Abstract
Background:
Up-front surgery followed by postoperative chemotherapy remains the standard paradigm for the treatment of patients with resectable pancreatic cancer. However, the risk for positive surgical margins, the poor recovery after surgery that often impairs postoperative treatment, and the common metastatic relapse limit the overall clinical outcomes achieved with this strategy. Polychemotherapeutic combinations are valid options for postoperative treatment in patients with good performance status. liposomal irinotecan (Nal-IRI) is a novel nanoliposome formulation of irinotecan that accumulates in tumor-associated macrophages improving the therapeutic index of irinotecan and has been approved for the treatment of patients with metastatic pancreatic cancer after progression under gemcitabine-based therapy. Thus, it remains of the outmost urgency to investigate introduction of the most novel agents, such as nal-IRI, in perioperative approaches aimed at increasing the long-term effectiveness of surgery.
Methods:
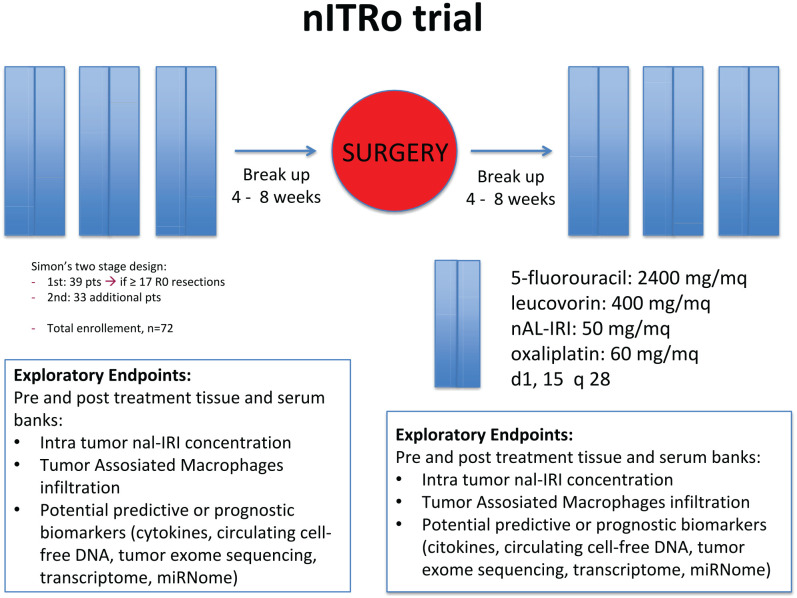
The nITRO trial is a phase II, single-arm, open-label study to assess the safety and the activity of nal-IRI with fluorouracil/leucovorin (5-FU/LV) and oxaliplatin in the perioperative treatment of patients with resectable pancreatic cancer. The primary tumor must be resectable with no involvement of the major arteries and no involvement or <180° interface between tumor and vessel wall of the major veins. A total of 72 patients will be enrolled to receive a perioperative treatment of three cycles before and three cycles after surgical resection with nal-IRI 50 mg/m2, oxaliplatin 60 mg/m2, leucovorin 200 mg/m2, and 5-fluorouracil 2400 mg/m2, days 1 and 15 of a 28-day cycle. The primary objective is to improve from 40% to 55% the proportion of patients achieving R0 resection after preoperative treatment.
Discussion:
The nITRO trial will contribute to strengthen the clinical evidence supporting perioperative strategies in resectable pancreatic cancer patients. Moreover, this study represents a unique opportunity for translational analyses aimed to identify novel immune-related prognostic and predictive factors in this setting.
Trial registration
Clinicaltrial.gov: NCT03528785. Trial registration data: 1 January 2018
Protocol number: CRC 2017_01
EudraCT Number: 2017-000345-46
Keywords: nal-irinotecan, peri-operative chemotherapy, R0, resectable pancreatic adenocarcinoma
Background
Pancreatic cancer has still the lowest 5-year relative survival rate among solid tumors at 7%, and is projected to become the second leading cause of cancer-related death by 2030 in western countries.1 The poor prognosis for patients affected by this disease could be mainly attributed to the limited efficacy of available systemic treatments, and to the early metastatic behavior demonstrated along the process of tumor progression that makes de facto pancreatic cancer a systemic disease at the time of diagnosis.2–6 More than 80% of patients present, indeed, with a locally advanced or metastatic disease,7 and even among patients with apparently localized disease who undergo surgery and postoperative therapy, metastatic recurrence remains extremely common.8
In this regard, evidence supports the vision of ours and other research groups that the current standard paradigm for the treatment of immediately resectable pancreatic cancer represented by up-front surgery followed by postoperative therapy has no future in further improving patients’ outcomes.9
Results of many trials contributed to set gemcitabine monotherapy or gemcitabine-containing regimens as valid options for postoperative therapy.8,10,11 More recently, the PRODIGE 24/CCTG PA.6 phase III trial demonstrated a statistically and clinically relevant advantage of the triple combination of irinotecan + fluorouracil/leucovorin (5-FU/LV) + oxaliplatin (mFOLFIRINOX) regimen if compared with gemcitabine monotherapy, setting a new postoperative treatment option in patients with good performance status. Nonetheless, the 3-year disease free survival (DFS) was of nearly 40%, indicating that even in this selected population of good performance status resected patients receiving a triplet chemotherapeutic, more than half of the patients experienced local or metastatic recurrence.12 In interpreting these results, it should, furthermore, be considered that they do not include the overall population of patients undergoing surgical resection. Several studies estimated that only 50–60% of this population of patients effectively receive a postoperative treatment, further contributing to the overestimation of the outcomes obtained with postoperative approaches.13–15
Different studies have demonstrated that a truly localized pancreatic cancer might indeed be very uncommon, and that the metastatic spread begins early along the progression of this disease. The molecular mechanisms that promote the early metastatic spread of pancreatic cancer have recently been elucidated. Recent studies using a mathematical modeling approach with radiological and pathological data on pancreatic cancer patients who underwent autopsy revealed that all patients are expected to harbor cells that are capable of metastasis in the primary tumor at the time of diagnosis, even when the size of the primary tumor is small.16 Further evidence supporting the model that metastasis is an early event in pancreatic carcinogenesis has been provided by using a genetically engineered murine model of pancreatic cancer in which the pancreatic epithelial cells could be tracked during tumor progression through the expression of YFP allele into the Kras plus p53 or p16 mutant background. In this model, even low-grade pancreatic intraepithelial neoplasia (PanINs) showed evidence of cells that have crossed the basement membrane, migrated from the glandular epithelium into the surrounding tissue and circulatory system, and seeded the liver prior to pancreatic cancer formation. This behavior was associated with an early epithelial-to-mesenchymal transition (EMT) in the premalignant lesions.17 These biological models represent the mechanistic bases for some clinical evidence showing that metastatic lesions may indeed be detectable within weeks from the first presentation of an initially resectable disease in at least 15–20% of patients.18,19
Because of this aggressive biological behavior and the high rate of recurrence after surgical resection and postoperative therapy, increasing interest is growing about pre or perioperative treatments in resectable pancreatic cancer with the aim of expanding the population of patients who may ultimately benefit from resection.20 These strategies might provide an interval of time to assess the biological aggressiveness and the chemoresistance of the disease to avoid an unnecessary surgery in patients who would relapse soon after resection. Moreover, preoperative therapy might allow the delivery of systemic therapy to a higher percentage of patients and with an improved tolerance than in a postoperative setting. A preoperative treatment could allow an earlier treatment of occult micrometastatic foci than a postoperative therapy. Delivering cytotoxic drugs to tumors that are intact and not altered by surgery with resultant local hypoxia, inflammation, and fibrosis, preoperative therapy has the potential to destroy tumor cells, particularly in the periphery of the tumor mass, thereby improving the likelihood of an ultimate R0 resection.
