Abstract
Background:
Apatinib, an oral small-molecule angiogenesis inhibitor, selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR-2), which inhibits vascular endothelial growth factor (VEGF) stimulated endothelial cell migration and proliferation and decreases tumour growth and metastasis. Recently, the efficacy of multi-target angiogenic drugs has been demonstrated for many cancers, including non-small-cell lung cancer (NSCLC). The aim of this retrospective study was to evaluate the clinical efficacy of apatinib in patients with advanced NSCLC.
Patients and methods:
We conducted a retrospective analysis of 70 patients with advanced NSCLC who received second-line and later treatment from November 2015 to July 2017 with poor results. Out of the 70 patients, 36 patients received apatinib treatment after second-line or later treatment, whereas 34 patients in the control group did not receive further treatment. The patients were treated with oral apatinib 500 mg once a day every day for 4 weeks per cycle. Treatment was continued in responding and stable patients until disease progression or intolerable toxicity. The objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and side effects of the drug were recorded and reviewed.
Results:
ORR, DCR, PFS, and OS were evaluated in 36 patients receiving apatinib and 34 patients in the control group. The ORR and DCR in patients receiving apatinib therapy were 22.2% and 77.8%, respectively. The median PFS and OS in the treatment group were 5.6 and 9.6 months, respectively. The median OS in the apatinib group was significantly longer than that in the control group (9.6 versus 3.8 months; p < 0.0001). In contrast, there were no differences in adverse reactions between the patients in the treatment and control groups.
Conclusion:
Apatinib showed favourable efficacy and safety and can thus be used as a treatment option for patients with advanced NSCLC.
Keywords: angiogenesis, apatinib, anti-VEGF, non-small-cell lung cancer, TKI, efficacy, safety
Introduction
Lung cancer has the highest associated morbidity and mortality of any cancer worldwide.1 According to the National Central Cancer Registry of China, the incidence of lung cancer in China was 51/100,000 in 2018, and the mortality rate was 40.71/100,000.2 The two main classifications of lung cancer are small-cell lung cancer and non-small-cell lung cancer (NSCLC). NSCLC accounts for approximately 80% of all lung cancer cases. Because of the difficulties associated with early NSCLC detection, most patients already display middle- or advanced-stage disease at the time of diagnosis, and surgical resection is no longer feasible. In the past decade, significant progress in the treatment of advanced NSCLC has been achieved using targeted immunotherapeutics.3 Nonetheless, for patients with no targetable genetic aberrations, platinum-based doublet chemotherapy is still the preferred treatment method. Following disease progression, second-line treatment mainly includes new-generation targeted drug therapy or chemotherapeutics such as docetaxel or pemetrexed. However, there is no standard treatment for patients with advanced metastatic NSCLC who have progressed after two or more lines of standard treatment, and current guidelines generally recommend clinical trials or palliative treatment.4–7
Angiogenesis is a key requirement of malignant tumour growth and metastasis. Tumour neovascularisation promotes tumour growth by facilitating the supply of nutrients to, and removal of metabolites from, tumour cells. Angiogenesis is also likely to facilitate the subsequent spread of tumour cells to other parts of the body from the primitive lesion via these new blood vessels. An important cytokine that induces tumour angiogenesis is vascular endothelial growth factor (VEGF).8 VEGF stimulates downstream signal transduction by binding to transmembrane receptor tyrosine kinases and promoting the proliferation, mitosis, and migration of endothelial cells to form new vascular cavities.9–11 In recent years, the efficacy of multi-target angiogenic drugs targeting VEGF and its receptor VEGFR has been demonstrated for many cancers, including NSCLC. Bevacizumab, a recombinant human monoclonal antibody, is the first anti-angiogenesis drug to be approved by the United States Food and Drug Administration for the treatment of patients with NSCLC. Bevacizumab was recommended as first-line therapy in combination with chemotherapy for the treatment of advanced NSCLC because of the prolonged overall survival (OS) associated with its use.10,12–17 Anlotinib is another anti-angiogenesis drug that has also been shown to prolong PFS in the third-line treatment of NSCLC.18
Apatinib, a tyrosine kinase inhibitor (TKI), is a new-generation oral anti-angiogenic drug that has been independently developed in China. Apatinib exerts its antitumour effects by blocking VEGFR-2, thus attenuating the downstream activation of mitogen-activated protein kinase (MAPK) and inhibiting the proliferation of vascular endothelial cells.19,20 Apatinib shows high antitumour activity in a variety of tumours, including cancers of the gastrointestinal tract and lung cancers.12,21–23 In the present study, we investigated the clinical effects and safety of apatinib as third- or further-line therapy in the treatment of advanced NSCLC.
