Abstract
Background:
Computer-aided detection (CADe) of colon polyps has been demonstrated to improve colon polyp and adenoma detection during colonoscopy by indicating the location of a given polyp on a parallel monitor. The aim of this study was to investigate whether embedding the CADe system into the primary colonoscopy monitor may serve to increase polyp and adenoma detection, without increasing physician fatigue level.
Methods:
Consecutive patients presenting for colonoscopies were prospectively randomized to undergo routine colonoscopy with or without the assistance of a real-time polyp detection CADe system. Fatigue level was evaluated from score 0 to 10 by the performing endoscopists after each colonoscopy procedure. The main outcome was adenoma detection rate (ADR).
Results:
Out of 790 patients analyzed, 397 were randomized to routine colonoscopy (control group), and 393 to a colonoscopy with computer-aided diagnosis (CADe group). The ADRs were 20.91% and 29.01%, respectively (OR = 1.546, 95% CI 1.116–2.141, p = 0.009). The average number of adenomas per colonoscopy (APC) was 0.29 and 0.48, respectively (Change Folds = 1.64, 95% CI 1.299–2.063, p < 0.001). The improvement in polyp detection was mainly due to increased detection of non-advanced diminutive adenomas, serrated adenoma and hyperplastic polyps. The fatigue score for each procedure was 3.28 versus 3.40 for routine and CADe group, p = 0.357.
Conclusions:
A real-time CADe system employed on the primary endoscopy monitor may lead to improvements in ADR and polyp detection rate without increasing fatigue level during colonoscopy. The integration of a low-latency and high-performance CADe systems may serve as an effective quality assurance tool during colonoscopy. www.chictr.org.cn number, ChiCTR1800018058.
Keywords: artificial intelligence, colonoscopy, computer-aided diagnosis, polyp
Background and objectives
Colonoscopy remains the gold standard examination for colorectal cancer (CRC) screening. However, colonoscopy can be technically demanding, and there is significant variation in how colonoscopy is performed. This variation in performance has been linked to important outcome measures. For example, interval cancers are more common in low adenoma detectors as compared with high adenoma detectors. Evidence has shown that each 1% increase in adenoma detection rate (ADR) predicts 3% decrease in interval CRC.1,2
Computer-aided detection (CADe)3 of colon polyps during colonoscopy has been demonstrated to improve colon polyp detection rates (PDRs) and ADRs by alarming polyps on a parallel monitor.4 Introducing a second monitor might unnecessarily add physician fatigue by changing gaze patterns and requiring that an endoscopist watches both screens, an issue that has come up in the study of similar technologies.5 Moreover, interestingly, some briefly visible polyps, especially those that appear on the edge of the screen or are partially obscured, might still be missed during the time interval when the endoscopist turns to look at the second monitor. A recent study showed that more than 43% of those polyps and adenomas initially missed by physicians were on the edge of the visual field.6 Thus, vigilance remains crucially important so that brief alarm flashes on the edge of the visual field may otherwise be missed (supplemental material Video S1, Video S2). This degree of vigilance may be easier if the CADe is embedded into the primary colonoscopy monitor so that the endoscopist’s attentions are not divided. Thanks to recent improvements in graphics processing unit (GPU) computation power and improvements in processing time, a CADe system may be embedded into the primary endoscopy monitor without introducing noticeable latency. A recent study7 showed an increase of adenoma detection using fully integrated CADe in the endoscopy workflow, avoiding a second display to show the artificial intelligence (AI) detection. However, the ADR of the control group was based on only high-risk CRC populations without representation of more extensive normal patients. Furthermore, false detections and missed polyps by the CADe and initially missed polyps by the endoscopist were not recorded nor analyzed in the recent one-monitor study. The concrete contributions and distractions caused by the real-time CADe system are crucially important to reveal the actual efficacy and risk of such systems.
In this study, we further hypothesized that this may also lead to decrease physician fatigue during endoscopy. The aim of this study was to investigate whether the integration of a CADe system into the primary monitor used during colonoscopy may increase polyp and adenoma detection without increasing physician fatigue. False detections and missed polyps by the CADe system and initially missed polyps by the endoscopist were recorded, analyzed and compared with the previous double-blinded study,6 in order to gain a concrete understanding of how a real-time CADe system makes efficacy during real-life colonoscopy.