Solid clinical evidence is emerging to demonstrate an improved tumor resectability or patient survival duration with preoperative therapy compared to standard treatment in resectable pancreatic cancer. A recent analysis of 15,237 patients with stage I and II pancreatic cancer identified in the US National Cancer Database compared overall survival (OS) of patients receiving preoperative treatment with that of patients who received up-front surgery. The first group showed a significantly better OS (26 versus 21 months, p < 0.01; Hazard Ratio (HR) = 0.72; 95% Confidence Interval (CI) = 0.68–0.78). Patients resected up-front demonstrated a higher pathological primary tumor (pT3 and T4: 86% versus 73%; p < 0.01), a higher rate of lymph node positivity (73% versus 48%, p < 0.01), and a higher rate of R1 resections (24% versus 17%; p < 0.01). Even when compared to the subpopulation of patients who had received postoperative chemotherapy after up-front surgery, patients who received preoperative treatment showed a better OS (HR = 0.83, 95% CI, 0.73–0.89).21 Moreover, several meta-analytical studies including immediately resectable patients sustained the benefit of preoperative treatment with gemcitabine-based and more recent chemotherapeutic regimens.22–24
The phase III PREOPANC trial was the largest prospective study to allocate patients randomly affected by resectable or borderline resectable pancreatic cancer to a perioperative chemoradiotherapy strategy with gemcitabine or to standard up-front surgery and postoperative gemcitabine. Although this study did not demonstrate a statistically significant advantage in OS in the intention-to-treat population, all the secondary endpoints, including disease-free survival and R0 resection rate, were significantly superior with preoperative chemoradiotherapy, suggesting a potential advantage for this approach.25
To improve further the outcome achieved with this strategy, more active combination regimens borrowed by the metastatic setting are currently under evaluation as effective preoperative treatments. FOLFIRINOX is an effective choice for first-line treatment in patients affected by advanced pancreatic cancer, and in this setting it achieved a disease control rate (DCR) of 70.2%.12 A small proof-of-concept pilot study recently demonstrated the feasibility of perioperative mFOLFIRINOX in resectable pancreatic cancer. Among the 21 patients enrolled 95% completed four cycles of preoperative mFOLFIRINOX, 81% completed resection with a high R0 resection rate and a promising survival.26 A systematic review and patient-level meta-analysis of 24 studies of neo-adjuvant FOLFIRINOX in borderline pancreatic cancer has showed a 83.9% of R0 and a patient level mOS of 22.2 months (95% CI: 18.8–25.6), without unexpected toxicities and deaths attributed to chemotherapy.27 The 84% of R0 after FOLFIRINOX was higher compared with 67% obtained with up-front surgery in a previous meta-analysis of Versteijne and colleagues.23 In this regard, FOLFIRINOX is currently explored as preoperative regimen in a number of clinical trials in resectable pancreatic cancer, including the SWOG 1505 trial (ClinicalTrials.com identifier: NCT02562716), the phase II trial of perioperative modified FOLFIRINOX conducted at Yale Cancer Center (ClinicalTrials.com identifier: NCT02047474), the PREOPANC-2 trial (Netherlands Trial Register identifier: NTR7292), the A021806 trial of Alliance for Clinical Trials in Oncology (ClinicalTrials.com identifier: NCT04340141), the NorPACT-1 trial (ClinicalTrials.com identifier: NCT02919787), the NEPAFOX trial (ClinicalTrials.com identifier: NCT02172976), and the PANACHE01-PRODIGE48 trial (ClinicalTrials.com identifier: NCT02959879).
Given the extreme biological aggressiveness of pancreatic cancer and the still unsatisfactory prognosis of patients undergoing major surgery for resectable disease, we aimed to investigate novel and more effective preoperative systemic treatments to increase the long-term effectiveness of surgery.
Liposomal irinotecan (Nal-IRI) is irinotecan hydrochloride encapsulated into a nanoliposome drug delivery system (nanoliposomal irinotecan; nal-IRI). Drug carrier technologies represent a rational strategy to improve the pharmacokinetics and biodistribution of irinotecan while protecting it from premature metabolism. Nal-IRI employs a novel intraliposomal drug stabilization technology for encapsulation of irinotecan into long-circulating liposome-based nanoparticles with high drug load and high in vivo stability. The stable nanoliposome formulation of irinotecan has several attributes that may provide an improved therapeutic index. The controlled and sustained release should improve activity of this schedule-dependent drug by increasing duration of exposure of tumor tissue to drug, an attribute that allows it to be present in a higher proportion of cells during the more sensitive S-phase of the cell cycle. The improved pharmacokinetics and the high intravascular drug retention in the liposomes may potentially result in site-specific drug delivery to solid tumors. Stromal targeting results from the subsequent depot effect, in which liposomes accumulating in tumor-associated macrophages (TAMs) release the active drug and convert it locally to the substantially more cytotoxic SN-38. The preferentially local bio-activation should result in reduced exposure to potential sites of toxicity and increased exposure to neighboring cancer cells within the tumor.
Nal-IRI demonstrated a significant activity in different preclinical models of solid tumors, including colon, pancreatic, gastric, cervical, non-small cell lung, small cell lung, ovarian, thyroid, and breast cancers, glioma, Ewing’s sarcoma, and neuroblastoma.28–30 Nal-IRI has shown potent anti-tumor activity, including durable tumor regressions, and was markedly superior to the equivalent dose of free drug in a bioluminescent-based orthotopic xenograft pancreatic model.31
The clinical efficacy of nal-IRI has been demonstrated in gemcitabine-refractory metastatic pancreatic cancer patients: in a randomized, phase III, international study (NAPOLI-1), nal-IRI was given as a monotherapy, or in combination with 5-FU/LV, compared to the control arm of 5-FU/LV alone.32–34 The nal-IRI+5-FU/LV combination significantly prolonged OS compared to 5-FU/LV treatment alone. The median OS for the nal-IRI+5-FU/LV combination arm was 6.1 months compared to 4.2 months for the 5-FU/LV alone control arm with a stratified hazard ratio (HR) of 0.57 (95% CI: 0.41–0.80; p = 0.0009; unstratified HR = 0.67; p = 0.012). The overall response rate (ORR) in the nal-IRI+5-FU/LV combination arm was 16% versus 1% on the control arm (p < 0.001). Based on the NAPOLI-1 trial, in October 2015, nal-IRI (Onivyde) has been approved by the US Food and Drug Administration (FDA), in combination with 5FU/LV, for the treatment of patients with metastatic pancreatic cancer after disease progression following gemcitabine-based therapy.35,36
In a recent phase I/II trial has been assessed the safety, tolerability, and dose-limiting toxicities of nal-IRI+5-FU/LV + oxaliplatin for the first-line treatment of patients with locally advanced and metastatic pancreatic cancer, and to determine phase III dosing (NCT02551991). A total of 56 patients were enrolled and treated. A recommended dose for the expansion (part 1B) was selected: nal-IRI 50 g/m2, oxaliplatin 60 mg/m2, LV 400 mg/m2, and 5-FU 2400 mg/m2 on days 1 and 15 of each 28-day cycle. The safety profile suggested a manageable regimen with a promising anti-tumor activity (DCR16wk of 71.9%, sum of Complete Response (CR) + Partiale Response (PR) + Stable Disease (SD): 81.3%, and ORR of 34%).37
A critical challenge in this field remains the introduction of the most novel and effective agents, such as nal-IRI, in peri-operative treatment of resectable pancreatic cancer in order to obtain a more profound tumor shrinkage, to increase the rate of R0 resections, to allow an early treatment of occult micro-metastatic disease, and eventually, to improve patients’ survival. This study proposal is designed to address this challenge.
Methods/design
Study design
nITRO is a phase II, single-arm, open-label trial carried out at two centers in Italy. Eligible patients with resectable pancreatic cancer will receive a peri-operative treatment with three cycles (3 months) with nal-IRI 50 mg/m2, oxaliplatin 60 mg/m2, LV 200 mg/m2, and 5-FU 2400 mg/m2, days 1 and 15 of a 28 day-cycle before and after surgical resection (Figure 1). The ethics committee of Verona University has approved the protocol. All patients will provide written informed consent before enrollment. Monitoring will be carried out in this trial.
Figure 1.
Study design diagram.
Endpoints
Primary endpoint
The number of patients achieving R0 resection, defined according to the International Study Group of Pancreatic Surgery and RCPath guidelines as a resection margin >1 mm (www.rcpath.org). Tumor clearance should be given for all of the following margins: anterior, posterior, medial, or superior mesenteric groove, superior mesenteric artery (SMA), pancreatic transection, bile duct, and enteric. Surgery will be performed after receiving at least two cycles of chemotherapy preoperatively planned at up to 12 weeks.