Patients and methods
Patient eligibility
Patients with advanced NSCLC receiving treatment at three different cancer centres from 1 November 2015 to 31 July 2017 were included in this retrospective study. The patients’ clinical and laboratory data were retrospectively retrieved from medical records collected during hospitalisation. The selection criteria were as follows: pathologically confirmed NSCLC; clinical stage IV; normal heart, liver, and kidney function, and normal blood count before treatment; no bleeding disorders; and at least one appraised lesion according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1.24 Overall, 70 patients were recruited for the study; they were split into a control group comprising 34 cases and an apatinib-treatment group comprising 36 cases. In the control group, after previous second-line and later standard treatments, no other active antitumour treatments were offered upon further progression. In the treatment group, after previous second-line and later standard treatments, patients were treated with apatinib upon further progression.
In total, 18 apatinib-treatment cases were excluded from this study (11 cases were found not to be in accordance with the inclusion criteria, two cases were excluded owing to lack of follow-up, and five cases were excluded owing to incomplete information). The study protocol was approved by the Ethics Committee of the First People’s Hospital of Foshan (2018 NO. 3), and the study was conducted in accordance with the Declaration of Helsinki.
Treatment methods
The patients were treated with oral apatinib 500 mg once a day (QD) every day for 4 weeks as a cycle. Dose reduction (250 mg QD) was permitted for drug-related toxicity (in cases of apatinib-related grade III haematologic or grade II non-haematologic toxicity). Tumour responses were assessed after every second cycle. Treatment was continued in responding and stable patients until disease progression or intolerable toxicity. The follow-up continued until the study cut-off date of 20 March 2018.
Efficacy and toxicity
Therapeutic responses to apatinib treatment and the presence of significant signs of disease progression were evaluated every second cycle. Objective response criteria in the tumours, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), were evaluated according to the RECIST 1.1 guidelines. The objective response rate (ORR) was calculated as CR plus PR. Similarly, the disease control rate (DCR) was calculated by adding CR, PR, and SD. Time from initial diagnosis to apatinib treatment was also recorded. Tumour responses and toxicities were assessed by reviewing the patients’ medical histories and laboratory records according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) guidelines for Adverse Events version 4.0 (CTC4.0).
Statistical analyses
Data were collected from patient enrolment (between November 2015 and July 2017) through to the final follow-up date (20 March 2018). Statistical analyses were performed using SPSS version 19.0 (IBM, Armonk, NY, USA). In the treatment group, OS was measured from the first day of apatinib administration until date of death from any cause or final follow-up. In the control group, OS was calculated as the time from disease progression after second-line treatment until date of death from any cause or final follow-up. Progression-free survival (PFS) was measured from the first day of apatinib administration until the date of tumour recurrence or final follow-up. Between-group comparisons were conducted using chi-square tests. Kaplan–Meier curves were constructed for OS and PFS, and the difference was compared using the log-rank test. A multivariate Cox regression model was used to estimate the treatment hazard ratios (HRs). Differences yielding a two-tailed p < 0.05 were designated as statistically significant in all tests.