Method
Study design
CADe system integration (EndoScreener, Shanghai Wision AI Co., Ltd. China)
The real-time automatic polyp detection system (Figure S1, supplemental material) was developed on a deep-learning architecture,8 which was previously validated to have a per-image sensitivity of 94.38%, per-image specificity of 95.92% and an area under the receiver operating characteristic curve of 0.984 to detect colon polyps in colonoscopy report images. Moreover, the algorithm was demonstrated to have a per-polyp sensitivity of 100.00% (per-image sensitivity of 91.64%) and a per-image specificity of 95.40% in real-world colonoscopy videos.3
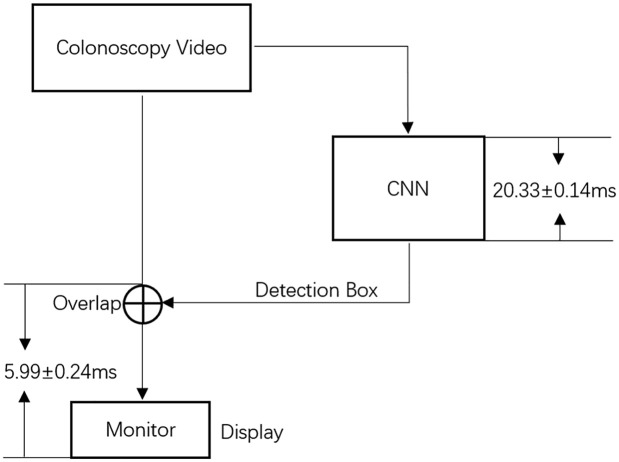
By changing the deep-learning framework from Caffe (Berkeley, CA) to TensorRT (Nvidia, CA), and combining some layers in SegNet, the system processes >30 frames per second on Ge-Force-1080ti (Nvidia, CA). To enhance user experience regarding latency, the colonoscopy video was unprocessed and displayed directly on the primary monitor without waiting for its detection result (the detection box), which was then placed on subsequent frames with a latency of 20.33 ± 0.14 ms. CPU/GPU was used for the overlap and display of colonoscopy video frames, which takes 5.99 ± 0.24 ms to overlap the detection box in the original colonoscopy video and display on the monitor. If FPGA or ASIC were used for the overlapping and displaying instead of CPU/GPU, colonoscopy video with detection can be displayed with a latency less than 1.00 ms (Figure 1).
Figure 1.
Latency diagram.
Prospective randomized study
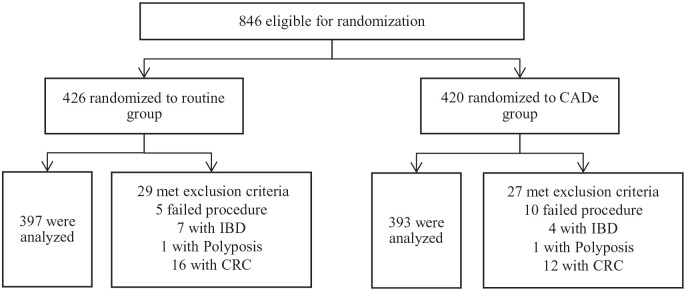
The prospective study was designed as a non-blinded randomized trial. This study was conducted in the Endoscopy Center of Sichuan Provincial People’s Hospital, China. Consecutive patients who underwent colonoscopy from September 2018 to February 2019 were eligible for enrollment (Figure 2). Written informed consent was obtained from all individual participants enrolled in the study. Bowel preparation method was 2 L of polyethylene glycol with 6 ml simethicone solution, given in split doses. Colonoscopies were performed with last-generation high image-quality colonoscopes (Olympus CF-Q260 and CF-H260) and high-definition monitors. We excluded patients with a history of inflammatory bowel disease (IBD), CRC, any polyposis syndromes, colorectal surgery and patients with a contraindication for biopsy. Patients with prior failed colonoscopy and high suspicion of polyposis syndromes, IBD and typical advanced CRC were also excluded.
Figure 2.
Flow diagram of enrollment.
Baseline demographic characteristics for each patient were recorded (Table S1, supplemental material). Any complication during the procedure or recovery was also recorded.
Eleven physicians from the division of gastroenterology participated in the study, including eight experienced endoscopists (four seniors, four mid-level) and three junior endoscopists.
Each patient was prospectively randomized into one of two groups by the research assistant, using a digital random number generator before the colonoscopy procedure. In the control group, a routine colonoscopy was performed. In the research group (CADe group), the real-time automatic polyp detection system was used to assist the endoscopist. The system was embedded in the endoscopy processor, processed each frame of the video stream synchronously and reported the detected polyp location with a hollow blue tracing box directly in the endoscopy monitor with a simultaneous sound alarm (Video S3). The system was employed during withdrawal only. For any area detected by the CADe system, the endoscopist was required to check and verify the area using his or her own clinical judgment. The staff assistant records whether the CADe system detects polyps before endoscopists every time encountering a polyp; this is based on the subjective judgment of the operating endoscopist. In this case, the CADe system detects a polyp prior to the endoscopist, which indicates not only the advantage of low latency but also the possibility of avoiding a missed diagnosis. If the endoscopist deemed that a polyp was first identified by himself or simultaneously with the CADe system, then it was not credited to the CADe system. After each procedure, the endoscopist rated his or her fatigue level from 0 to 10 using a 10-point Likert scale where 0 represented no fatigue and 10 represented extreme fatigue.
When a polyp was identified either by the endoscopist first or by CADe system first, the nurse assisted in performing cold forceps biopsy for histology and the staff assistant recorded the location, size and morphological features according to the Paris classification. In the CADe group, missed polyps by the system and consistent false detections were also recorded. A missed polyp was defined as a polyp confirmed by the endoscopist but undetected by the system. A consistent false detection was defined as a detected lesion, which was continuously traced by the system, deemed by the endoscopist not to be a polyp.6 This study was approved by the local review board and registered with the Chinese Clinical Trial Registry.
All authors had access to the study data and reviewed and approved the final manuscript.
