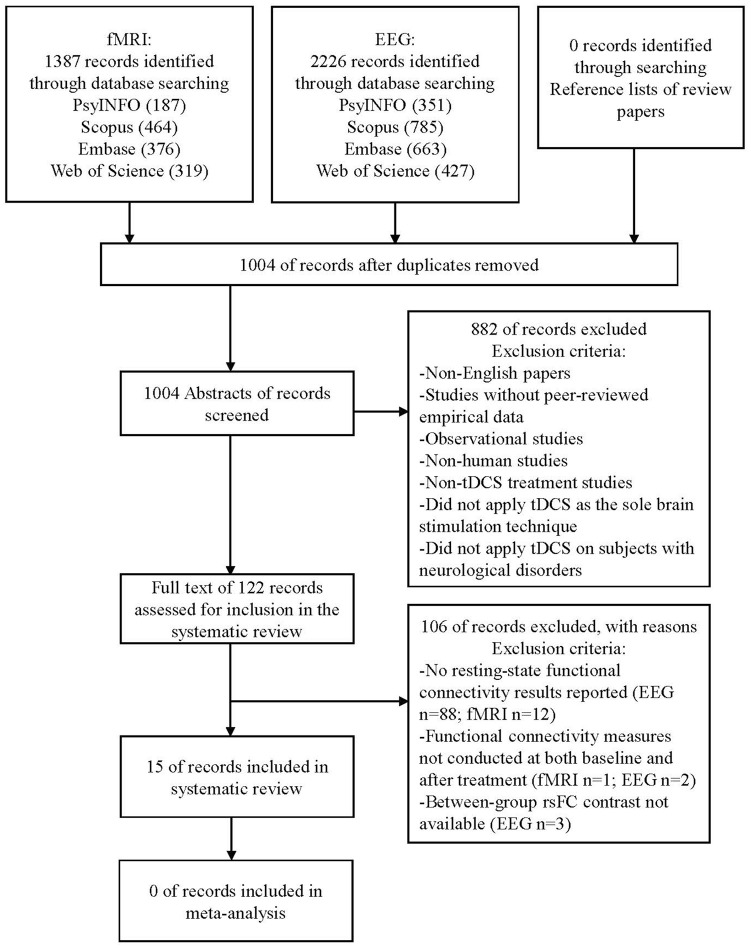
A total of 15 studies were included in this review. The electronic database search yielded a total of 3613 neurophysiological and neuroimaging studies (2226 EEG and 1387 fMRI records, respectively), with 2609 records remaining for abstract screening after the removal of 1004 duplicated records. A total of 2488 studies were excluded after exclusion criteria were applied at this stage. The full text of 121 records was further assessed for inclusion in the systematic review. One hundred studies were excluded as the primary outcome of our systematic review (ie, resting-state functional connectivity) was not reported in the papers (88 EEG and 12 fMRI records, respectively); 3 other studies were excluded because the primary outcome measure was not conducted at both baseline and after treatment. Between-group rsFC contrasts could not be obtained from 3 studies, and they were also excluded from the review. See Figure 1 for the diagram illustrating the article screening procedure and Supplemental Table A for the details of the 106 records excluded during full-text screening.
Figure 1.
Flowchart of the article screening process.
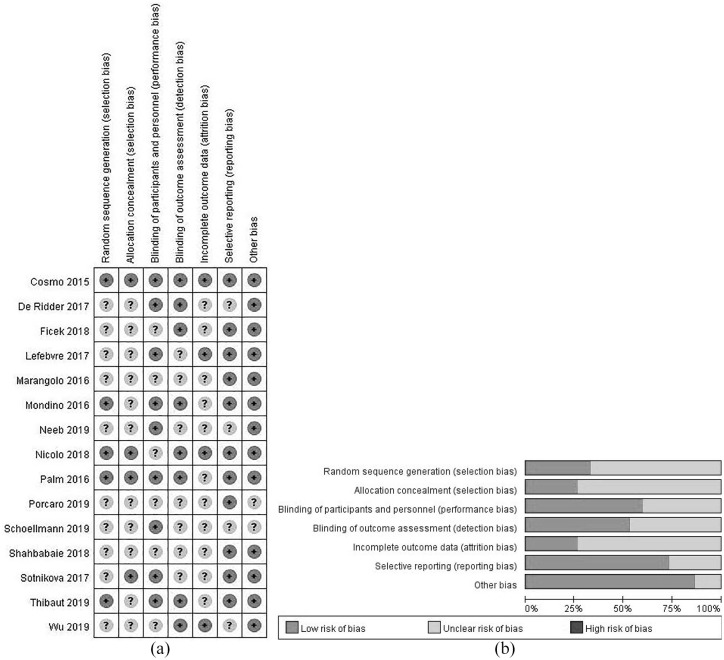
Risk of bias within studies
A majority of studies showed unclear bias in random sequence generation, allocation concealment, incomplete outcome data. More than half of the studies adopted blinding procedures during treatment administration and outcome assessments. Although most of the studies reported both significant and non-significant results of between- and within-group comparisons, 4 studies did not report their data as planned in the methods section. Figure 2a displays the risk of bias items presented as percentages across all included studies, and Figure 2b shows the risk of bias summary for each included study. Supplemental Table B presents all of the authors’ judgment details for each study.
Figure 2.
(a) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies. Note that an item was rated “+” (ie, low risk of bias) only when the measure taken to prevent certain risk of bias was clearly stated in the paper; when evidence/quotes or explanation were not available in the text or after clarifying with the authors of the included studies, a rating of “?” (ie, unclear risk of bias) was given and (b) review authors’ judgments about each risk of bias item for each included study. Note that “other bias” was defined as other sources of risk on top of selection, performance, detection, attrition, and reporting biases. Specifically, carry-over effects in cross-over trials and baseline imbalance between groups were considered as “other bias.”