Results
Patient characteristics
A retrospective analysis of 70 patients with advanced NSCLC who had received second-line and later treatment with poor results was performed. Depending on the presence/absence of sensitising gene mutations, all patients had previously received targeted treatment and/or platinum-based chemotherapy. While 34 patients in the control group received no further treatment, 36 patients in the treatment group received additional apatinib treatment. The baseline characteristics of the patients in the two groups are shown in Table 1. There were no differences between the two groups with regard to the median age and sex. In addition, there were no differences in smoking history, Eastern Cooperative Oncology Group performance status, pathological subtypes, epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase gene mutations, and tumour distant metastasis between the two groups.
Table 1.
Baseline patient characteristics.
| Characteristics | The treatment groupn = 36n (%) | The control groupn = 34n (%) | p value |
|---|---|---|---|
| Gender | |||
| Male | 21 (58.3) | 20 (58.8) | 0.967 |
| Female | 15 (41.7) | 14 (41.2) | |
| Age, years | |||
| <65 | 21 (58.3) | 21 (61.8) | 0.770 |
| ⩾65 | 15 (41.7) | 13 (38.2) | |
| Smoker | |||
| No | 28 (77.8) | 26 (76.5) | 0.896 |
| Yes | 8 (22.2) | 8 (23.5) | |
| ECOG performance status | |||
| ⩽2 | 29 (80.6) | 26 (76.5) | 0.677 |
| >2 | 7 (19.4) | 8 (23.5) | |
| Pathology type | |||
| Squamous carcinoma | 7 (19.4) | 6 (17.6) | 0.847 |
| Adenocarcinoma | 29 (80.6) | 29 (82.4) | |
| EGFR status | |||
| Mutation | 8 (22.2) | 7 (20.6) | 0.978 |
| Wild-type | 13 (36.1) | 13 (38.2) | |
| Unknown | 15 (41.7) | 14 (41.2) | |
| ALK status | |||
| Negative | 13 (36.1) | 20 (58.8) | 0.095 |
| Positive | 1 (2.8) | 2 (5.9) | |
| Unknown | 22 (61.1) | 12 (35.3) | |
| Line of treatment | |||
| 3rd line | 21 (58.3) | ||
| 4th line | 11 (30.6) | ||
| 5th line | 4 (11.1) | ||
| Brain metastases | |||
| No | 28 (77.8) | 28 (82.4) | 0.632 |
| Yes | 8 (22.2) | 6 (17.6) | |
| Previous treatment | 0.019 | ||
| Targeted therapy | 0 (0.0) | 5 (7.1) | |
| Chemotherapy | 27 (38.6) | 26 (37.1) | |
| Targeted therapy + chemotherapy | 9 (12.9) | 3 (4.3) | |
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Clinical efficacy
Of the 36 patients in the treatment group, eight (22.2%) achieved PR, 20 (55.6%) achieved SD, and eight (22.2%) achieved PD. The ORR and DCR were 22.2% and 77.8%, respectively (Table 2). For squamous cell carcinoma, the ORR and DCR were 14.3% and 85.7%, respectively, whereas for adenocarcinoma, the ORR and DCR were 24.1% and 75.9%, respectively. No statistically significant difference in therapeutic effect was observed between patients with different pathological types (ORR, p = 1.000; DCR, p = 1.000). In addition, no significant differences in efficacy were observed when mutant and wild-type EGFR gene status were compared (p = 0.618). The ORR and DCR of patients with mutant EGFR were 37.5% and 75.0%, respectively, whereas the ORR and DCR of patients with wild-type EGFR were 23.1% and 84.6%, respectively.
Table 2.
Tumour response.
| Tumour response | Number of patients (%) |
|---|---|
| CR | 0 (0) |
| PR | 8 (22.2) |
| SD | 20 (55.6) |
| PD | 8 (22.2) |
| ORR | 8 (22.2) |
| DCR | 28 (77.8) |
CR, complete response; DCR, disease control rate (CR + PR + SD); ORR, objective response rate (CR + PR); PD, progressive disease; PR, partial response; SD, stable disease.