Study endpoint
The primary endpoint was ADR, which was defined as proportion of individuals undergoing a complete colonoscopy who have one or more adenomas detected. The secondary endpoints were PDR, which was defined as the proportion of individuals undergoing a complete colonoscopy who have one or more polyps detected. Polyps per colonoscopy (PPC) and adenomas per colonoscopy (APC) were calculated by dividing the total number of polyps or adenomas detected by the total number of colonoscopies performed.
Statistical analysis
We prospectively designed this study to allow for 80% power or more to detect a 10% difference (30% versus 20%), in ADR, between colonoscopy procedures using a two-group Chi-square test with a two-sided level of 0.05. A sample size of 702 participants was needed, and the overall participant enrollment goal was 850 to allow for potential exclusions or dropouts.
Statistical analysis was performed with R studio V.3.4.0 or higher. Comparison of baseline clinical and demographic characteristics between the CADe and the control group was performed using a Chi-square test for categorical variables and using the two-sample t-test for continuous variables. Regarding the ADR and PDR, a logistic regression was performed to evaluate the effect of computer-assisted diagnosis for colonoscopy on the adenoma/polyp detection rate. The response variable was the binary outcome of whether an adenoma/polyp was detected or not. The covariate was the group variable indicating whether the patient belonged to the computer-assisted group. Regarding the number of detected adenomas and polyps, a Poisson regression was applied to evaluate the effect of computer-assisted diagnosis for colonoscopy. A two-sided p-value of 0.05 was used as the threshold for statistical significance. In the event of any baseline clinical and demographic characteristics showing a statistically significant difference between the two comparison groups, additional covariate adjusted logistics/Poisson regression models were built to address the possible confounding effect by adding those significant characteristics into the models as covariates.
Results
Patient enrollment and baseline data
A total of 846 consecutive patients were enrolled, among which 56 patients were excluded during colonoscopy due to meeting exclusion criteria. A total of 790 eligible patients were analyzed, with 397 patients randomized into the control group and 393 into the CADe group. Baseline characteristics are presented in Table 1. There were no statistically significant differences between the two groups in terms of demographic data and adenoma detection risk factors (Table S1). There were no complications reported. Withdrawal times when biopsy time was excluded from analysis were 6.62 min and 6.71 min in the control and CADe groups, respectively (p = 0.450).
Table 1.
Baseline information.
| Characteristics | Routine colonoscopy (n = 397) | CADe colonoscopy (n = 393) | p-value* |
|---|---|---|---|
| Age, mean (SD) | 48.79 (13.00) | 49.84 (13.11) | 0.253 |
| BMI, mean (SD) | 23.13 (3.02) | 23.08 (3.08) | 0.821 |
| Withdrawal time, mean (SD) | 6.94 (1.53) | 7.29 (1.98) | 0.007 |
| Withdrawal time excluding biopsy time | 6.62 (1.22) | 6.71 (1.63) | 0.450 |
| Total time, mean (SD) | 13.01 (4.64) | 13.37 (5.15) | 0.315 |
| Insertion time, mean (SD) | 6.07 (4.40) | 6.08 (4.70) | 0.975 |
| No polyp withdrawal time, mean (SD) | 6.54 (1.13) | 6.41 (1.27) | 0.249 |
| Indication | 0.231 | ||
| Screening, n (%) | 84 (21.16) | 98 (24.94) | |
| Symptomatic, n (%) | 313 (78.84) | 295 (75.06) | |
| Sex | 0.407 | ||
| Female, n (%) | 203 (51.13) | 213 (54.20) | |
| Male, n (%) | 194 (48.87) | 180 (45.80) | |
| BMI category | 0.920 | ||
| <25, n (%) | 296 (74.56) | 290 (73.79) | |
| 25 ⩽ BMI <30, n (%) | 93 (23.43) | 96 (24.43) | |
| ⩾30, n (%) | 8 (2.02) | 7 (1.78) | |
| Procedure time | 0.334 | ||
| AM, n (%) | 197 (49.62) | 209 (53.18) | |
| PM, n (%) | 200 (50.38) | 184 (46.82) | |
| Endoscope | 0.127 | ||
| H-260 | 183 (46.10) | 202 (51.40) | |
| Q-260 | 214 (53.90) | 191 (48.60) | |
| Anesthesia# | 0.835 | ||
| No, n (%) | 18 (4.53) | 19 (4.83) | |
| Yes, n (%) | 379 (95.47) | 374 (95.17) | |
| Boston Score, mean (SD) | 6.71 (1.38) | 6.72 (1.52) | 0.911 |
| Boston Score Rank | 0.067 | ||
| Inadequate (Sum < 6.0 or anyone < 2.0), n (%) | 63 (15.87) | 82 (20.87) | |
| Adequate (Sum ⩾ 6.0 and everyone ⩾ 2.0), n (%) | 334 (84.13) | 311 (79.13) | |
| Doctor level | 0.787 | ||
| Senior | 127 (31.99) | 120 (30.53) | |
| Mid-level | 234 (58.94) | 241 (61.32) | |
| Junior | 36 (9.07) | 32 (8.14) | |
No Polyp Withdrawal time: withdrawal time during those colonoscopies where no polyp was detected or removed.
p-value from χ2 test (or Fisher’s exact test, as appropriate) or t-test.
Anesthesia was administered with midazolam, fentanyl by an anesthesiologist there to monitor for complications.
BMI, body mass index.