Study characteristics
The included RCTs involved 348 patients in total from different age groups who suffered from a broad range of neurological disorders, including fibromyalgia,49 inflammatory bowel disorder50 and neuropsychiatric disorders (ie, schizophrenia,51,52 substance abuse53), neurodegenerative disorders (ie, multiple sclerosis,54 Parkinson’s disease,55 primary progressive aphasia56), neurodevelopmental disorders (ie, attention-deficit/hyperactivity disorder57,58), stroke,59,60 and disorders of consciousness.61 These studies adopted a great variety of tDCS protocols and adopted a variety of rsFC analytic methods. A brief summary of the demographic, experimental and outcome measurement details of individual studies is shown in Table 1.
Table 1.
Summary of the demographic, experimental, and outcome measurement details of the included studies.
| Reference | Study design | Participants characteristics | Experimental details | Outcome measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurological diagnosis | N | Age group | Concurrent medication | Stimulation type | Total stimulation time (min) | Current density (A/m2) | Concurrent task | Mean washout period (d) | Clinical outcome (description) | rsFC outcome | ||
| De Ridder and Vanneste49 | Parallel | Fibromyalgia | A:19 | Adult | Yes | Bilateral | 300 | 0.429 | Nil | N/A | Symptomatic relief (pain perception) | EEG phase lagged index |
| S:12 | ||||||||||||
| Neeb et al50 | Parallel | Inflammatory bowel disorder | A:24 | Adult | Yes | Anodal | 100 | 0.571 | Nil | N/A | fMRI whole-brain rsFC | |
| S:12 | ||||||||||||
| Porcaro et al54 | Crossover | Multiple sclerosis | 13 | Adult | No | Anodal | 75 | Varied | Nil | Not stated | Symptomatic relief (fatigue perception) | EEG mutual information |
| Mondino et al51 | Parallel | Schizophrenia | A:11 | Adult | Yes | Cathodal | 200 | 0.571 | Nil | N/A | Symptomatic relief (auditory hallucination) | fMRI seed-whole brain rsFC |
| S:12 | ||||||||||||
| Palm et al27 | Parallel | A:10 | Adult | Yes | Anodal | 200 | 0.571 | Nil | N/A | Symptomatic relief (psychiatric symptoms) | ||
| S:10 | ||||||||||||
| Shahbabaie et al53 | Crossover | Substance abuse | 15 | Adult | No | Bilateral | 20 | 0.571 | Nil | 7 | Symptomatic relief (drug craving intention) | fMRI whole-brain rsFC |
| Sotnikova et al57 | Crossover | ADHD | 13 | Adolescent | Yes | Anodal | 20 | 0.769 | n-back task | 14 | Enhancement of specific neurological function (working memory performance) | fMRI seed-whole brain rsFC |
| Cosmo et al58 | Parallel | A:30 | Adult | Not stated | Bilateral | 20 | 0.286 | Nil | N/A | N/A (only rsFC outcome measure were planned) | Weighted node degree | |
| S:30 | ||||||||||||
| Schoellmann et al55 | Crossover | Parkinson’s disease | 10 | Elderly | No | Anodal | 20 | 0.270 | Nil | Not stated | Enhancement of specific neurological function (motor function) | Imaginary corticocortical coherence |
| Marangolo et al62 | Crossover | Stroke-induced aphasia | 9 | Adult | No | Bilateral | 300 | 0.571 | Syllable/word pronunciation training | 14 | Enhancement of specific neurological function (motor speech ability of trained items) | fMRI Eigenvector centrality |
| Ficek et al56 | Parallel | Primary progressive aphasia | A:12 | Elderly | No | Anodal | 300 | 0.800 | Oral/written naming task training | N/A | Enhancement of specific neurological function (oral/written naming ability for trained and untrained items) | fMRI seed-seed rsFC |
| S:12 | ||||||||||||
| Lefebvre et al59 | Crossover | Stroke | 22 | Elderly | Not stated | Bilateral | 30 | 0.286 | Motor skill learning | N/A | Enhancement of specific neurological function (execution of learnt motor skill) | fMRI seed-whole brain rsFC |
| Nicolo et al60 | Parallel | A:12 | Elderly | Not stated | Bilateral | 200 | 0.286 | Nil | N/A | Enhancement of specific neurological function (upper limb function) | Weighted node degree | |
| S:12 | ||||||||||||
| Thibaut et al61 | Crossover | Disorders of consciousness | 14 | Adult | Yes | Bilateral | 20 | Unknown | Nil | 2 | Enhancement of specific neurological function (spasticity) | EEG phase lagged index |
| Wu et al63 | Parallel | A:5 | Adult | Yes | Anodal | 200 | 0.571 | Nil | N/A | Enhancement of global neurological function | Coherence | |
| S:5 | ||||||||||||
Abbreviations: A, active-tDCS; ADHD, attention-deficit/hyperactivity disorder; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; N, number of participants; N/A, not applicable; rsFC, resting-state functional connectivity; S, sham-tDCS.
Effects of tDCS on inducing changes in rsFC and its clinical relevance
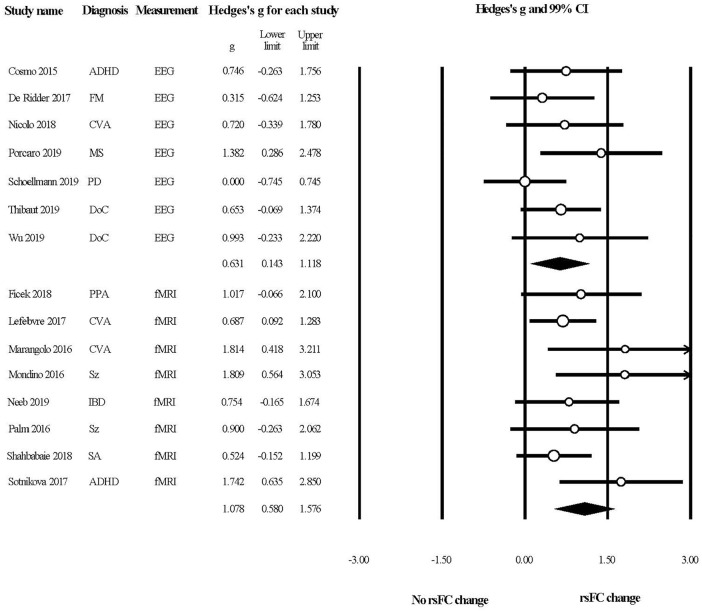
Figure 3 provides the overall summary of the included studies with a forest plot. All of the effect sizes were computed with the reported test statistics/P-values except for 4 studies,49,52,53,63 hypothetical P-values were assigned for these studies as planned. The plot indicated that tDCS could induce rsFC changes in patients with neurological disorders, as evident from neuroimaging and neurophysiological studies.