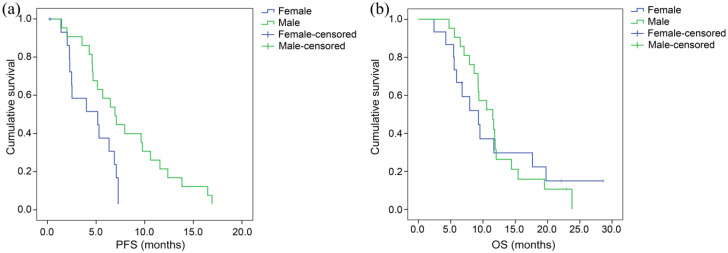
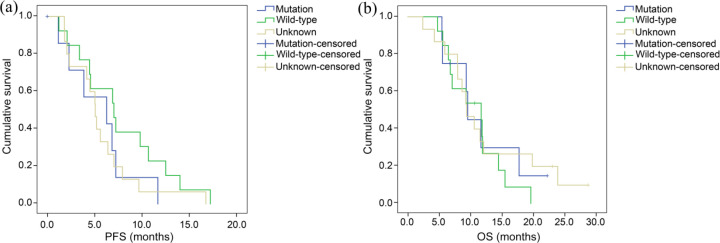
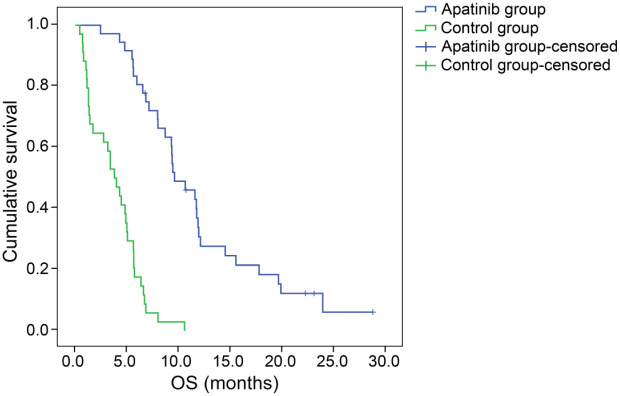
At the end of the follow-up period, five patients were still alive and one patient was still receiving apatinib treatment with no adjustments. The median PFS and median OS of all patients treated with apatinib as third-line (or later) therapy were 5.6 months [95% confidence interval (CI), 4.0–7.2 months] and 9.6 months (95% CI, 7.0–12.0 months), respectively (Figure 1). Among patients who were treated with apatinib, the PFS of male patients was 6.9 months (95% CI, 4.8–9.0), which was significantly longer than that of female patients: 4.3 months (95% CI, 0–3.9). Moreover, the OS of male patients, 11.5 months (95% CI, 8.3–14.7) was longer than that of female patients [9.3 months (95% CI, 4.9–13.8)], though the difference was not statistically significant (Figure 2). No significant differences were observed regarding PFS and OS between patients with mutant and patients with wild-type EGFR gene status (p = 0.293 and 0.779, respectively) (Figure 3). The PFS and OS of patients with different clinical features are shown in Table 3. The median time to apatinib treatment was 11.7 months from diagnosis (95% CI, 9.9–13.5 months), which was significantly correlated with PFS (p = 0.049). The median OS of patients in the control group was 3.8 months (95% CI, 2.5–5.1 months), which was significantly shorter than the median OS of patients in the treatment group (p < 0.0001) (Figure 4). Furthermore, multivariate analysis demonstrated that the use of apatinib was an independent factor of OS for patients with advanced NSCLC who received second-line and later standard regimens (OS, HR = 9.487; 95% CI, 4.717–19.080, p < 0.0001). The results are listed in Table 4.
Figure 1.
Kaplan–Meier estimates of the (a) progression-free survival (PFS) and (b) overall survival (OS) of patients with advanced non-small-cell lung cancer receiving apatinib treatment.
Figure 2.