Secondary endpoints
OS (time frame up to 2 years), time from enrolment to death from any cause.
Disease free survival (DFS) (time frame up to 2 years), time from enrolment to recurrence (locoregional or distant) or death due to any cause.
Incidence of serious adverse events (SAEs), using common toxicities adverse events criteria (CTCAE) version 4.0 (time frame every 2 weeks during treatment).
ORR after preoperative chemotherapy with RECIST 1.1 (time frame up to 2 years).
Overall resection rate (time frame immediately after surgery).
Number of participants achieving pathological complete response (pCR) (time frame up to 2 years).
Lymph node status (time frame immediately after surgery).
Biochemical response rate by CA 19.9 decrease (time frame after 3 months of induction therapy).
Number of participants experiencing perioperative (30-day) mortality or morbidity (time frame up to 30 days from surgery).
Exploratory endpoints
Pre and post-treatment tissue and serum banks:
Intra-tumor nal-IRI concentration.
Immuno-histochemical (IHC) analyses of TAMs enrichment and their polarization, transdifferentiation of fibroblasts into activated myofibroblasts, pericyte coverage of the tumor vasculature and microvessel density, fibrotic and collagen content (in biopsy specimens at baseline and surgical specimens).
Potential predictive and/or prognostic biomarkers (cytokines concentration will be measured using multiplex xMAP/Luminex technology, at baseline and after treatment).
Recruitment
Patients with resectable, histologically confirmed pancreatic cancer will be recruited. All the patients should take following examinations at baseline: computed tomography (CT) scan and abdominal magnetic resonance imaging (MRI). A total of 72 patients will be enrolled.
Inclusion criteria
In order to be included in the program, patients must have/be:
Able to understand and provide written informed consent.
⩾18 years of age.
Histologically or cytologically confirmed pancreatic ductal adenocarcinoma.
Adequate hepatic, renal and hematological function.
Patients must have measurable disease in the pancreas, with no evidence of metastatic disease on imaging of the chest, abdomen and pelvis at contrast-enhanced CT and MRI abdomen with contrast performed within 28 days before dosing; positron-emission tomography (PET) scans alone will not be adequate alternatives.
- The primary tumor must be surgically resectable, defined as:
- - no involvement (abutment or encasement) of the major arteries (celiac, common hepatic and/or SMA);
- - no involvement or <180° interface between tumor and vessel wall of the portal vein, superior mesenteric vein and/or portal vein/splenic vein confluence.
Exclusion criteria
Patients must meet all the inclusion criteria listed previously and none of the following exclusion criteria:
Serum total bilirubin ⩾2 × Upper Normal Limit (UNL) (biliary drainage is allowed for biliary obstruction).
Severe renal impairment (creatinine clearance (CLcr) ⩽30 ml/min).
- Inadequate bone marrow reserves as evidenced by:
- - Absolute neutrophil count (ANC) ⩽ 1500 cells/μl; or
- - Platelet count ⩽ 100,000 cells/μl; or
- - Hemoglobin (Hb) ⩽ 9 g/dL.
Karnofsky performance score (KPS) <60.
Any clinically significant disorder impacting the risk–benefit balance negatively per physician’s judgment.
Any clinically significant gastrointestinal disorder, including hepatic disorders, bleeding, inflammation, occlusion, or diarrhea >grade 2.
Severe arterial thromboembolic events (myocardial infarction, unstable angina pectoris, stroke) in past 6 months.
New York Heart Association (NYHA) class III or IV congestive heart failure, ventricular arrhythmias or uncontrolled blood pressure or known abnormal electrocardiogram (ECG) with clinically significant abnormal findings.
Active infection or an unexplained fever >38.5°C (excluding tumor fever), which in the physician’s opinion might compromise the patient’s health.
Current use or any use in past 2 weeks of strong cytochrome P450 3A4 (CYP3A) enzyme inducers/inhibitors and/or strong DP-glucorosil-transferase A1 (UGT1A) inhibitors.
Known hypersensitivity to any of the components of nal-IRI other liposomal irinotecan formulations, irinotecan, fluoropyrimidines, or LV.
Breast feeding, known pregnancy, positive serum pregnancy test or unwillingness to use an effective method of contraception, during therapy and for 3 months following the last dose of nal-IRI. Women of childbearing potential must either agree to use and be able to take effective contraceptive birth control measures (Pearl index <1) or agree to practise complete abstinence from heterosexual intercourse during the course of the study and for at least 3 months after last application of program treatment. A female subject is considered to be of childbearing potential unless she is aged ⩾50 years and naturally amenorrhoeic for ⩾2 years, or unless she is surgically sterile. Men must agree not to father a child (including not donating sperm) during the course of the trial and for at least 6 months after last administration of study drugs.
Patients who received previous chemotherapy or radiotherapy for pancreatic cancer.
Chemotherapy
Patients will receive a treatment scheme of three cycles (3 months) with nal-IRI 50 mg/m2, oxaliplatin 60 mg/m2, leucovorin 200 mg/m2, and 5-fluorouracil 2400 mg/m2, days 1 and 15 of a 28-day cycle. Patients achieving stable disease or better will undergo surgical resection 4–8 weeks after completion of the first three cycles of treatment. Within 4–8 weeks following pancreatectomy, patients will receive an additional three cycles (3 months) of the same regimen in the absence of disease progression or unacceptable toxicity. The first cycle day 1 is a fixed day; subsequent doses should be administered on the first day of each cycle ± 2 days.
Patients will be treated until disease progression (radiological or clinical deterioration), intolerable toxicity, excessive treatment delay or non-compliance, patient’s decision/consent withdrawn, until the product is commercially available and/or reimbursed in the local market, or reasons for program termination.
Description of nal-IRI
Nal-IRI is supplied as sterile, single-use vials containing 10 mL of nal-IRI at a concentration of 5 mg/mL. Each vial is expected to have a 9.5 ml extractable amount of nal-IRI. One 10 ml vial of concentrate contains the equivalent of 50 mg irinotecan hydrochloride trihydrate (as sucrosofate salt in a liposomal formulation) which corresponds to 43 mg irinotecan.
Combination regimen and dosage and administration
Nal-IRI, oxaliplatin, LV and 5-FU will be administered sequentially. The recommended dose and regimen of nal-IRI is 50 mg/m2 intravenously (iv) over 90 min (±10 min), oxaliplatin 60 mg/m2 over 120 min (±10 min), LV 200 mg/m2 intravenously over 30 min (±5 min) followed by 5-FU 2400 mg/m2 intravenously over 46 h. Nal-IRI must not be administered as a bolus injection or an undiluted solution. Prior to administration, the appropriate dose of nal-IRI must be diluted with 5% glucose solution for injection or sodium chloride 9 mg/ml (0.9%) solution for injection to prepare a solution of the appropriate dose of nal-IRI diluted to a final volume of 500 ml. Mix the diluted solution by gentle inversion. Care should be taken not to use in-line filters or any other diluents. The actual dose of nal-IRI to be administered should be determined by calculating the patient’s body surface area (BSA) at the beginning of each cycle. A ±5% variance in the calculated total dose should be allowed for ease of dose administration.
Premedication
It is recommended that patients receive premedication for nausea and vomiting prior to chemotherapy infusion with standard doses of dexamethasone (or an equivalent corticosteroid) together with a 5-hydroxytryptamine (5-HT3) antagonist (or other antiemetic), unless contraindicated for the individual patient. Premedication should be given on the day of treatment, starting at least 30 min before administration of the therapy. Atropine may be prescribed prophylactically for patients who experience acute cholinergic symptoms in previous cycles. Physicians should also consider providing patients with an antiemetic regimen for subsequent use, as well as loperamide (or equivalent) for treatment of late diarrhea, if necessary.
Dose modification requirements
In clinical studies, dosing may be held for up to 3 weeks from when it was due, to allow for recovery from toxicity related to the study treatments. If the time required for recovery from toxicity is more than 3 weeks, the patient should be discontinued from the program, unless the patient is benefiting from the program treatment. If a patient’s dose is reduced during the program due to toxicity, it should remain reduced for the duration of the program; dose should not be re-escalated to an earlier dose. Any patient who has two dose reductions and experiences an adverse event that would require a third dose reduction should be discontinued from program treatment.