Figure 3.
A forest plot summarizing the effects of tDCS in modulating rsFC in patients with neurological disorders.
Abbreviations: ADHD, attention deficit/hyperactivity disorders; CVA, cerebral vascular accident; DoC, disorders of consciousness; EEG, electroencephalography; FM, fibromyalgia; fMRI, functional magnetic resonance imaging; IBD, inflammatory bowel disorder; MS, multiple sclerosis; PD, Parkinson’s disease; PPA, primary progressive aphasia; SA, substance abuse; Sz, schizophrenia.
The results from electrophysiological studies (Table 2) showed that rsFC near electrodes could be modulated by tDCS. Thibaut et al61 reported an increase in rsFC between C3 and C4 electrodes (where the cathodes were placed), as well as between C4 (cathode placement) and F3 (anode placement) among patients with disorders of consciousness with multichannel bilateral stimulation. Similar changes could be found in the study conducted by Nicolo et al60 on stroke patients, who reported an increase in the weighted node degree of C3/4 (cathode placement) for the beta, but not the alpha frequency band. However, negative results were reported by Cosmo et al58 in which stimulation was applied to adults with ADHD over the dorsolateral prefrontal cortex. The local rsFC showed nonsignificant changes after active tDCS when compared to the sham group although this study was of the largest sample size (N = 30 for both the active and sham groups) among all included studies.
Table 2.
Summary of resting-state functional connectivity results from the functional magnetic resonance imaging (fMRI) studies.
| Reference | Significant changes in primary clinical outcome? | rsFC changes correlated with primary clinical outcome? | Montage placement | Brain regions reported significant rsFC differences (t-test FWE corrected P < .05) between active and sham tDCS (classified in terms of resting-state network and direction of effect) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual | Somatomotor | Dorsal attention | Ventral attention | Limbic | Frontoparietal | Default | ||||||||||||
| Anode | Cathode | A > S | S > A | A > S | S > A | A > S | S > A | A > S | S > A | A > S | S > A | A > S | S > A | A > S | S > A | |||
| Lefebvre et al59 | Yes | Yes | Ipsi-lesional M1 | Contra-lesional M1 | L/R PMC | L/R ACC | ||||||||||||
| Marangolo et al62 | Yes | Yes | Ipsi-lesional IFG | Contra-lesional IFG | L/R PMC cerebllm | R SMA, L MFG, cerebllm | L ACC, L Precuneus, R MPFC | |||||||||||
| Ficek et al56 | Yes | Yes | L IFG | R cheek | L MFG | L MTG | ||||||||||||
| Mondino et al51 | Yes | Yes | L DLPFC | L Temporo-parietal junction | R IFG, insula | L AG, precuneus | ||||||||||||
| L DLPFC | ||||||||||||||||||
| Neeb et al50 | Yes | n.r. | L/R M1 | L/R supra-orbital region | L/R FG | L/R post-CG | L/R insula | R ACC, L/R PCC, L MTG | ||||||||||
| Palm et al27 | Yes | No | L DLPFC | R supra-orbital region | L MTG | |||||||||||||
| Sotnikova et al57 | Yes | n.r. | L DLPFC | Cz | L/R MFG | R AG, ACC, L ITG | ||||||||||||
| Shahbabaie et al53 | Yes | Yes | R DLPFC | L DLPFC | R insula, L IFG | L/R LG, R precuneus, L MTG, R PCC, L/R MPFC, L/R ACC, L/R OFC | ||||||||||||
Abbreviations: A, active tDCS; ACC, anterior cingulate cortex; AG, angular gyrus; cerebllm, cerebellum; Cz, labelling in the EEG 10 to 20 system; DLPFC, dorsolateral prefrontal cortex; FG, fusiform gyrus; FEW, family-wise error; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; L, left; M1, primary motor cortex; MFG, middle frontal gyrus; MPFC, medial prefrontal gyrus; MTG, middle temporal gyrus; n.r., not reported; PCC, posterior cingulate cortex; PMC, premotor cortex; post-CG, postcentral gyrus; R, right; rsFC, resting state functional connectivity; S, sham tDCS; SFG, superior frontal gyrus.
Changes in rsFC are also shown within the brain regions that are functionally connected with the stimulated region. An EEG study by Porcaro et al54 which applied tDCS to individuals with multiple sclerosis over the somatosensory region using a personalized anode, reported an increase in interhemisphere rsFC over the bilateral primary motor cortex. For fMRI studies (Table 3), when electrodes are placed over the primary motor cortex (M1), the rsFC of the somatomotor network, comprising brain regions such as the premotor cortex and the postcentral gyrus that are functionally connected to M1, increases50,59; when electrodes are placed over the inferior frontal gyrus,56,62 a brain region within the dorsal attention network, rsFC increased in the dorsal attention network.
Table 3.
Summary of resting-state functional connectivity results from the electroencephalography (EEG) studies.