Kaplan–Meier estimates of the (a) progression-free survival (PFS) and (b) overall survival (OS) of patients according to gender.
Figure 3.
Kaplan–Meier estimates of the (a) progression-free survival (PFS) and (b) overall survival (OS) of patients according to epidermal growth factor receptor gene status.
Table 3.
Log-rank analysis of progression-free survival (PFS) and overall survival (OS) with different clinical features in the apatinib group.
| Characteristics | mPFS in months (95% CI) | p value | mOS in months (95% CI) | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 6.9 (4.8–9.0) | 0.009 | 11.5 (8.3–14.7) | 0.960 |
| Female | 4.3 (0–3.9) | 9.3 (4.9–13.8) | ||
| Age, years | ||||
| <65 | 5.2 (0.2–10.2) | 0.546 | 9.6 (5.3–13.9) | 0.906 |
| ⩾65 | 5.6 (4.0–7.2) | 10.6 (8.0–13.2) | ||
| Smoker | ||||
| No | 5.2 (4.2–6.2) | 0.596 | 11.5 (7.8–15.3) | 0.797 |
| Yes | 6.4 (3.0–9.8) | 9.3 (9.1–9.5) | ||
| ECOG performance status | ||||
| ⩽2 | 5.1 (2.6–7.5) | 0.775 | 9.4 (9.0–9.8) | 0.822 |
| >2 | 5.6 (4.6–6.6) | 11.7 (11.3–12.0) | ||
| Pathology type | ||||
| Squamous carcinoma | 5.0 (3.7–6.4) | 0.766 | 10.6 (7.5–13.7) | 0.511 |
| Adenocarcinoma | 5.6 (3.9–7.3) | 9.6 (6.7–12.4) | ||
| EGFR status | ||||
| Mutation | 6.3 (0.1–12.4) | 0.293 | 9.6 (9.0–10.1) | 0.779 |
| Wild-type | 7.0 (3.9–10.2) | 11.7 (4.1–19.3) | ||
| Unknown | 5.1 (4.2–6.0) | 9.4 (7.0–11.8) | ||
| ALK status | ||||
| Negative | 5.1 (2.2–7.9) | 0.855 | 9.3 (6.7–11.9) | 0.441 |
| Positive | 6.9 | 11.8 | ||
| Unknown | 8.7 (3.6–7.6) | 11.5 (8.4–14.7) | ||
| Line of treatment | ||||
| 3rd line | 6.4 (3.8–9.0) | 0.173 | 11.5 (9.2–13.8) | 0.261 |
| 4th line | 4.5 (0–11.7) | 7.9 (3.9–11.9) | ||
| 5th line | 5.0 (3.2–7.0) | 9.6 (7.0–12.0) | ||
| Brain metastases | ||||
| No | 5.2 (4.2–6.2) | 0.293 | 9.3 (9.1–9.6) | 0.694 |
| Yes | 6.9 (4.4–9.4) | 11.7 (8.7–14.6) | ||
CI, confidence interval; ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; mOS, median overall survival; mPFS, median progression-free survival.
Figure 4.
Kaplan–Meier estimates of overall survival in the apatinib and control groups.
Table 4.
Multivariate analysis of overall survival.
| Factors | B | SE | HR | 95% CI | p |
|---|---|---|---|---|---|
| Gender | −0.327 | 0.314 | 0.721 | 0.389–1.335 | 0.298 |
| Group | 2.250 | 0.356 | 9.487 | 4.717–19.080 | 0.000 |
| Age | 0.348 | 0.281 | 1.417 | 0.816–2.459 | 0.215 |
| Smoke | −0.383 | 0.351 | 0.682 | 0.342–1.358 | 0.276 |
| Histology | −0.474 | 0.372 | 0.622 | 0.300–1.291 | 0.203 |
| EGFR mutation | 0.978 | ||||
| Mutation | −0.065 | 0.637 | 0.937 | 0.269–3.268 | 0.919 |
| Wild-type | 0.069 | 0.540 | 1.071 | 0.372–3.089 | 0.898 |
CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio.