Dosage adjustments
Guidelines for dose adjustments of each individual treatment within the regimen are found in the tables in the following for hematological toxicities (Table 1), and for non-hematological toxicities (Table 2), and were based on published dose modifications for the established FOLFIRINOX regimen. For all tables in the following, patients should be withdrawn from study treatment if more than two dose reductions are required. LV dose does not require adjustment. LV should be given immediately prior to each 5-FU dose; hence, if the 5-FU dose is held, LV should be held as well.
Table 1.
Dose modifications for hematological toxicities.
| Worst toxicity by CTCAE grade | Nal-IRI | 5-FU | Oxaliplatin |
|---|---|---|---|
| Grade 2 neutropenia (ANC <1500–1000 cells/mm3) | 100% of previous dose | 100% of previous dose | 1st occurrence: 100% of previous dose; 2nd occurrence: reduce dose to 50 mg/m2 |
| Grade 3 or 4 neutropenia (ANC <1000/mm3) or febrile neutropenia | 1st occurrence: reduce dose to 40 mg/m2; 2nd occurrence: reduce dose to 30 mg/m2 |
1st occurrence: reduce dose by 25%; 2nd occurrence: reduce dose another 25% (50% of original dose) |
1st occurrence: 100% of previous dose; 2nd occurrence: reduce dose to 50 mg/m2 |
| >Grade 2 thrombocytopenia (Grade 2: platelets <75,000–50,000/mm3 OR Grade 3–4: platelets <50,000/mm3) |
If grade 2: 100% of previous dose If >grade 3: 1st occurrence: reduce dose to 50 mg/m2; 2nd occurrence: reduce dose to 40 mg/m2 |
If grade 2: 100% of previous dose If >grade 3: 1st occurrence: reduce dose by 25%; 2nd occurrence: reduce dose another 25% (50% of original dose) |
1st occurrence: reduce dose to 60 mg/m2; 2nd occurrence: maintenance of the reduced dose of 50 mg/m2 |
| Other hematological toxicities not specifically listed above | If <grade 2: 100% of previous dose If >grade 3: 1st occurrence: reduce dose to 40 mg/m2; 2nd occurrence: reduce dose to 30 mg/m2 |
If <grade 2: 100% of previous dose If >grade 3: 1st occurrence: reduce dose by 25%; 2nd occurrence: reduce dose another 25% (50% of original dose) |
If <grade 2: 100% of previous dose If >grade 3: 1st occurrence: reduce dose to 50 mg/m2; 2nd occurrence: maintenance of the reduced dose of 50 mg/m2 |
ANC, absolute neutrophil count; CTCAE, common toxicities adverse events criteria; 5-FU, 5-fluorouracil; Nal-IRI, nanoliposomal irinotecan.
Table 2.
Dose modifications for non-hematological toxicities other than asthenia and grade 3 anorexia.
| Worst toxicity by CTCAE grade | Nal-IRI | 5-FU | Oxaliplatin |
|---|---|---|---|
| Grade 1 or 2, including diarrhea | 100% of previous dose | 100% of previous dose, except for grade 2 hand foot syndrome, grade 2 cardiac toxicity, or any grade neurocerebellar toxicity | 100% of previous dose |
| Grade 3 or 4, including diarrhea (except nausea and vomiting) | 1st occurrence: reduce dose to 40 mg/m2
2nd occurrence: reduce dose to 30 mg/m2 |
1st occurrence: reduce dose by 25% 2nd occurrence: reduce dose another 25% (50% of original dose)* except for grade 3 or 4 hand foot syndrome |
1st occurrence: 100% of previous dose 2nd occurrence: reduce dose to 50 mg/m2 |
| Grade 3 or 4 nausea and/or vomiting despite antiemetic therapy | Optimize antiemetic therapy AND reduce dose to 40 mg/m2; if the patient is already receiving 50 mg/m2, reduce dose to 40 mg/m2 | Optimize antiemetic therapy AND reduce dose by 25%; if the patient is already receiving a reduced dose, reduce dose an additional 25%f | 1st occurrence; 100% of previous dose 2nd occurrence: reduce dose to 50 mg/m2 |
| Grade 2 hand foot syndrome | 100% of previous dose | 1st occurrence: reduce dose by 25% 2nd occurrence: reduce dose another 25% (50% of original dose) |
100% of previous dose |
| Grade 3 or 4 hand foot syndrome | 1st occurrence: reduce dose to 40 mg/m2
2nd occurrence: reduce dose to 30 mg/m2 |
Discontinue therapy | No dose modifications required |
| Any grade neurocerebellar or >grade 2 cardiac toxicity | No dose modifications required | Discontinue therapy | No dose modifications required |
| Sensory neuropathy | No dose modifications required | No dose modifications required | Grade 2, persistent: reduce dose to 50 mg/m2
Grade 3: recovers prior to next cycle and reduce dose to 50 mg/m2 Grade 3, persistent: discontinue therapy Grade 4: discontinue therapy |
CTCAE, common toxicities adverse events criteria; 5-FU, 5-fluorouracil; Nal-IRI, nanoliposomal irinotecan.
Concomitant therapy
All concurrent medical conditions and complications of the underlying malignancy will be treated at the discretion of the treating physician, according to acceptable local standards of medical care. Patients should receive analgesics, antiemetics, antibiotics, antipyretics, and blood products as necessary. Although warfarin-type anticoagulant therapies are permitted, careful monitoring of coagulation parameters is imperative, in order to avoid complications of any possible drug interactions. Institutional guidelines for the treatment of these conditions may also be used. The concomitant therapies that warrant special attention are discussed in the following.
Antiemetic medications: Dexamethasone and a 5-HT3 blocker (e.g. ondansetron or granisetron) should be administered to all patients as premedications unless contraindicated for the individual patient. Antiemetics should also be prescribed as clinically indicated during the program period.
Colony stimulating factors: Use of granulocyte colony-stimulating factors (G-CSFs) is permitted to treat patients with neutropenia or neutropenic fever; prophylactic use of G-CSFs can be considered in those patients who have had at least one episode of grade 3 or 4 neutropenia or neutropenic fever while receiving program therapy or have had documented grade 3 or 4 neutropenia or neutropenic fever while receiving prior antineoplastic therapy.
Therapy for diarrhea: Diarrhea can occur early (onset in less than 24 h after starting nal-IRI) or late (more than 24 h). Early onset diarrhea may be accompanied by cholinergic symptoms: sweating, abdominal cramping, myosis and salivation. Patients should be made aware of the risk of delayed diarrhea which can be debilitating and, on rare occasions, life threatening because persistent loose or watery stools can result in dehydration, electrolyte imbalance, colitis, gastrointestinal ulceration, infection or sepsis.
As soon as the first liquid stool occurs, the patient should start drinking large volumes of beverages containing electrolytes and an appropriate antidiarrheal therapy must be initiated immediately.
Prophylactic or therapeutic treatment with atropine in patients experiencing early onset diarrhea with cholinergic symptoms (0.25 mg to 1 mg, administered intravenously or subcutaneously), should be considered unless contraindicated.
Patients should have loperamide (or equivalent) readily available to begin treatment for late diarrhea. Loperamide should be initiated at first occurrence of poorly formed or loose stools or at the earliest onset of bowel movements more frequent than normal. Loperamide should be given until patient is without diarrhea for at least 12 h. If diarrhea persists while patient is on loperamide for more than 24 h, adding oral antibiotic support (fluoroquinolone for 7 days) should be considered. Loperamide should not be used for more than 48 consecutive hours due to the risk of paralytic ileus. If diarrhea persists for more than 48 h, stop loperamide, monitor and replace fluid electrolytes and continue antibiotic support until resolution for accompanying symptoms. Nal-IRI treatment should be delayed until diarrhea resolves to ⩽ grade 1 (2–3 stools/day more than pre-treatment frequency). Nal-IRI must not be administered to patients with bowel obstruction, until it is resolved.
Other treatments: Symptomatic treatment for other toxicities should be per institutional guidelines. Prevention of alopecia with cold cap or of stomatitis with iced mouth rinses is allowed.