| Reference | Significant changes in primary clinical outcome? | rsFC changes correlated with primary clinical outcome? | Montage placement | Between-group contrast in rsFC | |||||
|---|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | Delta | Theta | Alpha | Beta | Gamma | |||
| Nicolo et al60 | No | No | Ipsilesional supraorbital region | Contralesional M1 | n.r. | n.r. | Weighted node degree of M1: n.s. | Weighted node degree of M1: A > S (t24 = 2.78, P = .01) | n.r. |
| De Ridder and Vanneste49 | Yes | Yes | L occipital lobe | R occipital lobe | n.s. | n.s. | n.s. | A > S (t-value n.r.; P < .05) | A > S (t-value n.r.; P < .05) |
| Cosmo et al58 | N/A | N/A | L DLPFC | R DLPFC | Whole-brain weighted node degree: n.s. (U = 319.00; P = .92) | ||||
| Porcaro et al54 | Yes | Yes | Primary sensory cortex | Occipital lobe | Treatment (pre,post) × condition (real,sham): F(1,11) = 13.235, P = .004 | ||||
| Post-hoc t-test: increase of FC between L and R M1 was significant (P = .0002), while remained non-significant for sham (P = .274) | |||||||||
| Schoellmann et al55 | Yes | n.r. | L primary motor cortex | R supraorbital region | n.r. | n.r. | n.r. | n.s. (t-value n.r.; P > .05) | n.r |
| Thibaut et al61 | Yes | n.r. | Bilateral DLPFC | Bilateral M1 | n.s. | n.s. | n.s. | A > S; C3 to C4 (t27 = 2.56, P = .02) | n.r. |
| A > S; C4 to F3 (t27 = 2.30, P = .04) | |||||||||
| Wu et al63 | No | n.r. | L DLPFC | R supraorbital region | A > S (t-value n.r.; P < .05) | ||||
| R DLPFC | L supraorbital region | n.s. | |||||||
Abbreviations: A, active-tDCS; DLPFC, dorsolateral prefrontal cortex; F3, labeling in the EEG 10 to 20 system; L, left; M1, primary motor cortex; n.r., not reported; n.s., non-significant; N/A, not applicable; R, right; rsFC, resting state functional connectivity; S, Sham-tDCS.
Notably, rsFC modulation was found in brain networks that were not functionally connected to the brain regions directly under the scalp electrodes. Notably, all of the included fMRI studies induced rsFC changes to the default mode network (DMN; Table 3) regardless of the mode of stimulation and electrode placements. Another study conducted by De Ridder and Vanneste49 reported an increase in lagged phase synchronization specifically at the beta and gamma frequency bands over the pregenual and dorsal anterior cingulate cortex when electrodes were placed over the bilateral occipital lobes for patients with fibromyalgia. Studies that applied tDCS over the dorsolateral prefrontal cortex, a brain region that has been considered to be part of the ventral/dorsal attention network, showed a more diverse pattern of rsFC modulation. For instance, Sotnikova et al57 found that the dorsal attention network was modulated in patients with ADHD, while the ventral attention network was modulated in patients with schizophrenia and substance abuse.51,53
At the whole-brain level, Wu et al63 identified an increase in EEG coherence after applying stimulation over the left dorsolateral prefrontal cortex among 5 patients with disorders of consciousness. In contrast, another study of a larger sample size conducted by Schoellmann et al55 found no significant differences between active and sham tDCS groups in rsFC, as indicated by imaginary corticocortical coherence across beta frequency bands among patients with Parkinson’s disease.
Regarding the correlation between rsFC changes and clinical outcomes, 14 studies involved clinical assessments as their outcome measures (Table 1), with 7 out of 14 studies reporting a significant correlation between rsFC changes and clinical outcomes, 2 out of 14 of studies reporting a nonsignificant correlation, and 5 out of 14 studies did not report such correlation as such analysis was not planned in their studies (Tables 2 and 3).
Effects of different tDCS parameters on changes in rsFC
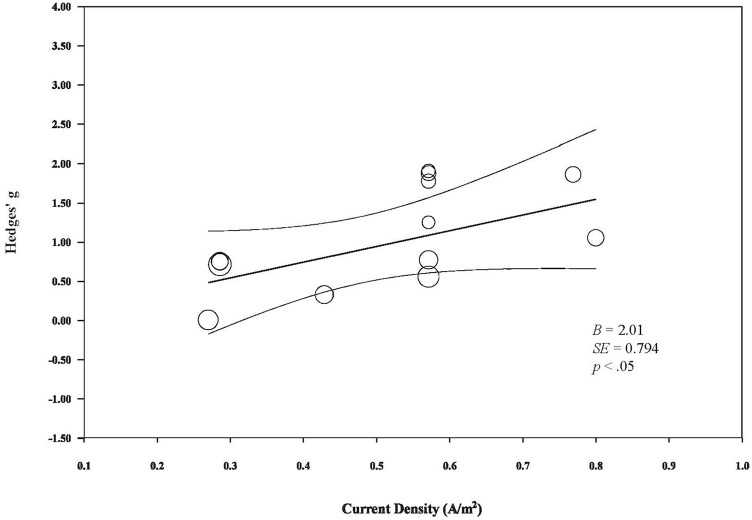
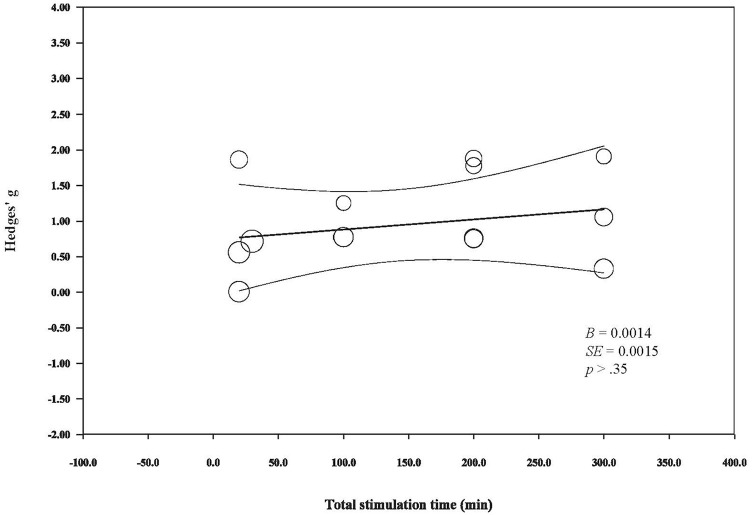
Meta-regressions reveal a statistically significant, positive relationship between current density and changes in rsFC (B = 2.01, SE = 0.794, P < .05; Figure 4). Meanwhile, total stimulation does not appear to modulate changes in rsFC, as indicated by a non-significant correlation between these 2 factors (B = 0.0014, SE = 0.0015, P < .35; Figure 5). Of note, due to the limited number of studies available and the marked heterogeneity across studies, these observations should be interpreted with caution.