Safety
Most of the observed side effects were mild and tolerable (Table 5). Apatinib was reduced to 250 mg/day in 12 patients because of adverse events, primarily hypertension and hand–foot syndrome. Apatinib treatment was discontinued because of intolerance in only two patients. The most common adverse events were proteinuria (44.4%), hypertension (30.6%), and hand–foot syndrome (25.0%). The frequencies of grade 3/4 adverse events were as follows: hand–foot syndrome (13.9%); hypertension (5.6%); proteinuria (2.8%); thrombocytopenia (2.8%); bleeding from primary peptic ulcer (2.8%); and hepatic injury (2.8%). The grade 4 hepatic injury was caused by obstructive jaundice related to tumour compression rather than drug administration. No deaths related to apatinib treatment were observed.
Table 5.
Analysis of adverse events.
| Adverse event | Grade 1/2 n (%) |
Grade 3/4 n (%) |
Total n (%) |
|---|---|---|---|
| Proteinuria | 8 (22.2) | 1 (2.8) | 9 (25.0) |
| Hypertension | 9 (25.0) | 2 (5.6) | 11 (30.6) |
| Hand–foot syndrome | 11 (30.6) | 5 (13.9) | 16 (44.4) |
| Hepatic injury | 1 (2.8) | 1 (2.8) | 2 (5.6) |
| Thrombocytopenia | 0 (0) | 1 (2.8) | 1 (2.8) |
| Oral ulcer | 1 (2.8) | 0 (0) | 1 (2.8) |
| Anaemia | 1 (2.8) | 0 (0) | 1 (2.8) |
| Bleeding from primary peptic ulcer | 0 (0) | 1 (2.8) | 1 (2.8) |
Discussion
This study assessed the efficacy and safety of apatinib as third- or further-line therapy for patients with advanced NSCLC. The results showed that the ORR was 22.2% and the DCR was 77.8%. The median PFS was 5.6 months, and the OS was 9.6 months. The median OS in the apatinib group was significantly longer than that in the control group (p < 0.0001).
Apatinib is a novel protein TKI that targets VEGFR-2, c-kit, RET, and c-Src. VEGF-2 promotes the proliferation of vascular endothelial cells by activating the MAPK signalling pathway. By blocking VEGFR-2, apatinib reduces MAPK activation, thus inhibiting vascular endothelial cell proliferation.25,26 Apatinib has been approved in China for use in patients with advanced gastric cancer who have already received at least two chemotherapy drugs before progression or recurrence.21 In addition, apatinib has demonstrated high efficacy in the treatment of many other types of tumours, including liver, lung, and bowel cancers.12,21,23 A study by Fang et al. demonstrated that apatinib was more effective than single-agent chemotherapy in the third- or further-line treatment of patients with advanced NSCLC with wild-type EGFR.22 In 36 patients, the ORR was 16.7% and the DCR was 75%. Moreover, the median OS and PFS were 8.2 and 4.5 months, respectively. In another study by Song et al., apatinib was used as salvage treatment for patients with advanced NSCLC.25 While the ORR was 9.5% and the DCR was 61.9%, the median OS and PFS were 6.0 and 4.2 months, respectively. The present study resulted in an ORR of 22.2% and a DCR of 77.8%, and the PFS and OS were 5.6 and 9.6 months, respectively. The ORR and PFS were slightly better than those obtained in previous reports, and the DCR and OS were similar.
Although the remission rate achieved with traditional chemotherapy is high, traditional chemotherapy is associated with distant metastasis and acquired drug resistance.27 Compared with traditional chemotherapy, apatinib treatment yields longer survival data (the median PFS and OS were 5.6 and 9.6 months, respectively),28 and the survival data obtained with apatinib are close to that of third-generation EGFR-TKIs.29 Interestingly, the survival results obtained with apatinib reported in the present study were longer than the survival results obtained in a previous third-line study of apatinib.5,25,29
The TKI anlotinib is another anti-angiogenesis drug used in the treatment of NSCLC. Anlotinib has also been shown to inhibit tumour angiogenesis and proliferative signalling. The ALTER0302 study demonstrated that anlotinib used as third-line therapy for patients with advanced NSCLC significantly prolonged PFS compared with that of placebo, and the OS was longer than that of the placebo group, though not significantly.18 The PFS and OS of our study were similar to the ALTER0302 study, but our study showed that the OS was significantly longer than that of the control group.