Adenoma characteristics, adenomas detected per colonoscopy and ADR
There were 304 (48.64%) adenomas and 12 (1.92%) serrated adenomas detected. The ADR were 20.91% and 29.01% in the control and CADe arms, respectively [odds ratio (OR) = 1.546, 95% confidence interval (CI) 1.116–2.141, p = 0.009]. The APCs were 0.29 and 0.48, respectively (Change Folds = 1.64, 95% CI 1.299–2.063, p < 0.001). The average number of sessile serrated lesions (SSLs) was 0.0049 versus 0.0261 in the control versus CADe groups, p = 0.021 (Tables 2 and 3).
Table 2.
Polyp and adenoma characteristics.
| Characteristics | Routine colonoscopy (n = 204) | CADe colonoscopy (n = 421) | p-value* |
|---|---|---|---|
| Pathology | 0.006 | ||
| Carcinoma, n (%) | 0 (0.00) | 0 (0.00) | 1.000 |
| SSL, n (%) | 1 (0.49) | 11 (2.61) | 0.021 |
| Adenoma, n (%) | |||
| Advanced adenoma, n (%) | 8 (3.92) | 6 (1.43) | 0.607 |
| Others, n (%) | 108 (52.94) | 182 (43.23) | <0.001 |
| Benign lesions, n (%) | |||
| Hyperplastic and inflammatory | 87 (42.65) | 222 (52.73) | <0.001 |
| Hamartoma | 0 (0.00) | 0 (0.00) | 1.000 |
| Normal colon mucosa | 0 (0.00) | 0 (0.00) | 1.000 |
| Polyp location | 0.505 | ||
| Cecum, n (%) | 5 (2.45) | 8 (1.90) | 0.400 |
| Ascending, n (%) | 37 (18.14) | 87 (20.67) | <0.001 |
| Transverse, n (%) | 48 (23.53) | 76 (18.05) | 0.011 |
| Descending, n (%) | 25 (12.25) | 45 (10.69) | 0.017 |
| Sigmoid, n (%) | 38 (18.63) | 98 (23.28) | <0.001 |
| Rectum, n (%) | 51 (25.00) | 107 (25.42) | <0.001 |
| Polyp shape | 0.016 | ||
| Pedunculated, n (%) | 18 (8.82) | 22 (5.23) | 0.507 |
| Sessile, n (%) | 182 (89.22) | 398 (94.54) | <0.001 |
| LST, n (%) | 4 (1.96) | 1 (0.24) | 0.218 |
| Polyp size (mm), mean (SD) | 4.97 (3.03) | 3.85 (2.05) | <0.001 |
| Polyp size category | 0.001 | ||
| 0–5 mm, n (%) | 149 (73.04) | 359 (85.27) | <0.001 |
| 6–10 mm, n (%) | 47 (23.04) | 56 (13.30) | 0.349 |
| >10 mm, n (%) | 8 (3.92) | 6 (1.43) | 0.607 |
| Adenoma location | 0.253 | ||
| Cecum, n (%) | 4 (3.45) | 4 (2.13) | 0.989 |
| Ascending, n (%) | 25 (21.55) | 52 (27.66) | 0.002 |
| Transverse, n (%) | 31 (26.72) | 33 (17.55) | 0.771 |
| Descending, n (%) | 16 (13.79) | 25 (13.30) | 0.154 |
| Sigmoid, n (%) | 22 (18.97) | 49 (26.06) | 0.002 |
| Rectum, n (%) | 18 (15.52) | 25 (13.30) | 0.273 |
| Adenoma shape | 0.093 | ||
| Pedunculated, n (%) | 17 (14.66) | 19 (10.11) | 0.716 |
| Sessile, n (%) | 97 (83.62) | 169 (89.89) | <0.001 |
| LST, n (%) | 2 (1.72) | 0 (0.00) | 0.996 |
| Adenoma size (mm), mean (SD) | 5.83 (3.15) | 4.55 (2.26) | <0.001 |
| Adenoma size category | 0.004 | ||
| 0–5 mm, n (%) | 69 (59.48) | 146 (77.66) | <0.001 |
| 6–10 mm, n (%) | 41 (35.34) | 37 (19.68) | 0.683 |
| >10 mm, n (%) | 6 (5.17) | 5 (2.66) | 0.776 |
p-value from χ2 test (or Fisher’s exact test, as appropriate).
LST, laterally spreading tumor; SSL, sessile serrated lesion.
Table 3.
Main outcomes.
| Routine colonoscopy (n = 397) | CAD colonoscopy (n = 393) | p-value** | FC/OR | 95% CI | |
|---|---|---|---|---|---|
| PDR | 0.3325 | 0.4707 | <0.001 | 1.786* | 1.339–2.381 |
| ADR | 0.2091 | 0.2901 | 0.009 | 1.546* | 1.116–2.141 |
| SDR | 0.0025 | 0.0076 | 0.336 | 3.046 | 0.316–29.404 |
| PPC | 0.5139 | 1.0712 | <0.001 | 2.085# | 1.764–2.464 |
| APC | 0.2922 | 0.4784 | <0.001 | 1.637# | 1.299–2.063 |
| Fatigue level | 3.2821 | 3.4020 | 0.357 | 1.037* | 0.960–1.119 |
OR, odds ratio.