Figure 4.
The relationship between tDCS current density and its modulatory effects on rsFC.
Figure 5.
The relationship between tDCS total stimulation time and its modulator effects on rsFC.
Regarding the effects of stimulation type on rsFC, both anodal and bilateral stimulation were found to enhance rsFC, while cathodal stimulation resulted in mixed findings. For instance, when cathodal stimulation was given to patients with schizophrenia, the rsFC of the DMN increased and rsFC in the ventral attention network was reduced. However, the beta coherence was found to be higher than that of the sham-stimulated group in another study.60
Risk of bias across studies
The funnel plot (Figure 6) showing the effect sizes of both EEG and fMRI studies plotted against standard error was asymmetric, which illustrated the presence of publication bias. Egger’s test was highly significant (P < .01, 2-tailed), which was a clear indication of a small-study effect.
Figure 6.
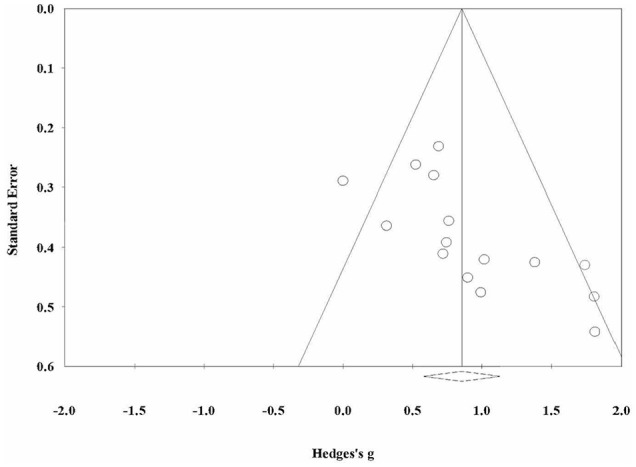
A funnel plot for the inspection of publication bias across studies.
Discussion
This systematic review aimed to investigate the effectiveness of active tDCS in modulating resting-state functional connectivity among individuals with neurological disorders. By conducting a comprehensive literature search using multiple electronic databases and performing manual searches in reference lists of previously published papers, 15 RCTs with either crossover or parallel group designs, comparing active tDCS with sham stimulation, that also reported between-group resting-state functional connectivity results were included. In conclusion, this systematic review indicated 2 main points. First, the currently available neurophysiological and neuroimaging studies provided some evidence that active tDCS can induce changes in both localized (ie, brain regions under the transcranial electrodes) and diffused (ie, brain regions not directly stimulated by the transcranial electrodes) rsFC. Interestingly, fMRI studies showed that the DMN was enhanced regardless of patients’ diagnoses, the stimulation paradigms used or the rsFC analytical methods utilized. Second, stimulation intensity, but not duration, appeared to positively influence the effect of tDCS on rsFC.
Effects of tDCS on functional connectivity
Despite the great heterogeneity in the rsFC analytical methods used among studies included in this review, the data provided converging evidence that tDCS is effective in modulating rsFC in patients suffering from various kinds of neurological disorders. For instance, rsFC was not only found to be modulated in the local brain network where the electrodes were placed, but a network that encompasses brain regions not directly stimulated by the electrodes also exhibited alterations in rsFC. Consistent with previous studies that investigated the rsFC effects of tDCS in healthy individuals,64 we demonstrated that tDCS is capable of inducing network-based effects in patients with various kinds of neurological disorders. Given that many neural functions are supported by functional networks rather than a particular brain region29 and functional impairments among people with neurological disorders are usually found to be influenced by the disordered connectivity,16 the network modulatory effects engendered by tDCS can serve as an important rationale that supports the clinical use of such neurostimulation techniques. Regarding the clinical relevance of rsFC, of the 9 papers that reported relevant data for analysis, the majority of studies showed a significant correlation between rsFC and clinical outcome changes. This observation lays a preliminary foundation for future studies to regard tDCS as a clinically relevant and effective technique for the betterment of patients with neurological disorders.
Possible physiological and biophysical mechanisms of tDCS in functional connectivity modulation
As evident from the EEG studies, enhancement in local rsFC (ie, rsFC confined to the stimulated brain regions only) induced by anodal tDCS were particularly reflected in the higher frequency bands (ie, beta and gamma) but not in the lower (ie, delta, theta, alpha) frequency bands, in which these findings are in line with the results from previous studies in healthy individuals during task-based FC studies65 and animal studies.66 The rsFC enhancement particularly at the higher frequency bands might be an important finding supporting the effects of anodal tDCS on local inhibitory network activity. Previous study reported that anodal tDCS applied over the primary motor cortex reduce local gamma-aminobutyric acid (GABA) concentration67 over the stimulated brain region, of which GABA concentration was negatively correlated with rsFC in the somatomotor network.35,68 Interestingly, synaptic activities at the GABA type A (GABAA) receptors was found to be positively associated with beta peak frequency69 and gamma power,70 but not for other frequency bands, in which both beta/gamma peak frequency71 and gamma power72 around the anodal electrode have been found to be reduced by tDCS over the primary motor cortex. Importantly, our results further show that the rsFC enhancement effect by anodal tDCS does not limit to studies that applied anodal stimulation over the primary motor cortex but those applying tDCS over other brain regions (ie, prefrontal/occipital cortex). These studies and our results collectively postulate that, anodal tDCS suppresses local inhibitory synaptic network activity to bring about network-level changes (ie, enhancement in rsFC). Further studies are warranted to validate the relationships between GABA synaptic activity, beta/gamma oscillatory power/frequency, rsFC and anodal tDCS applied beyond the primary motor cortex, the negative association between beta/gamma power and peak frequency with beta/gamma functional connectivity, as well as to investigate the physiological mechanisms of cathodal tDCS stimulation.