In the present study, the median OS in the apatinib group was significantly longer than that in the control group, indicating that patients showing tumour progression after second-line treatment may benefit from apatinib treatment. The ORR, DCR, PFS, and OS associated with apatinib were similar to those associated with anlotinib, and our data were also similar to those previously reported.25,30 Moreover, the time from diagnosis to apatinib treatment was observed to be positively correlated with PFS (p = 0.049), indicating that the longer the time from diagnosis to apatinib treatment, the better the prognosis. While the OS of male and female patients was similar in this study (p = 0.08), the PFS was longer in male patients than in female patients (p = 0.002). The observed difference in PFS may be attributed to the fact that seven male patients had squamous cell carcinoma, whereas all female patients had adenocarcinoma. No significant association between survival and pathological type was observed, probably because only seven cases of squamous cell carcinoma were enrolled compared with 29 cases of adenocarcinoma. EGFR mutation status was also not significantly associated with PFS or OS. In addition, age, smoking history, performance status, pathological type, line of therapy, and the presence of brain metastases were not significantly correlated with PFS or OS.
It has been reported that apatinib may reverse EGFR-TKI resistance. Li et al. showed that apatinib exerted antitumour effects in patients with advanced NSCLC exhibiting acquired resistance to EGFR-TKIs.30 The ORR and DCR in the apatinib-treated patients were 25% and 100%, respectively. The median PFS in the treatment group was 4.6 months, suggesting that EGFR-TKI and apatinib combination therapy may be an alternative therapeutic approach for patients with acquired resistance to EGFR-TKIs. A study by Fang et al. recommended apatinib as third- or further-line therapy for patients with advanced NSCLC.22 In our study, no significant differences were observed regarding PFS or OS in patients with mutant or wild-type EGFR. Our results suggest that further studies are warranted to investigate whether apatinib should be used alone or in combination with EGFR-TKIs in patients with acquired resistance to EGFR-TKIs. Additional studies are also required to confirm the effectiveness of apatinib in the treatment of patients with advanced lung cancer.
There were no apatinib treatment-related deaths in this study. The most frequent side effects were hypertension, hand–foot syndrome, and proteinuria.
Conclusion
Apatinib shows high efficacy and safety in the treatment of patients with advanced NSCLC and may represent a new therapeutic option. This study confirmed that the administration of an initial dose of 500 mg/day apatinib caused only mild or moderate adverse reactions in most cases. The most frequent side effects were hypertension, hand–foot syndrome, and proteinuria. Further large-scale studies are required to determine additional clinical effects of this drug. Moreover, several important issues remain unresolved, including the pathologies and gene status that may benefit from this treatment and the effectiveness of single-drug or combination regimens. In addition, predictive indicators of efficacy are required. Finally, new clinical studies should offer further guidelines for clinical treatment.
Acknowledgments
The authors wish to acknowledge all the medical personnel from the three cancer centres for their assistance during data collection. We would like to thank Dr Liao S. for assistance in the statistical analyses. We would also like to acknowledge all the patients, staff, and researchers who contributed to this study. We would like to thank SAGE language service (http://languageservices.sagepub.com) for English language editing.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Special Fund Project for Technology Innovation of Foshan City 2014AG10003 (to Yanming Deng).
Contributor Information
Jianmiao Liang, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Weiguang Gu, Oncology Department, Nanhai People’s Hospital/The Second School of Clinical Medical, Southern Medical University, Foshan, Guangdong, China.