FC, fold change.
p-value from χ2 test (or Fisher’s exact test, as appropriate) or t-test.
ADR, adenoma detection rate; APC, adenoma per colonoscopy; PDR, polyp detection rate; PPC, polyp per colonoscopy; SDR, sessile serrated lesion detection rate.
The increase in detection was mainly due to non-advanced diminutive adenomass, serrated adenoma and hyperplastic polyps. Among which, the detection of SSL was significantly higher in CADe group (2.61%, 11/421) than that of routine group (0.49%, 1/204) (P=0.021). Moreover, in CADe group, 6 out 11 SSLs were detected in right colon, the remaining were detected in sigmoid colon and rectum, whereas there was only one SSL detected in right colon in routine group.
Polyp characteristics, polyps detected per colonoscopy and PDR
A total of 625 polyps were detected. The PDRs of the control and CADe groups were 33.25% and 47.07%, respectively (OR = 1.786, 95% CI 1.339–2.381, p < 0.001). The PPCs were 0.51 and 1.07, respectively (Change Folds = 2.09, 95% CI 1.764–2.464, p < 0.001) (Tables 2 and 3).
There was no statistically significant difference between the two groups in terms of baseline clinical and demographic variables. Thus, covariate adjusted models were not considered to address the potential confounding effect.
Consistent false detections by the CADe system
There was a total of 29 consistent false detections (based on endoscopist judgment of non-polyp objects which were continuously traced) in the CADe group, averaging at 0.074 per colonoscopy, mostly wrinkled mucosa. One extra consistent false detection was found after histology exam as normal colon mucosa (Table 4).
Table 4.
Statistical results of false-alarms and missed polyp.
| CAD colonoscopy* | |
|---|---|
| False-alarm | 29 (100.00) |
| Bubble | 3 (10.34) |
| Feces | 1 (3.45) |
| Undigested debris | 3 (10.34) |
| Wrinkled mucosa | 12 (41.38) |
| Local inflammation | 6 (20.69) |
| Local bleeding | 0 (0.00) |
| Rounded drug capsules | 3 (10.34) |
| Other (circular blood vessel, scar, diverticulum, etc) | 1 (3.45) |
| Missed Polyp | 0 (0.00) |
n (%).
Of all the detected polyps in the CADe group, none was missed by the CADe system.
Polyps detected first by CADe system before endoscopists in the CADe group
In total, 165 polyps, including 73 adenomas and one SSLs, averaging 0.19 adenomas and 0.42 polyps per patient, were detected by CADe system prior to endoscopists in the CADe group (Table 5). These polyps were generally small in size [mean polyp size 3.55 mm (SD 1.58); mean adenoma size 3.82 mm (SD 1.23)], isochromatic [149 (90.3%) of 165 polyps; 61 (83.6%) of 73 adenomas], flat in shape [122 (73.9%) polyps; 48 (65.8%) adenomas; Video S3], unclear boundary [33 (20%) polyps; 11 (15.1%) adenomas], partly behind colon folds [32 (19.4%) polyps; 16 (21.9%) adenomas], and on the edge of the visual field [78 (47.3%) polyps; 36 (49.3%) adenomas; Video S1].
Table 5.
Characteristics of polyps missed at endoscopy among patients in computer-aided detection group.
| Endoscopist-missed polyp in CADe colonoscopy | Polyp (n = 165) | Adenoma (n = 73) | SSL (n = 1) |
|---|---|---|---|
| Polyp characteristics | |||
| Isochromatic | 149 (90.3) | 61 (83.6) | 0 (0.0) |
| Flat | 122 (73.9) | 48 (65.8) | 0 (0.0) |
| Unclear boundary | 33 (20.0) | 11 (15.1) | 0 (0.0) |
| Partially occluded by colon folds | 32 (19.4) | 16 (21.9) | 1 (100.0) |
| On the edge of visual field | 78 (47.3) | 36 (49.3) | 1 (100.0) |
| Colon condition | |||
| Insufficient air inflation | 27 (16.4) | 8 (11.0) | 1 (100.0) |
| Partially occluded by Liquid feces or debris | 35 (21.2) | 18 (24.7) | 1 (100.0) |
| Endoscopist | |||
| Withdraw too fast | 20 (12.1) | 7 (9.6) | 0 (0.0) |
| Obvious polyp missed diagnosis | 11 (6.7) | 6 (8.2) | 0 (0.0) |
| Endoscopy | |||
| Overexposure | 112 (67.9) | 52 (71.2) | 1 (100.0) |
| Blurred lens | 20 (12.1) | 7 (9.6) | 0 (0.0) |
| Insufficient light condition | 21 (12.7) | 12 (16.4) | 0 (0.0) |
Fatigue level
The fatigue score for each procedure was 3.28 versus 3.40 for routine and CADe groups, p = 0.357.
Discussion
With the assistance of CADe system, ADR was found significantly increased from 20.91% in routine group to 29.01% in CADe group (P=0.009), APC was also found significantly increased from 0.29 in routine group to 0.48 in CADe group (P<0.001) without introducing more fatigue to endoscopists, which was demonstrated by similar fatigue scores in routine and CADe group (3.28 vs 3.40, p=0.357).