Our review presented studies showing enhancements in the resting-state networks that were functionally connected to the stimulated brain region. For instance, stimulation in the primary motor cortex resulted in changes in rsFC within the somatomotor network,54 while stimulation at the IFG resulted in changes in the dorsal attention network.51 In line with the GABA hypothesis, increasing evidence, although limited to the somatomotor network, has shown that tDCS could enhance the connectivity of highly correlated brain regions that were associated with the local GABA reduction at the stimulated site.64,73 Surprisingly, all of the included studies showed the enhancement of the DMN, a resting-state network that has been found to be anti-correlated with task-based networks such as the somatomotor and ventral/dorsal attention networks,74 regardless of the differences in tDCS protocol and patients’ diagnoses between studies. It might be possible that local beta/gamma oscillation enhancement modulates long-range functional connectivity.75 It might also be possible that tDCS induces consistent electric fields not only limited to the coverage of the electrodes but also far from the electrodes. A computer simulation study that estimated the electrical field changes for different montage placements also indicated that the medial brain structures, where most of the key DMN regions are situated,40,76 exhibited consistent strength and direction of the electric field regardless of the mode of stimulation and electrode placements whether it was placed at C3, C4, F3, or F4.77 Furthermore, a recently published study has shown that tDCS-induced changes in rsFC within and between networks appear to be related to the electric field strength over the targeted brain regions.73 Therefore, it is conceivable that that the neuromodulatory effects on DMN may be results of the electrical fields generated in the brain by tDCS over the intended target. Future studies investigating spatial specificity are needed to verify this speculation and to elucidate the complex interaction between electrical field intensity and modulation of rsFC by tDCS.
Factors contributing to the differential tDCS rsFC effects
We inspected how current density and stimulation time affect tDCS rsFC outcomes by conducting meta-regressions and showed that current density, but not total stimulation time positively and linearly affect the rsFC effect by tDCS. In line with the discussion that the electric field is associated with the observed rsFC effects, given that the current density is directly proportional to the electric field as defined by Ohm’s law (, where J represents the current density, E represents the electrical field and represents a material’s conductivity), our observation from the scattered plot, that stimulation intensity positively correlate with rsFC change, is theoretically valid. However, a previous study has shown that individuals respond to tDCS differently given the same current intensity,78 implying that the relationship between the electric field and current density can be subject to interindividual variability (eg, thickness of cortical bone, cortical folding22) and might not be purely linear. Regarding stimulation time and rsFC, our observation echoed a behavioral study showing that repetitive tDCS might not outperform single-session tDCS in terms of memory enhancement.79 Although it might be possible that total stimulation might not modulate, at least linearly, tDCS effects, a previous study has shown that the electrode size might play a role in modulating the stimulation effect.80 For instance, it was reported the cumulative increase in cortical excitability was seen only in participants stimulated with a larger, but not a smaller electrode in their study. In addition, by qualitative analysis, mixed results were seen when cathodal stimulation was used, although we cannot draw any conclusion because only 2 studies were available. It would be encouraging to revisit the effects of stimulation type on rsFC effects regarding cathodal stimulation when more studies are available.
Limitations
As planned, we conducted a systematic review to summarize the current research trends in EEG and fMRI research that investigates the effects of tDCS on functional connectivity among people with neurological disorders. However, some limiting factors hinder us from giving a firm conclusion regarding the effects of tDCS in modulating rsFC. First, it should be noted that the number of previously published papers relevant to this topic, as well as for the respective brain diseases, is limited. Second, the marked heterogeneity among available studies, including differences in clinical diagnoses and gender of participants, variations in study outcomes and methodological issues such as tDCS protocol, could mediate patients’ responses to tDCS. Third, as our understanding of neurological disorders are still evolving, the etiology of some disease groups included in our review (ie, inflammatory bowel disorder50) might not be considered strictly neurological. Further empirical studies investigating tDCS effects on rsFC in brains with specific neurological diseases, how gender difference mediates tDCS effects and how various tDCS treatment protocols modulate the brains differentially, are warranted to address these questions; due to the inherent variations in brains with various neurological disorders, further studies on healthy brains will also be helpful for us to understand the modulatory effects of tDCS on rsFC. In addition, the possible underestimation of the effect sizes for some studies in which P-values could only be assumed for the calculation of effect sizes. We have taken several measures to minimize such effects and to avoid misinterpretation. For instance, we did not attempt to provide a summary estimate of the effect; the forest plot we presented showed the maximum confidence intervals (ie, 99%) of g for each study. Moreover, incomplete reporting of some of the primary studies may pose another limitation. Although we have contacted the authors of eligible studies to obtain additional information on unreported or missing data, some research data remained unavailable for our analyses. Specifically, between-group rsFC differences were not reported in 3 of the primary studies, we had to exclude these studies as a result. Additionally, the analysis for clinical relevance was not completed as over 30% of reports omitted the necessary data. To scrutinize this issue, we suggest that future studies can minimize the risks of bias by reporting both significant and nonsignificant results as planned in the methods, as well as investigate and report the brain-behavior relationship, which is particularly essential for the development of advanced and effective evidence-based treatments for the betterment of this group of patients.
Conclusion
To the best of our knowledge, this is the first systematic review that investigates the tDCS effects on rsFC among patients with neurological disorders. Here we provide some evidence that tDCS can enhance rsFC, which is correlated with functional enhancements. Preliminary observations also support that higher current density contribute to greater rsFC changes. We recommend that future studies could investigate the effect of tDCS on rsFC, especially for cathodal stimulation. For clinicians, we suggest that anodal stimulation at a higher stimulation intensity, which is within the safety limit, might maximize tDCS effects in modulating aberrant functional connectivity, a shared problem among patients with different neurological diagnoses that contribute to the manifestations of their physical/cognitive dysfunctions.
Supplemental Material
Supplemental material, sj-docx-1-cns-10.1177_1179573520976832 for The Effect of Transcranial Direct Current Stimulation in Changing Resting-State Functional Connectivity in Patients With Neurological Disorders: A Systematic Review by Melody MY Chan and Yvonne MY Han in Journal of Central Nervous System Disease
Supplemental material, sj-docx-2-cns-10.1177_1179573520976832 for The Effect of Transcranial Direct Current Stimulation in Changing Resting-State Functional Connectivity in Patients With Neurological Disorders: A Systematic Review by Melody MY Chan and Yvonne MY Han in Journal of Central Nervous System Disease
Acknowledgments
We thank Miss Kris Chan, Mr Marco Cheng, Mr Alex Chu, Mr Paul Hui, Mr Eddie Li, and Ms Michelle Cheung for their efforts in supporting the study inclusion and data extraction processes.