Interactions
Information about drug interactions with nal-IRI is referenced from the published scientific literature for non-liposomal irinotecan. No dedicated drug interaction studies were conducted with nal-IRI.
Strong CYP3A4 inducers: Patients receiving concomitant non-liposomal irinotecan and CYP3A4 enzyme-inducing anticonvulsants phenytoin, phenobarbital or carbamazepine have substantially reduced exposure to irinotecan (AUC reduction by 12% with St John’s wort, 57–79% with phenytoin, phenobarbital, or carbamazepine) and SN38 (area under the curve [AUC] reduction by 42% with St John’s wort, 36–92% with phenytoin phenobarbital, or carbamazepine).
Strong CYP3A4 inhibitors and UGT1A1 inhibitors: Patients receiving concomitant non-liposomal irinotecan and ketoconazole, a CYP3A4 and UGT1A1 inhibitor, have increased SN-38 exposure by 109%. Therefore, co-administration of nal-IRI with other inhibitors of CYP3A4 (e.g. grapefruit juice, clarithromycin, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telaprevir, voriconazole) may increase systemic exposure of nal-IRI. Based on the drug interaction of non-liposomal irinotecan and ketoconazole, co-administration of nal-IRI with other inhibitors of UGT1A1 (e.g. atazanavir, gemfibrozil, indinavir) may also increase systemic exposure of nal-IRI. Treatment with these agents and any others that interact with irinotecan should be avoided whenever possible. Co-administration of nal-IRI with 5-FU/LV does not alter the pharmacokinetics of nal-IRI based on the population pharmacokinetic analysis.
Program procedures (Table 3)
Table 3.
Study procedures.
| Procedure | Screening visit (up to 28 days) |
Cycle 1-2-3 (28 days) |
C3D28 (–7 Days) |
Cycle 4-5-6 (28 days) |
Follow-up (C6D28) or ET | Follow-up (q3 months) | ||
|---|---|---|---|---|---|---|---|---|
| Preoperative |
Postoperative |
|||||||
| Day 1 | Day 15 | Day 1 | Day 15 | |||||
| Revision criteria INC/EXC | X | X (only C1D1) | X (only C4D1) | |||||
| Informed consent | X | |||||||
| Medical history | X | |||||||
| Demographics | X | |||||||
| Physical exam | X | Xc | Xc | Xc | Xc | X | ||
| Vital signs | X | Xc | Xc | Xc | Xc | X | ||
| KPS | X | Xc | Xc | Xc | Xc | X | ||
| CBC with differential | X | Xc | Xc | Xc | Xc | X | ||
| Serum chemistry | X | Xc | Xc | Xc | Xc | X | ||
| CA 19.9 | X | Xc | Xc | |||||
| Serum pregnancy testa | X | |||||||
| Urine pregnancy testa | X | X | X | |||||
| ECG | X | Xc (only C1) | Xc (only C1) | X | ||||
| Tumor assessment | X | X | X | |||||
| Concomitant medications | X | X | X | X | X | X | ||
| Concomitant procedures | X | X | X | X | X | X | ||
| Administration of nal-IRI, Oxaliplatin, 5-FU, leucovorin, including premedication | X | X | X | X | ||||
| Adverse event reportingb | X | X | X | X | X | X | ||
| Overall survival | X | |||||||
If indicated.
Healthcare professionals are asked to report any suspected drug adverse reactions.
Can be performed up to 3 days prior to drug.
CA, carbohydrate antigen; CBC, complete blood count; ECG, electrocardiogram; 5-FU, 5-fluorouracil; KPS, Karnofsky performance score; nal-IRI, nanoliposomal irinotecan; Oxa, oxaliplatin.
Protocol visits
Screening visit: the screening phase will begin once the patients sign the informed consent form (ICF).
On study visit: patients who are confirmed to meet all inclusion and exclusion criteria will be enrolled in treatment period. C1D1 is a fixed day; cycle 1 day 15 and day 1 and day 15 of all subsequent cycles should be performed with a window of ± 2 days.
Follow up visit: all patients must be complete a follow-up assessment at the end of study treatment (cycle 6 day 28 ± 2 days) or at the time the investigator removes the patient from treatment.
Survival follow-up: after the follow-up visit, the patient should continue to be followed for survival status once every 3 months (±14 days) via telephone, email, clinic visit, or medical record review until 36 months after the end of treatment, death, lost to follow-up, withdrawal of consent or study closure, whichever occurs first.
Clinical procedures
Proper execution and documentation of the clinical procedures is the responsibility of the treating physician. A careful assessment and review is needed to check eligibility and continued participation in the program.
Medical history
A medical history should include all pertinent prior medical conditions, treatments for pancreatic cancer, surgeries or other medical procedures.
Physical examination
Physical examination should include a careful assessment of all body systems, including the skin; central and peripheral nervous system; eyes; ears, nose and throat; respiratory, musculoskeletal and cardiovascular systems; abdomen and extremities. Particular attention should be paid to areas of possible neoplastic involvement.
Vital signs
Vital signs include height (only at screening), weight, resting blood pressure, pulse, respiratory rate and temperature.
Karnofsky performance score
KPS should be obtained by the treating physician by questioning the patient about their functional capabilities.
Electrocardiogram
A 12-lead ECG will be performed at screening, day 1 and day 15 of cycle 1 and follow-up.
Adverse event reporting
Investigators should complete all routine and standard of care assessments to evaluate for toxicity and symptoms of drug-induced adverse events. This may include, but is not limited to, verbal report from the patient and/or caregiver, physical examinations and laboratory findings. In addition, information on patient hospitalizations and/or hospital visits should also be collected, whether or not associated with any adverse event.
Disease assessment
Tumor response should be evaluated according to RECIST version 1.1 to establish disease progression by CT or MRI scan with contrast. In addition, other radiographic or scintigraphy procedures, as deemed appropriate by the treating physician, may be performed to assess sites of neoplastic involvement. Investigators should select target and non-target lesions in accordance with RECIST version 1.1 guidelines. Follow-up measurements and overall response should be in accordance with these guidelines. The first post-baseline tumor assessment must be performed after 12 weeks from start of treatment within a 7 day window (C3D28) and the second on follow-up (C6D28). Baseline CT and CT performed after three cycles of pre-operatory chemotherapy will be evaluated with computed texture analysis as previously described in Ciaravino et al.38 At tumor texture analysis the following parameters will be analyzed: variance, skewness, kurtosis and entropy. This analysis supplies an evaluation of the tumor at imaging pixel or voxel-gray levels distribution and could improve the assessment of resectability after chemotherapy treatment in those cases with no evident downsizing after chemotherapy.
Tumor removal evaluation is performed with analysis of microscopic surgical margins.
Laboratory procedures
Proper execution and documentation of the laboratory procedures is the responsibility of the treating physician. A careful assessment and review is needed to check eligibility and continued participation in the program.
Complete blood count
A complete blood count (CBC) will be performed locally, and should include white blood count (WBC) and differential, hemoglobin, hematocrit and platelet count.
Serum chemistry
Serum chemistry will be performed locally. Serum chemistry should include electrolytes (sodium, potassium, chloride and bicarbonate), Blood Urea Nitrogen (BUN), serum creatinine, clearance creatinine, glucose, direct and total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), uric acid, total protein, albumin, calcium, magnesium and phosphate.
Urine or serum pregnancy test
A urine or serum pregnancy test will be obtained for all women of childbearing potential at screening, at the start of each cycle during study treatment and at the follow-up.
CA 19-9
CA 19-9 biomarker levels will be measured locally for all patients.
Translational research analyses
Liquid biopsy specimens from each patient will be collected on the first day of pre and postoperative chemotherapy, at the end of pre and postoperative chemotherapy and after resection. This bio-bank of blood samples will be available for the screening of a wide panels of circulating proteins, by using a bead-based suspension array system for multi analyte profiling (Bio-Plex, BioRad), and for detection of cell-free circulating tumor DNA, using droplet digital polymerase chain reaction (PCR) technology (BioRad).Tumor tissue specimens will be collected at diagnosis and after surgical resection and will be available for protein and RNA expression analysis. Data obtained from matched liquid biopsies and tumor tissue samples will be used for correlation with clinical outcomes.