Jun Jin, Department of Oncology, Guangdong Province Hospital of Combination of Traditional Chinese and Western Medicine, Foshan, Guangdong, China.
Hua Zhang, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Zecheng Chen, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Yicong Tang, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Shunda Zhang, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Shuang Yang, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Yanming Deng, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, Foshan, Guangdong, China.
Weineng Feng, Department of Head and Neck/Thoracic Medical Oncology, The First People’s Hospital of Foshan, No. 81, North Lingnan Avenue, Chancheng District, Foshan City, Guangdong Province 528041, China.
References
- 1. Chen W, Zheng R, Zeng H, et al. Epidemiology of lung cancer in China. Thorac Cancer 2015; 6: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 2019; 39: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi Y, Sun Y, Yu J, et al. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 version). Asia Pac J Clin Oncol 2017; 13: 87–103. [DOI] [PubMed] [Google Scholar]
- 4. Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer 2012; 77: 371–375. [DOI] [PubMed] [Google Scholar]
- 5. Bareschino MA, Schettino C, Rossi A, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis 2011; 3: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 7. Casaluce F, Sgambato A, Maione P, et al. ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC. Target Oncol 2013; 8: 55–67. [DOI] [PubMed] [Google Scholar]
- 8. Krzywinska E, Kantari-Mimoun C, Kerdiles Y, et al. Loss of HIF-1α in natural killer cells inhibits tumour growth by stimulating non-productive angiogenesis. Nat Commun 2017; 8: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al Farsi A, Ellis PM. Anti-angiogenic therapy in advanced non-small cell lung carcinoma (NSCLC): is there a role in subsequent lines of therapy? J Thorac Dis 2015; 7: 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontanella C, Ongaro E, Bolzonello S, et al. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med 2014; 2: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xue JM, Astere M, Zhong MX, et al. Efficacy and safety of apatinib treatment for gastric cancer, hepatocellular carcinoma and non-small cell lung cancer: a meta-analysis. Onco Targets Ther 2018; 11: 6119–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384: 665–673. [DOI] [PubMed] [Google Scholar]
- 13. Hall RD, Le TM, Haggstrom DE, et al. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2015; 4: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 15. Stinchcombe TE. Targeted therapy of advanced non-small cell lung cancer: the role of bevacizumab. Biologics 2007; 1: 185–194. [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol 2015; 33: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 17. Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010; 5: 1416–1423. [DOI] [PubMed] [Google Scholar]
- 18. Ding J, Chen X, Gao Z, et al. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humans. Drug Metab Dispos 2013; 41: 1195–1210. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi T, Yamaguchi S, Chida K, et al. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J 2001; 20: 2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010; 10: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016; 34: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 22. Fang S, Zhang M, Wei G, et al. Apatinib as a third- or further- line treatment in patients with advanced NSCLC harboring wild-type EGFR. Oncotarget 2018; 9: 7175–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gou M, Si H, Zhang Y, et al. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer: a real-world retrospective study. Sci Rep 2018; 8: 4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 25. Song Z, Yu X, Lou G, et al. Salvage treatment with apatinib for advanced non-small-cell lung cancer. Onco Targets Ther 2017; 10: 1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu F, Liu X, Li X, et al. [Clinical investigation of efficacy of third-line and beyond pemetrexed treatment in advanced non-squamous non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi 2012; 15: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oronsky B, Caroen S, Zeman K, et al. A partial response to reintroduced chemotherapy in a resistant small cell lung cancer patient after priming with RRx-001. Clin Med Insights Oncol 2016; 10: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, Shi M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol 2012; 30: 7548. [Google Scholar]
- 29. Cao Y, Qiu X, Xiao G, et al. Effectiveness and safety of osimertinib in patients with metastatic EGFR T790M-positive NSCLC: an observational real-world study. PLoS One 2019; 14: e0221575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li F, Zhu T, Cao B, et al. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer 2017; 84: 184–192. [DOI] [PubMed] [Google Scholar]