Footnotes
Author Contributions: MC designed the study, performed literature search and screening, conducted statistical analysis, interpreted the data and drafted the manuscript. YH contributed to the conception of the study and assisted with study inclusion, data extraction and data interpretation. All authors read and approved the final manuscript.
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partly supported by the Health and Medical Research Fund [HMRF06173096] from the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region.
Declaration of conflicting interests:The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Melody MY Chan  https://orcid.org/0000-0001-9493-0134
https://orcid.org/0000-0001-9493-0134
Supplemental material: Supplemental material for this article is available online.
References
- 1. World Health Organization. Neurological Disorders: Public Health Challenges. World Health Organization; 2006. [Google Scholar]
- 2. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raj A, Powell F. Models of network spread and network degeneration in brain disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:788-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13-36. [DOI] [PubMed] [Google Scholar]
- 5. Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832-837. [DOI] [PubMed] [Google Scholar]
- 7. Hermundstad AM, Bassett DS, Brown KS, et al. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proc Natl Acad Sci. 2013;110:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huster RJ, Debener S, Eichele T, Herrmann CS. Methods for simultaneous EEG-fMRI: an introductory review. J Neurosci. 2012;32:6053-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rojas GM, Alvarez C, Montoya CE, de la Iglesia-Vayá M, Cisternas JE, Gálvez M. Study of resting-state functional connectivity networks using EEG electrodes position as seed. Front Neurosci. 2018;12:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. da Silva FL. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112-1128. [DOI] [PubMed] [Google Scholar]
- 11. Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-541. [DOI] [PubMed] [Google Scholar]
- 12. Bastos AM, Schoffelen J-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci. 2016;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lv H, Wang Z, Tong E, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol. 2018;39:1390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes JJ, Woolrich MW, Baker K, Colclough GL, Astle DE. Electrophysiological measures of resting state functional connectivity and their relationship with working memory capacity in childhood. Dev Sci. 2016;19:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plitt M, Barnes KA, Wallace GL, Kenworthy L, Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc Natl Acad Sci. 2015;112:E6699-E6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44:168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uddin LQ, Kelly AC, Biswal BB, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249-254. [DOI] [PubMed] [Google Scholar]
- 19. Lv Y, Margulies DS, Cameron Craddock R, et al. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Ann Neurol. 2013;73:136-140. [DOI] [PubMed] [Google Scholar]
- 20. Luo C, Song W, Chen Q, et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiol Aging. 2014;35:431-441. [DOI] [PubMed] [Google Scholar]
- 21. Fallon N, Chiu Y, Nurmikko T, Stancak A. Functional connectivity with the default mode network is altered in fibromyalgia patients. PLoS One. 2016;11:e0159198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polania R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174-187. [DOI] [PubMed] [Google Scholar]
- 23. Higgins ES, George MS. Brain Stimulation Therapies for Clinicians. American Psychiatric Publishing; 2019. [Google Scholar]
- 24. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Physiol J. 2000;527:633-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899-1901. [DOI] [PubMed] [Google Scholar]
- 26. Regner GG, Pereira P, Leffa DT, et al. Preclinical to clinical translation of studies of transcranial direct-current stimulation in the treatment of epilepsy: a systematic review. Front Neurosci. 2018;12:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266:681-694. [DOI] [PubMed] [Google Scholar]
- 28. Allman C, Amadi U, Winkler AM, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8:330re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J ECT. 2018;34:144-152. [DOI] [PubMed] [Google Scholar]
- 30. Marceglia S, Mrakic-Sposta S, Rosa M, et al. Transcranial direct current stimulation modulates cortical neuronal activity in Alzheimer’s disease. Front Neurosci. 2016;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo Y, Bai Y, Xia X, et al. Effects of long-lasting high-definition transcranial direct current stimulation in chronic disorders of consciousness: a pilot study. Front Neurosci. 2019;13:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cummiford CM, Nascimento TD, Foerster BR, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Res Ther. 2016;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122:114-120. [DOI] [PubMed] [Google Scholar]
- 34. Thair H, Holloway AL, Newport R, Smith AD. Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci. 2017;11:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bachtiar V, Near J, Johansen-Berg H, Stagg CJ. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 2015;4:e08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;151:264-269. [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization. International Statistical Classification of Diseases and Related Health Problems. Vol. 1 World Health Organization; 2004. [Google Scholar]
- 38. Nasseri P, Nitsche MA, Ekhtiari H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci. 2015;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jackson MP, Rahman A, Lafon B, et al. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol. 2016;127:3425-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schaefer A, Kong R, Gordon EM, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28:3095-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons; 2011. [Google Scholar]
- 42. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2-18. [DOI] [PubMed] [Google Scholar]
- 43. Rosenthal R. Meta-analytic procedures for social science research Sage Publications: Beverly Hills, 1984, 148 pp. Educ Res. 1986;15:18-20. [Google Scholar]
- 44. Hofmann SG, Wu JQ, Boettcher H. Effect of cognitive-behavioral therapy for anxiety disorders on quality of life: a meta-analysis. J Consult Clin Psychol. 2014;82:375-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fox NA, Bakermans-Kranenburg MJ, Yoo KH, et al. Assessing human mirror activity with EEG mu rhythm: a meta-analysis. Psychol Bull. 2016;142:291-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Ridder D, Vanneste S. Occipital nerve field transcranial direct current stimulation normalizes imbalance between pain detecting and pain inhibitory pathways in fibromyalgia. Neurother. 2017;14:484-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neeb L, Bayer A, Bayer K-E, et al. Transcranial direct current stimulation in inflammatory bowel disease patients modifies resting-state functional connectivity: a RCT. Brain Stimul. 2019;12:978-980. [DOI] [PubMed] [Google Scholar]