Missed polyps during colonoscopy might lead to subsequent CRC, which is one of the leading causes of cancer-related death.9,10 From a quality-control perspective, small increments in the quality of screening colonoscopies could have significant impact on the net gains of large-scale CRC screening programs.11 Furthermore, truthful awareness of adenoma numbers for each patient leads to better net gains from screening colonoscopy, including prompt resection of precancerous lesions and a better understanding of the surveillance time.6,12
Studies have shown that missed polyps fall into one of two categories: those polyps that remain outside the visual field and those polyps that are in the visual field but are unrecognized by the endoscopist.13 Although polyps that remain outside of the visual field contribute to the majority of missed polyps, polyps that remain unrecognized are also a major issue, and until now only second-observer strategies seemed helpful in increasing the PDR via this pathway.14–16 However, it is likely that adding additional human observers beyond one would lead to limited gain in ADR and would not fully address the many factors that affect intra-observer variability for a given colonoscopy such as “inattentional blindness”17,18 and “change blindness.”19 However, a high-performance CADe20 system has the advantages of high reproducibility, fidelity and uniformity. The CADe system3 is thus an ideal way to address unrecognized polyps and has shown good performance in our previous study,4 especially for those polyps that appear only briefly on the screen, those on the edge of the screen or those that remain partially occluded. The system has also demonstrated good performance in the detection of polyps with subtle visual features including flat and isochromatic polyps, and polyps with unclear boundaries. However, for these polyps, gaze patterns that rely on shuttling back and forth between two screens may lead to missing polyps that do appear in the visual field. Integration of the CADe system into the primary endoscopy monitor may mitigate these issues.21
A dual-screen set-up allows for specific study designs in a research setting, but it is not conducive to clinical practice. Dual-monitor set-ups in general may lead to increased fatigue for physician participants, and dual-monitor set-ups have been shown to increase fatigue via eye fatigue22 and overall energy and attention consumption. Similarly, in a vigilance task study,23 more workload and tense arousal were reported when participants performed actively in a dual-task research scenario. In addition, there appears to be a positive correlation between a center-looking visual gaze pattern (VGP) and ADR,24 and wider VGP has been demonstrated to allow an increased PDR.25 These also indicate that frequent shift of sight might harm the best VGP and thus lead to a decrease of ADR or PDR. Therefore, it is crucially important to minimize latency. There are three independent components of latency for a CADe system: the Display Latency of the colonoscopy video overlapped with detection box; the Computation Latency that the convolutional neural network (CNN) takes to produce the detection box for a certain video frame; and the Algorithm Latency due to per-frame sensitivity of the AI algorithm (time discrepancy between the first frame of polyp appearance and the first frame of detection on the polyp). For single-monitor set-up, the display latency should be imperceptible. How fast the CADe system reacts to a polyp appearance mainly depends on the Algorithm Latency due to per-frame sensitivity and Computation Latency. In this study, the Display Latency of the colonoscopy video overlapped with detection box is 5.99 ± 0.24 ms, the Computation Latency is 20.33 ± 0.14 ms, and the per-frame sensitivity is 95.4% as validated in the preclinical study.3
In this study, the endoscopists could identify the blue box promptly without having to switch between two monitors. Results demonstrated an equal fatigue level between standard endoscopy and endoscopy using the integrated CADe system. Furthermore, the rare consistent false detections, attributed to the high specificity of the CADe system, did not increase the fatigue level of the operating endoscopists in this study. This was also demonstrated by an equal withdrawal time when biopsy time was excluded in two groups.
In this study, ADR (20.91% versus 29.01%, p = 0.009) and APC (0.29 versus 0.48, p < 0.001) were shown to have similar increases from the control to the experimental arm as our previous study.4 The ADR in the control group is much higher than that reported extensively on a low ADR population.26–30
That indicates the CADe system can contribute to an increased detection rate even when the baseline ADR of participating endoscopists was already qualified, instead of being only useful to endoscopists with low detection rate.
The population is representative for normal people with various reasons to receive a colonoscopy compared with a recent study that only included high-risk older populations, mostly patients with CRC symptoms and positive results from a fecal immunochemical test.7
There was likely a lower limit in ADR in both the control and experimental groups, as the study site utilized older generation Olympus CF-Q260 colonoscopes; new colonoscopes may increase baseline ADR.31 An integrated CADe system should be further investigated in newer colonoscopy models. As in previous studies, the increase in ADR seen in the experimental group was largely due to the increased detection of diminutive adenomas and hyperplastic polyps. However, in this study, the detection of SSLs was higher in the CADe group in both proximal and distal colon, a fact which may be attributed to the nature of an integrated system. Such a single-monitor system may allow endoscopists to promptly check any quick, subtle alarms and identify more subtle features associated with SSL in real time, whereas in the two-monitor set-up those SSL might be missed during the time interval when the endoscopist turns to look at the second monitor.
Consistent false detections by the CADe system (Table 4) and polyps detected first by CADe system before endoscopist in the CADe group (Table 5) were consistent with the previous double-blinded study,6 which provide concrete evidence of how CADe system makes efficacy during colonoscopy. Moreover, data show that 26.4% (165/625) polyps, 24.0% (73/304) adenomas and 8.3% (1/12) SSLs were detected first by CADe system which was prior to the reaction of the endoscopist’s eye, which indicates not only that the glass-to-glass latency is imperceptibly low, but also proves the high sensitivity of the algorithm that could capture subtle features32 of a polyp even when it appears briefly on the screen or in suboptimal conditions including insufficient light, blurred lens and so on (Table 5). Also, the super-fast detection by the CADe system indicates a possibility that fewer missed diagnoses would happen with the aid of such a high-performance CADe system.