Tumor response and toxicity criteria
Tumor responses will be assessed after three cycles (3 months) of preoperative treatment and then regularly per institutional guidance, or sooner if the treating physician suspects disease progression based on clinical signs and symptoms. All treatment decisions will be based on the local radiologist’s and/or treating physician’s assessment of disease status. All these evaluations are done according to the RECIST 1.1. Adverse events were assessed according to CTCAE version 4.0.
Safety assessment
All adverse events observed have to be reported in the patients’ medical records and in electronic case report forms (eCRFs). All serious adverse events (SAEs) occurring during the study treatment period must be reported within 24 h.
Follow-up
Follow-up of all patients will be carried out according to our protocol (every 3 months for at least 2 years, every 6 months for years 3–5, then every 12 months for life). Physical examination, tumor marker examination, and CT scan were given every 3 months.
Discontinuation of patients
A patient may withdraw from the study at any time and for any reason.
Some possible reasons for early discontinuation of study treatment include, but are not limited to, the following:
patient has radiological evidence of disease progression per RECIST 1.1
patient shows symptomatic deterioration
patient experiences intolerable toxicity, or an adverse event requiring treatment discontinuation
treatment to be withheld for more than 21 days from the start of next cycle, unless, in the opinion of the treating physician, the patient is receiving benefit from program treatment
the patient experiences an adverse event which requires more than two dose reductions
patient is significantly non-compliant with treatment procedures in the opinion of the treating physician
withdrawal of consent
the treating physician, for any reason, but considering the rights, safety and wellbeing of the patient(s) and in accordance with appropriate guidelines and local regulations, stops the program or stops the patient’s participation in the program. A patient who does not meet eligibility criteria and is inadvertently included in this program can continue program treatment if he/she has a clinical benefit according to physician opinion.
Discontinuation of the program
The program may be discontinued at any time if the sponsor deems it necessary for various legitimate medical reasons including, but not limited to, significant safety concerns, or logistic/administrative reasons, considering rights, safety and wellbeing of the patient(s) and following appropriate guidelines and local regulations.
Sample size calculation
A Simon’s two-stage design will be used. The null hypothesis that the true R0 resection rate is 40% will be tested against a one-sided alternative. In the first stage, 39 patients will be accrued. If there are 17 or fewer R0 resections in these 39 patients, the study will be stopped. Otherwise, 33 additional patients will be accrued for a total of 72. The null hypothesis will be rejected if 35 or fewer R0 resections are observed in 72 patients. This design yields a type I error rate of 0.05 and power of 0.8 when the true R0 resection rate is 55%. Accrual time is 2 years followed by 3 years of follow-up.
Statistical analyses
Demographic and clinical characteristics
All characteristics (demographics, medical history, physical examination, vital signs, KPS, ECG, laboratory procedures, tumor assessment, concomitant medication and procedure) will be provided as descriptive statistics in summary tables both at baseline and for each time visit, when appropriate.
Descriptive statistics will be provided according to the type of variable summarized:
for quantitative variables: standard quantitative statistics (N, mean, standard deviation, median, minimum and maximum)
for qualitative variables: frequency distribution [number of non-missing observations (N) and percentages (%)].
R0 will be calculated as a binary outcome. Exact 95% confidence intervals will be calculated for binary outcomes. DFS is calculated from the date of enrollment to the date of detected disease recurrence. The following events are defined as recurrence: primary cancer recurrence and death. OS is calculated from the date of enrollment to the date of death or date of last follow-up. Survival will be estimated by using the Kaplan–Meier method.
Adverse events
Adverse events will be tabulated in a descriptive manner at each time visit. Toxicity evaluation will be enumeration of all major toxicities, with proportions calculated for each.
Follow-up
Survival data will be reported descriptively and graphically, for each time of follow-up visit (up to 2 years).
Interim analysis
An interim analysis is planned when the first 39 patients are accrued.
Data management, control of data consistency, quality control, monitoring and audits
The investigator is responsible for ensuring data quality. All information required in the protocol are entered in the eCRF. Periodic monitoring visits at the center are planned. Monitoring procedures will be adapted to the study-specific risks for patients. Standard operating procedures (SOPs) will be interpreted to ensure patient safety and the integrity of the clinical data. The investigator or a designated representative is obliged to provide clarification or respond to queries. If no further corrections are to be made in the database, it will be locked and used for statistical analysis.
Patient informed consent
The treating physician is responsible for ensuring that the patient understands the risks and benefits of participating in the program, including answering any questions the patient may have throughout the program and sharing any new information that may be relevant to the patient’s willingness to continue his or her participation in the program in a timely manner.
No program-related procedures will be performed until a patient or a patient’s legal representative has given written informed consent. The informed consent document must clearly describe the potential risks and benefits of the program, and each prospective participant must be given adequate time to discuss the program with the treating physician to decide whether or not to participate. Each patient who agrees to participate in the program and who signs the informed consent should be given a copy of the signed and dated document. The provision of informed consent should be documented in the patient’s medical record.
As used in this protocol, the term ‘informed consent’ includes all informed consent given by patients or their legal representatives (for informed consents see Appendices 1 and 2).
Investigational review board or ethics committee approval
The treating physician is responsible for obtaining investigational review board (IRB)/ethics committee (EC) approvals as locally required, unless a central EC approval has been obtained.
Discussion
The theme of pre or perioperative versus postoperative therapy for the treatment of resectable pancreatic cancer is a major source of dispute in this disease.39 Currently, the recommended treatment for the 10–20% of patients with potentially resectable pancreatic cancer is surgical resection followed by adjuvant chemotherapy.8,40 However, the administration of adjuvant treatment is often limited by surgical complications and a significant rate of early disease recurrence.
In this present study, we want to validate prospectively the safety and the activity of a novel preoperative regimen of chemotherapy with nal-IRI in combination with 5-FU and oxaliplatin, in particular in terms of improving R0 rates, defined according to a standardized international R classification. As a matter of fact, determination of the resection status is part of the pathological examination and it is a critical step of correct staging and planning of postoperative treatments. Moreover, it has been shown to have a prognostic value for pancreatic cancer in several studies.41–44 Despite the high R0 resection rates commonly reported in the literature, local and distal recurrence is frequent in patients with pancreatic cancer. This discrepancy is well highlighted in a recent retrospective study of 360 patients that reported 66% of local recurrence rates in R0 patients and 68% in the R1 group.45 Thus, the R1 rate is probably underestimated in certain studies. The R0 resection rate ranges from 15% to 83% across different studies.41,43–51 This heterogeneity in reporting R0 rates may be attributed to the lack of an international definition of the relevant resection margins, and of standardized protocols for pathological reporting.