- 51. Mondino M, Jardri R, Suaud-Chagny M-F, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Palm U, Keeser D, Hasan A, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42:1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shahbabaie A, Ebrahimpoor M, Hariri A, et al. Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav. 2018;8:e00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Porcaro C, Cottone C, Cancelli A, Rossini PM, Zito G, Tecchio F. Cortical neurodynamics changes mediate the efficacy of a personalized neuromodulation against multiple sclerosis fatigue. Sci Rep. 2019;9:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schoellmann A, Scholten M, Wasserka B, et al. Anodal tDCS modulates cortical activity and synchronization in Parkinson’s disease depending on motor processing. Neuroimage Clin. 2019;22:101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ficek BN, Wang Z, Zhao Y, et al. The effect of tDCS on functional connectivity in primary progressive aphasia. Neuroimage Clin. 2018;19:703-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sotnikova A, Soff C, Tagliazucchi E, Becker K, Siniatchkin M. Transcranial direct current stimulation modulates neuronal networks in attention deficit hyperactivity disorder. Brain Topogr. 2017;30:656-672. [DOI] [PubMed] [Google Scholar]
- 58. Cosmo C, Ferreira C, Miranda JGV, et al. Spreading effect of tDCS in individuals with attention-deficit/hyperactivity disorder as shown by functional cortical networks: a randomized, double-blind, sham-controlled trial. Front Psychiatry. 2015;6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lefebvre S, Dricot L, Laloux P, et al. Increased functional connectivity one week after motor learning and tDCS in stroke patients. Neuroscience. 2017;340:424-435. [DOI] [PubMed] [Google Scholar]
- 60. Nicolo P, Magnin C, Pedrazzini E, et al. Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch Phys Med Rehabil. 2018;99:862-872.e1. [DOI] [PubMed] [Google Scholar]
- 61. Thibaut A, Piarulli A, Martens G, Chatelle C, Laureys S. Effect of multichannel transcranial direct current stimulation to reduce hypertonia in individuals with prolonged disorders of consciousness: a randomized controlled pilot study. Ann Phys Rehabil Med. 2019;62:418-425. [DOI] [PubMed] [Google Scholar]
- 62. Marangolo P, Fiori V, Sabatini U, et al. Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: preliminary data from aphasia. J Cogn Neurosci. 2016;28:724-738. [DOI] [PubMed] [Google Scholar]
- 63. Wu M, Yu Y, Luo L, et al. Efficiency of repetitive transcranial direct current stimulation of the dorsolateral prefrontal cortex in disorders of consciousness: a randomized sham-controlled study. Neural Plast. 2019;2019:7089543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Amadi U, Ilie A, Johansen-Berg H, Stagg CJ. Polarity-specific effects of motor transcranial direct current stimulation on fMRI resting state networks. Neuroimage. 2014;88:155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. 2011;32:1236-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krause MR, Zanos TP, Csorba BA, et al. Transcranial direct current stimulation facilitates associative learning and alters functional connectivity in the primate brain. Curr Biol. 2017;27:3086-3096.e3. [DOI] [PubMed] [Google Scholar]
- 67. Patel HJ, Romanzetti S, Pellicano A, Nitsche MA, Reetz K, Binkofski F. Proton magnetic resonance spectroscopy of the motor cortex reveals long term GABA change following anodal transcranial direct current stimulation. Sci Rep. 2019;9:2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stagg CJ, Bachtiar V, Amadi U, et al. Local GABA concentration is related to network-level resting functional connectivity. Elife. 2014;3:e01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baumgarten TJ, Oeltzschner G, Hoogenboom N, Wittsack H-J, Schnitzler A, Lange J. Beta peak frequencies at rest correlate with endogenous GABA+/Cr concentrations in sensorimotor cortex areas. PLoS One. 2016;11:e0156829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hall SD, Stanford IM, Yamawaki N, et al. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56:1506-1510. [DOI] [PubMed] [Google Scholar]
- 71. Boonstra TW, Nikolin S, Meisener A-C, Martin DM, Loo CK. Change in mean frequency of resting-state electroencephalography after transcranial direct current stimulation. Front Hum Neurosci. 2016;10:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacobson L, Ezra A, Berger U, Lavidor M. Modulating oscillatory brain activity correlates of behavioral inhibition using transcranial direct current stimulation. Clin Neurophysiol. 2012;123:979-984. [DOI] [PubMed] [Google Scholar]
- 73. Antonenko D, Hayek D, Netzband J, Grittner U, Flöel A. tDCS-induced episodic memory enhancement and its association with functional network coupling in older adults. Sci Rep. 2019;9:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Esposito R, Cieri F, Chiacchiaretta P, et al. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018;12:127-141. [DOI] [PubMed] [Google Scholar]
- 75. Cabral J, Hugues E, Sporns O, Deco G. Role of local network oscillations in resting-state functional connectivity. Neuroimage. 2011;57:130-139. [DOI] [PubMed] [Google Scholar]
- 76. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Laakso I, Tanaka S, Mikkonen M, Koyama S, Sadato N, Hirata A. Electric fields of motor and frontal tDCS in a standard brain space: a computer simulation study. Neuroimage. 2016;137:140-151. [DOI] [PubMed] [Google Scholar]
- 78. Chew T, Ho K-A, Loo CK. Inter-and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimul. 2015;8:1130-1137. [DOI] [PubMed] [Google Scholar]
- 79. Talsma LJ, Kroese HA, Slagter HA. Boosting cognition: effects of multiple-session transcranial direct current stimulation on working memory. J Cogn Neurosci. 2017;29:755-768. [DOI] [PubMed] [Google Scholar]
- 80. Ho K-A, Taylor JL, Chew T, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated sessions. Brain Stimul. 2016;9:1-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cns-10.1177_1179573520976832 for The Effect of Transcranial Direct Current Stimulation in Changing Resting-State Functional Connectivity in Patients With Neurological Disorders: A Systematic Review by Melody MY Chan and Yvonne MY Han in Journal of Central Nervous System Disease
Supplemental material, sj-docx-2-cns-10.1177_1179573520976832 for The Effect of Transcranial Direct Current Stimulation in Changing Resting-State Functional Connectivity in Patients With Neurological Disorders: A Systematic Review by Melody MY Chan and Yvonne MY Han in Journal of Central Nervous System Disease