This study has several limitations. First, the study was unblinded, and awareness of the CADe system may have led participating endoscopists to either put more attention into either endoscopy with a “competitive spirit,”11 or they might relax and unduly rely on the CADe system for polyp detection. Both cases might affect the ADR and lead to a slight overestimation or underestimation of the effectiveness of such CADe system. Similarly, endoscopist scoring of fatigue may also have been affected by the unblinded nature of the study. Second, the control group in this study was routine colonoscopy instead of CADe-assisted colonoscopy with a second monitor, and we thus cannot draw any direct conclusion that an integrated CADe system is more effective or less fatiguing than a dual-monitor system. Third, the fatigue score was subjective and susceptible to factors other than the visual alarms, including insertion difficulty, bowel preparation level, case load per day and so on. Although randomization could balance the overall baseline fatigue factors, larger sample sizes (and a blinded component) might be needed to make the result more robust. Fourth, whether a polyp was first detected by CADe before the endoscopist was based on the operating endoscopist’s own judgment; this might introduce two subjective biases: first man may be reluctant to admit his failure to AI, especially when the operating endoscopist saw the polyp quickly after CADe’s detection box. Second, it is difficult for the endoscopist to tell if the CADe system is a little ahead when they identify a polyp almost at the same time. Thus, the result of how many polyps were initially detected by the CADe system before the endoscopists might be underestimated.
Fifth, The fact that the CADe system detected a polyp prior to endoscopists does not necessarily means that the endoscopists would have missed that lesion, and that is hard to be objectively measured, however, faster detection capability of AI is always believed a strong guarantee for endoscopist to miss less.
In conclusion, real-time visual alarms provided by a high-performance CADe system embedded into the primary colonoscopy monitor, with nearly unnoticeable latency, have been shown to cause a significant improvement in ADR due to an increased detection of diminutive adenomas without increasing physician fatigue level during colonoscopy. Implementation of a single-screen, augmented-reality CADe system may be an important step toward quality assurance during screening and surveillance colonoscopy.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-3-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-4-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Acknowledgments
We thank Dr. Wenfei Zhang for her professional advice on the design of statistical analysis.
Footnotes
Author contributions: Pu Wang, Peixi Liu, Guanyu Zhou contributed to study concept and design. Xiaogang Liu, Liangping Li, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong contributed to acquisition of data. Pu Wang, Tyler M. Berzin and Jeremy R. Glissen Brown contributed to interpretation of data and drafting of the manuscript.
All authors read and approved the final manuscript.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Tyler Berzin has received consulting fees from Shanghai Wision AI Co., Ltd. The CADe system (EndoScreener) was developed by Shanghai Wision AI Co., Ltd. The system was provided free of charge for the purpose of this study. Employees in the company were not involved in the clinical trial in any way, including in study design, statistical analysis or manuscript writing. The CADe system (EndoScreener, Shanghai Wision AI Co., Ltd.) is not a commercial product approved by the FDA; it was used in this study for a clinical benefit exploration under the supervision of IRB in Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital.
Ethical statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional IRB and with the Helsinki declaration. Written informed consent was obtained from all individual participants enrolled in the study. This study does not involve vulnerable groups such as women in pregnancy, minors, mental patients.
ORCID iDs: Pu Wang  https://orcid.org/0000-0002-1234-309X
https://orcid.org/0000-0002-1234-309X
Weihui Liu  https://orcid.org/0000-0003-2871-8316
https://orcid.org/0000-0003-2871-8316
Data acquisition: Deidentified data are available within 1 year of publication at the Chinese Clinical Trial Registry website: http://www.chictr.org.cn/showproj.aspx?proj=29921.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Peixi Liu, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Pu Wang, Department of Gastroenterology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, No.32 West Second Section First Ring Road, Chengdu, Sichuan, China.
Jeremy R. Glissen Brown, Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
Tyler M. Berzin, Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
Guanyu Zhou, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Weihui Liu, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Xun Xiao, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Ziyang Chen, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Zhihong Zhang, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Chao Zhou, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Lei Lei, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Fei Xiong, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Liangping Li, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
Xiaogang Liu, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, China.