According to the British Royal College of Pathology (RCPath) guidelines (www.rcpath.org), microscopic tumor clearance for at least 1 mm in all transection (the pancreatic duct margin, the bile duct margin, the proximal duodenal/stomach margin, the distal duodenal margin) and circumferential margins (the posterior pancreatic surface, the medial margin and the anterior surface) is required to confirm radicality and be considered as a meaningful prognostic and predictive factor. In the case of a vascular resection, the entire transection margins of the vessel should be validated.48 In more recent studies, the introduction of a standardized protocol for the evaluation of pancreatic cancer resection specimens led to significantly lower R0 resection rates than expected.41,49,52 In this regard, we recently conducted a retrospective evaluation by using these guidelines of 131 patients who underwent up-front resection for pancreatic cancer from February 2018 to March 2019. Based on these guidelines, we measured a 60% (81/131) of R1 and only a 40% (50/131) of R0 resection in this series, and this result was considered a benchmark for designing this present study. This result is perfectly in line with the R0 resection rate (40%) measured in the immediate surgery group within the recent prospective phase III PREOPANC trial.25
Despite recent biological insight and therapeutic advances, the prognosis of pancreatic cancer patients remains poor even in the resectable setting and this is largely correlated to the limited efficacy of available treatments. TAMs are a key component of the cancer microenvironment and can limit the activity of chemotherapies if programmed towards the M2 form by specific signals.53 We contributed to this field by demonstrating that several cytokines involved in myeloid cell attraction and M2 polarization, including IL-4 and MIP1-α, are the most significant negative prognostic factors in patients affected by resectable or advanced pancreatic cancer.54–56 As this subgroup of patients with higher levels of macrophage-attractant factors have probably the worst prognosis in this already devastating clinical setting and nal-IRI uses macrophages for its activity, we will investigate the relevance of these cytokines in the mechanisms of cancer resistance to nal-IRI, such as TAMs’ recruitment and activation, with the aim of improving the selection of pancreatic cancer patients with the worst prognosis for the most suitable and effective treatment. Moreover, one of the most common features of pancreatic cancer is a desmoplastic stromal microenvironment. The differentiation of mesenchymal precursors in myofibroblasts leads to increased collagen deposition and extracellular matrix remodelling as well as increased interstitial fluid pressure (IFP). This affects the efficiency of particle-based chemotherapeutic drug delivery to the tumor, as they cannot penetrate tissue under positive IFP. As an exploratory objective, our phase II study will correlate the pre and post-treatment levels of several biomarkers of tumor microenvironment remodeling with response to preoperative 5-FU, nal-IRI, oxaliplatin treatment. Blood samples and archived tumor tissues from the patients of the study will be collected and analyzed to survey potential predictive/prognostic biomarkers that may correlate with nal-IRI PK, toxicity, and/or disease response. The study will also evaluate the relationship between plasma PK of nal-IRI (total irinotecan, SN-38), oxaliplatin, 5-FU and efficacy and safety endpoints in resectable pancreatic cancer patients. We will evaluate pre and post-treatment levels of transdifferentiation of fibroblasts into activated myofibroblasts, pericyte coverage of the tumor vasculature and microvessel density, fibrotic and collagen content in tumor specimen sections, intratumor nal-IRI, SN-38, CPT-11 concentration. The TAMs population and their modulation during nal-IRI treatment will be measured and characterized in pre and post-treatment sample tissues. We aim to confirm prospectively the prognostic and/or predictive role of IL-4, CCL3/MIP-1α and TAMs enrichment for nal-IRI activity and detect potential predictive/prognostic biomarkers that could help clinicians to understand which patients will benefit more from the treatment, thus avoiding unnecessary toxicities in supposedly resistant patients.
The possibility to measure the presence of local or disseminated minimal residual disease even after radical resection remains of the utmost importance for the prognosis of patients. The detection of circulating cell-free DNA derived from tumor cells, or ctDNA, right after surgery and its longitudinal monitoring is one of the most promising approaches for predicting prognosis in this setting.57 We will perform deep sequencing [CAncer Personalized Profiling by deep Sequencing, (CAPP-Seq)] analyses58 of plasma cell-free DNA collected before and after resection to determine whether detection of ctDNA is associated with the risk of tumor recurrence.
Nonetheless, we acknowledge some limitations of this study. In order to have the chance fully to endorse the use of the nITRO regimen for the perioperative treatment of resectable pancreatic cancer patients, this trial should have had a randomized design by including a standard treatment arm with surgery followed by mFOLFIRINOX postoperative treatment. The novelty of the treatment explored in this present study is, indeed, both in the chemotherapeutic regimen explored itself for the first time in pancreatic cancer, as well as in the treatment paradigm shift proposed from a standard postoperative to a novel perioperative strategy. The acquisition of knowledge and meaning is rarely a linear process. The scientific knowledge progresses by a process of continuous paradigm shifts that produce a quantum leap in understanding rather than the assimilation of new knowledge into an existing paradigm. However, individual enquirers can experience significant steps at critical stages and, thus, gain greater insight and understanding of the complexity of the phenomenon under investigation.59 Several large randomized trials are currently addressing the potential advantage of pre or perioperative versus standard postoperative strategies by using regimens more commonly approved for the metastatic setting (ClinicalTrials.com identifiers: NCT02172976, NCT04340141, NCT02919787, NCT02959879). These are generally conducted by comprehensive cooperative groups with the advantage of counting on a large pool of potential patients to be enrolled in such randomized trials that generally require large sample sizes. Moreover, the agreement among surgical units in a given geographical area to offer the enrollment in the same clinical trial of pre or perioperative treatment to immediately resectable pancreatic cancer patients is essential to avoid the expected migration of patients towards different surgical units that might propose resection up-front, impairing, in turn, the enrollment rate in the trial. Thus, only the momentum given by a large cooperative group could protect from the limited patient population cohorts and the slow enrollment rate sometimes faced by randomized trials in this setting and that could limit the relevance of their conclusions.60 In this regard, we aimed the nITRO trial to explore the safety and activity of a chemotherapeutic regimen that to our knowledge is one of the most novel and potentially active in this disease. Whether this present trial will satisfy its hypothesis, we will have more solid evidence to support a future randomized clinical trial design within a nationwide cooperative group.
In conclusion, the nITRO trial will contribute to strengthen the clinical evidence supporting perioperative strategies in immediately resectable pancreatic cancer patients. Moreover, this study represents a unique opportunity for translational analyses aimed to identify novel immune-related prognostic and predictive factors in this clinical setting.
Acknowledgments
The authors sincerely thank all the patients and their families for participating in our study and for contribution to medicine and science. Work in the Digestive Molecular Clinical Oncology Research Unit is partially supported by investigator grants nos. 19111 and 23719 and 5x1000 grant no. 12182 through the Associazione Italiana per la Ricerca sul Cancro (AIRC), by the Ricerca Finalizzata 2016 grant GR-2016-02361134 through the Italian Ministry of Health, and by the ‘Nastro Viola’ and ‘Voglio il Massimo’ associations of patients’ donations to D. Melisi. Part of the work is performed at the Laboratorio Universitario di Ricerca Medica (LURM) Research Center, University of Verona. The authors also thank Dr. Hayley Louise Salt for data entry and administrative support.
Appendix
Appendix 1.
Informed consent for screening and enrolling patients in the nITRO trial.
Appendix 2.
Informed consent for translational research studies for patients enrolled in the Nitro trial.
Footnotes
Author contributions: DM is the principal investigator for this study. He ideated, initiated, holds the intellectual property of the project and was a major contributor in writing the protocol. Francesca S, CZ, VM contributed to the study design and writing the protocol. Francesca S, CZ, VM, AC, SC, MG, MD, Giuseppe M, LL, AE, Giovanni M, LC, MT, SP, Filippo S, Alessandro G, IF, PR, PC, Stefano G, Armando G, LB, CP, Serena G, MP, MG, GB, CB contributed to the study conduct. IR and SM were involved in protocol writing and approval. VF contributed to the translational studies conduction. All authors read and approved the manuscript.
Conflict of interest: DM declares research funding from Shire, Incyte, Evotec, Celgene, iOnctura and consulting role with Eli Lilly, Shire, Evotec, Baxter, Incyte, iOnctura.
Ethics approval and consent to participate: This study is conducted in accordance with the Declaration of Helsinki and ethical guidelines for medical and health research involving human subjects. The study received approval on 7 May 2019 by the ethics committee of Verona and Rovigo (protocol number CRC2017_01, v.1.1). All patients will provide written informed consent before enrollment.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study has been provided by an unrestricted research grant by Shire. Shire did not have any role in the design of the study and will not have any role in the conduction of the study.
ORCID iDs: Marina Gaule  https://orcid.org/0000-0001-6008-8495
https://orcid.org/0000-0001-6008-8495
Davide Melisi  https://orcid.org/0000-0002-4031-7585
https://orcid.org/0000-0002-4031-7585
Contributor Information
Francesca Simionato, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Camilla Zecchetto, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Valeria Merz, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Alessandro Cavaliere, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Simona Casalino, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Marina Gaule, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, Verona, Italy.
Mirko D’Onofrio, Department of Radiology, University and Hospital Trust of Verona, Verona, Italy.
Giuseppe Malleo, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Luca Landoni, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Alessandro Esposito, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Giovanni Marchegiani, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Luca Casetti, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Massimiliano Tuveri, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Salvatore Paiella, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Filippo Scopelliti, Department of Surgery, Pancreatic Surgery Unit, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Alessandro Giardino, Department of Surgery, Pancreatic Surgery Unit, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Isabella Frigerio, Department of Surgery, Pancreatic Surgery Unit, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Paolo Regi, Department of Surgery, Pancreatic Surgery Unit, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Paola Capelli, Department of Pathology, University and Hospital Trust of Verona, Verona, Italy.