References
- 1. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 3. Wang P, Xiao X, Glissen Brown JR, et al. Development and validation of a deep-learning algorithm for the detection of polyps during colonoscopy. Nat Biomed Eng 2018; 2: 741–748. [DOI] [PubMed] [Google Scholar]
- 4. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut 2019; 68: 1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol 2014; 15: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang P, Liu X, Berzin TM, et al. Effect of a deep-learning Computer-Aided Detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol 2020; 5: 343–351. [DOI] [PubMed] [Google Scholar]
- 7. Repici A, Badalamenti M, Maselli R, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. Epub ahead of print 1 May 2020. DOI: 10.1053/j.gastro.2020.04.062. [DOI] [PubMed] [Google Scholar]
- 8. Glissen Brown JR, Bilal M, Wang P, et al. Introducing computer-aided detection to the endoscopy suite. VideoGIE 2020; 5: 135–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Cancer Society. Cancer facts and figures: 2017. Atlanta, Georgia: American Cancer Society, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-fgures-2017.html (2017, accessed 15 August 2017). [Google Scholar]
- 10. Fang J-Y, Zheng S, Jiang B, et al. Consensus on the prevention, screening, early diagnosis and treatment of colorectal tumors in China: Chinese Society of Gastroenterology, October 14–15, 2011, Shanghai, China. Gastrointest Tumors 2014; 1: 53–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut 2015; 64: 982–990. [DOI] [PubMed] [Google Scholar]
- 12. Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut 2015; 64: 982–990. [DOI] [PubMed] [Google Scholar]
- 13. Mahmud N, Cohen J, Tsourides K, et al. Computer vision and augmented reality in gastrointestinal endoscopy. Gastroenterol Rep 2015; 3: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aslanian HR, Shieh FK, Chan FW, et al. Nurse observation during colonoscopy increases polyp detection: a randomized prospective study. Am J Gastroenterol 2013; 108: 166–172. [DOI] [PubMed] [Google Scholar]
- 15. Lee CK, Park DI, Lee SH, et al. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: a multicenter, prospective, randomized study. Gastrointest Endosc 2011; 74: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 16. Buchner AM, Shahid MW, Heckman MG, et al. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc 2011; 73: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 17. Memmert D, Unkelbach C, Ganns S. The impact of regulatory fit on performance in an inattentional blindness paradigm. J Gen Psychol 2010; 137: 129–139. [DOI] [PubMed] [Google Scholar]
- 18. Simons DJ, Chabris CF. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception 1999; 28: 1059–1074. [DOI] [PubMed] [Google Scholar]
- 19. Simons DJ, Rensink RA. Change blindness: past, present, and future. Trends Cogn Sci 2005; 9: 16–20. [DOI] [PubMed] [Google Scholar]
- 20. Alagappan M, Brown JRG, Mori Y, et al. Artificial intelligence in gastrointestinal endoscopy: the future is almost here. World J Gastrointest Endosc 2018; 10: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang P, Liu P, Glissen Brown JR, et al. Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Gastroenterology. Epub ahead of print 17 June 2020. DOI: 10.1053/j.gastro.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 22. Yoo WG. Comparison of orbicularis oculi muscle activity during computer work with single and dual monitors. J Phys Ther Sci 2014; 26: 1807–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Head J, Helton WS. Passive perceptual learning versus active searching in a novel stimuli vigilance task. Exp Brain Res 2015; 233: 1481–1489. [DOI] [PubMed] [Google Scholar]
- 24. Lami M, Singh H, Dilley JH. Gaze patterns hold key to unlocking successful search strategies and increasing polyp detection rate in colonoscopy. Endoscopy 2018; 50: 701–707. [DOI] [PubMed] [Google Scholar]
- 25. Almansa C, Shahid MW, Heckman MG, et al. Association between visual gaze patterns and adenoma detection rate during colonoscopy: a preliminary investigation. Am J Gastroenterol 2011; 106: 1070–1074. [DOI] [PubMed] [Google Scholar]
- 26. Chen HD, Li N, Ren JS, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut 2019; 68: 1450–1457. [DOI] [PubMed] [Google Scholar]
- 27. Jia H, Pan Y, Guo X, et al. Water exchange method significantly improves adenoma detection rate: a multicenter, randomized controlled trial. Am J Gastroenterol 2017; 112: 568–576. [DOI] [PubMed] [Google Scholar]
- 28. Bai Y, Fang J, Zhao SB, et al. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: a multicenter, endoscopist-blinded randomized controlled trial. Endoscopy 2018; 50: 128–136. [DOI] [PubMed] [Google Scholar]
- 29. Xu Y, Chen K, Xu L, et al. Diagnostic yield is not influenced by the timing of screening endoscopy: morning versus afternoon. Scand J Gastroenterol 2018; 53: 365–369. [DOI] [PubMed] [Google Scholar]
- 30. Cai B, Liu Z, Xu Y, et al. Adenoma detection rate in 41,010 patients from Southwest China. Oncol Lett 2015; 9: 2073–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adler A, Aminalai A, Aschenbeck J, et al. Latest generation, wide-angle, high-definition colonoscopes increase adenoma detection rate. Clin Gastroenterol Hepatol 2012; 10: 155–159. [DOI] [PubMed] [Google Scholar]
- 32. Zhou GY, Xiao X, Tu MT, et al. Computer aided detection for laterally spreading tumors and sessile serrated adenomas during colonoscopy. PLoS One 2020; 15: e0231880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-2-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-3-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-pdf-4-tag-10.1177_1756284820979165 for The single-monitor trial: an embedded CADe system increased adenoma detection during colonoscopy: a prospective randomized study by Peixi Liu, Pu Wang, Jeremy R. Glissen Brown, Tyler M. Berzin, Guanyu Zhou, Weihui Liu, Xun Xiao, Ziyang Chen, Zhihong Zhang, Chao Zhou, Lei Lei, Fei Xiong, Liangping Li and Xiaogang Liu in Therapeutic Advances in Gastroenterology