Stefano Gobbo, Department of Pathology, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Armando Gabbrielli, Endoscopy Unit, University and Hospital Trust of Verona, Verona, Italy.
Laura Bernardoni, Endoscopy Unit, University and Hospital Trust of Verona, Verona, Italy.
Vita Fedele, Digestive Molecular Clinical Oncology Research Unit, Department of Medicine, University of Verona, Verona, Italy.
Irene Rossi, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Cristiana Piazzola, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Serena Giacomazzi, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Martina Pasquato, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Morena Gianfortone, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Stefano Milleri, Centro Ricerche Cliniche di Verona, University and Hospital Trust of Verona, Verona, Italy.
Michele Milella, Medical Oncology Unit, University and Hospital Trust of Verona, Verona, Italy.
Giovanni Butturini, Department of Surgery, Pancreatic Surgery Unit, Hospital P. Pederzoli, Peschiera del Garda, Italy.
Roberto Salvia, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Claudio Bassi, Department of Surgery, University and Hospital Trust of Verona, Verona, Italy.
Davide Melisi, Digestive Molecular Clinical Oncology Unit, Section of Medical Oncology, Department of Medicine, University of Verona, AOUI Verona – Policlinico “G.B. Rossi”, Piazzale L.A. Scuro, 10, Verona 37134, Italy; Medical Oncology Unit, University and Hospital Trust of Verona, Verona 37134, Italy.
References
- 1. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2. Vaccaro V, Melisi D, Bria E, et al. Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin Ther Targets 2011; 15: 1183–1196. [DOI] [PubMed] [Google Scholar]
- 3. Tamburrino A, Piro G, Carbone C, et al. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front Pharmacol 2013; 4: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaccaro V, Sperduti I, Vari S, et al. Metastatic pancreatic cancer: is there a light at the end of the tunnel? World J Gastroenterol 2015; 21: 4788–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Melisi D, Calvetti L, Frizziero M, et al. Pancreatic cancer: systemic combination therapies for a heterogeneous disease. Curr Pharm Des 2014; 20: 6660–6669. [DOI] [PubMed] [Google Scholar]
- 6. Bazzichetto C, Conciatori F, Luchini C, et al. From genetic alterations to tumor microenvironment: the Ariadne’s string in pancreatic cancer. Cells 2020; 9: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004; 363: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 8. Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010; 304: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 9. Wolff RA. Adjuvant or neoadjuvant therapy in the treatment in pancreatic malignancies: where are we? Surg Clin North Am 2018; 98: 95–111. [DOI] [PubMed] [Google Scholar]
- 10. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013; 310: 1473–1481. [DOI] [PubMed] [Google Scholar]
- 11. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011–1024. [DOI] [PubMed] [Google Scholar]
- 12. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 13. Tzeng CWD, Cao HST, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg 2014; 18: 16–24; discussion 24–25. [DOI] [PubMed] [Google Scholar]
- 14. Aloia TA, Lee JE, Vauthey JN, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007; 204: 347–355. [DOI] [PubMed] [Google Scholar]
- 15. Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012; 214: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012; 148: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolff RA, Varadhachary GR, Evans DB. Adjuvant therapy for adenocarcinoma of the pancreas: analysis of reported trials and recommendations for future progress. Ann Surg Oncol 2008; 15: 2773–2786. [DOI] [PubMed] [Google Scholar]
- 19. Glant JA, Waters JA, House MG, et al. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery 2011; 150: 607–616. [DOI] [PubMed] [Google Scholar]
- 20. Seufferlein T, Ettrich TJ. Treatment of pancreatic cancer-neoadjuvant treatment in resectable pancreatic cancer (PDAC). Transl Gastroenterol Hepatol 2019; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol 2017; 35: 515–522. [DOI] [PubMed] [Google Scholar]
- 22. Gillen S, Schuster T, Büschenfelde CMZ, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7: e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018; 105: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhir M, Malhotra GK, Sohal DPS, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol 2017; 15: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020; 38: 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de W, Marsh R, Talamonti MS, Baker MS, et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: a pilot study. J Surg Oncol 2018; 117: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst 2019; 111: 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drummond DC, Noble CO, Guo Z, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 2006; 66: 3271–3277. [DOI] [PubMed] [Google Scholar]
- 29. Kang MH, Wang J, Makena MR, et al. Activity of MM-398, nanoliposomal irinotecan (nal-IRI), in Ewing’s family tumor xenografts is associated with high exposure of tumor to drug and high SLFN11 expression. Clin Cancer Res 2015; 21: 1139–1150. [DOI] [PubMed] [Google Scholar]
- 30. Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res 2014; 74: 7003–7013. [DOI] [PubMed] [Google Scholar]
- 31. Tsai CS, Park JW, Chen LT. Nanovector-based therapies in advanced pancreatic cancer. J Gastrointest Oncol 2011; 2: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 33. Pelzer U, Blanc JF, Melisi D, et al. Quality-adjusted survival with combination nal-IRI+5-FU/LV vs 5-FU/LV alone in metastatic pancreatic cancer patients previously treated with gemcitabine-based therapy: a Q-TWiST analysis. Br J Cancer 2017; 116: 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hubner RA, Cubillo A, Blanc JF, et al. Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5-fluorouracil and leucovorin. Eur J Cancer 2019; 106: 24–33. [DOI] [PubMed] [Google Scholar]
- 35. Taieb J, Prager GW, Melisi D, et al. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open 2020; 5: e000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aprile G, Negri FV, Giuliani F, et al. Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol 2017; 115: 1–12. [DOI] [PubMed] [Google Scholar]
- 37. Wainberg ZA, Boland PM, Lieu CH, et al. A phase 1/2, open-label, dose-expansion study of liposomal irinotecan (nal-IRI) plus 5-fluorouracil/leucovorin (5-FU/LV) and oxaliplatin (OX) in patients with previously untreated metastatic pancreatic cancer (mPAC). World GI Annual Meeting, Barcelona, Spain, 2019. [Google Scholar]
- 38. Ciaravino V, Cardobi N, DE Robertis R, et al. CT texture analysis of ductal adenocarcinoma downstaged after chemotherapy. Anticancer Res 2018; 38: 4889–4895. [DOI] [PubMed] [Google Scholar]
- 39. Li D, O’Reilly EM. Adjuvant and neoadjuvant therapy for pancreatic cancer. Surg Oncol Clin N Am 2016; 25: 311–326. [DOI] [PubMed] [Google Scholar]
- 40. Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis 2018; 50: 1257–1271. [DOI] [PubMed] [Google Scholar]
- 41. Jamieson NB, Foulis AK, Oien KA, et al. Positive mobilization margins alone do not influence survival following pancreatico-duodenectomy for pancreatic ductal adenocarcinoma. Ann Surg 2010; 251: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 42. Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001; 234: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 2003; 27: 324–329. [DOI] [PubMed] [Google Scholar]
- 44. Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004; 91: 586–594. [DOI] [PubMed] [Google Scholar]
- 45. Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007; 246: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993; 217: 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol 2008; 15: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 48. Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006; 93: 1232–1237. [DOI] [PubMed] [Google Scholar]
- 49. Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009; 55: 277–283. [DOI] [PubMed] [Google Scholar]
- 50. Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226: 248–257; discussion 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006; 244: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaedcke J, Gunawan B, Grade M, et al. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg 2010; 395: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017; 14: 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piro G, Simionato F, Carbone C, et al. A circulating TH2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. Oncoimmunology 2017; 6: e1322242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Melisi D, Garcia-Carbonero R, Macarulla T, et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol 2019; 83: 975–991. [DOI] [PubMed] [Google Scholar]
- 56. Melisi D, Garcia-Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 2018; 119: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nagai M, Sho M, Akahori T, et al. Application of liquid biopsy for surgical management of pancreatic cancer. Ann Gastroenterol Surg 2020; 4: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med 2014; 20: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Popper K. Conjectures and refutations: the growth of scientific knowledge. London: Routledge, 1963. [Google Scholar]
- 60. Reni M, Balzano G, Zanon S, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol 2018; 3: 413–423. [DOI] [PubMed] [Google Scholar]