Abstract
Postpneumonectomy syndrome is a rare complication in patients who have previously had a pneumonectomy. Over time, the mediastinum may rotate toward the vacant pleural space, which can cause extrinsic airway and esophageal compression. As such, these patients typically present with progressive dyspnea and dysphagia. There is a paucity of reports in the anesthesiology literature regarding the intraoperative anesthetic approach to such rare patients. We present a case of an 18-year-old female found to have postpneumonectomy syndrome requiring thoracotomy with insertion of tissue expanders. Our case report illustrates the complexities involved in the care of these patients with regards to airway management, ventilation concerns, and potential for hemodynamic compromise. This case report underscores the importance of extensive multidisciplinary planning.
Keywords: pneumonectomy, postpneumonectomy syndrome, thoracic surgery, cardiac anesthesia, congenital heart disease
Introduction
Postpneumonectomy syndrome consists of a constellation of symptoms, including acute respiratory distress, progressive dyspnea, stridor, or dysphagia that can occur months to years after a pneumonectomy. The symptomatology is the result of extrinsic airway compression and esophageal compression from extreme mediastinal shift and rotation.1 It is a rare complication of a pneumonectomy with an incidence of 0.16%.2 Pneumonectomy is indicated for malignancy, congenital abnormalities, and trauma. Following surgery, a number of anatomical changes may be appreciated.3 Immediately after surgery, the postpneumonectomy space fills with air, which is resorbed and replaced with serosanguinous fluid at a rate of approximately 2 rib spaces per day. On average, it takes more than 2 weeks for the fluid to completely fill the postpneumonectomy space. Concurrently, elevation of the hemidiaphragm and hyperinflation of the contralateral lung occurs.
Following a left pneumonectomy, the heart rotates counterclockwise in the left postpneumonectomy space.4 Following a right pneumonectomy, the heart and mediastinum shifts laterally into the right postpneumonectomy space. Over time, the fluid in the postpneumonectomy space is partially or completely resorbed as the space is obliterated by the elevated hemidiaphragm, hyperinflated contralateral lung, and shifted mediastinum. Rarely, the mediastinum can shift too far and rotate, causing postpneumonectomy syndrome. For reasons that have yet to be elucidated, postpneumonectomy syndrome is significantly more common following a right pneumonectomy than a left pneumonectomy.5 The trachea and/or main bronchus can be compressed between the left pulmonary artery and descending aorta or vertebral body after a right-sided surgery and between the right pulmonary artery and aorta or vertebral body after a left-sided surgery. With long-standing airway compression, tracheobronchomalacia can result, which further worsens the respiratory symptoms.1,6
The diagnosis is challenging and requires exclusion of more common diagnoses, such as, pulmonary embolism and cancer recurrence. The workup includes chest computed tomography (CT), pulmonary function tests, and bronchoscopy. One treatment option involves surgical repositioning of the mediastinum, sometimes done with the use of tissue expanders, which serve to restore the normal anatomical architecture of the hemithorax to prevent reoccurrence of mediastinal shift and rotation.1,2,7 Risks of the surgery include hypotension from overexpansion of the saline implants, worsening of respiratory symptoms (ie, from compression of the remaining lung), and pneumonia. One follow-up study by Shen et al demonstrated that tissue expander placement provides long-lasting symptomatic relief with significant improvement in quality of life and low morbidity and mortality.5 In this retrospective study that included 18 patients with a median follow-up time of 32 months, 10 had symptomatic improvement, 1 had no improvement, and 1 had worsening of their symptoms. There was 1 death that occurred postoperatively following a complicated repair that involved aortic division and bypass. A less common surgical technique involves fixing the mediastinum to a pericardial patch. Tracheobronchial stent placement is a treatment option for poor surgical candidates. Complications of stents include malpositioning or migration, stent obstruction, and recurrent infection.8 Tracheobronchial stent placement can be performed prior to planned surgical repair, particularly in patients who have developed tracheobronchomalacia because these patients may not have symptomatic improvement after surgical repair alone.6 The concern in patients with stents is that intubation can cause stent migration. A conservative approach for airway management would be to inspect the stent with fiber-optic bronchoscopy prior to final endotracheal tube (ETT) placement.
There are several concerns for anesthesiologists when managing patients with postpneumonectomy syndrome in the operating room; however, the low incidences of these cases yield few reports on management of this complex cardiopulmonary pathology. There are only 2 published case reports in the anesthesiology literature.9,10 Airway compression caused by the twisted mediastinum can make both intubation and ventilation challenging. As previously mentioned, these patients often have lung hyperinflation, which can decrease venous return and put these patients at risk for hemodynamic compromise. We describe a case of an 18-year-old female with postpneumonectomy syndrome who underwent a thoracotomy for insertion of tissue expanders and the anesthetic considerations for the case. Informed written consent was obtained from the patient.
Case Report
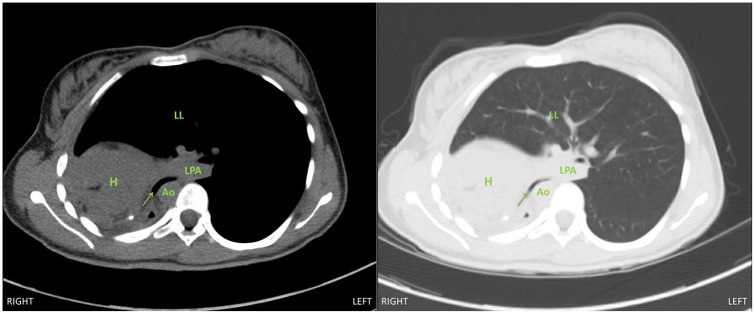
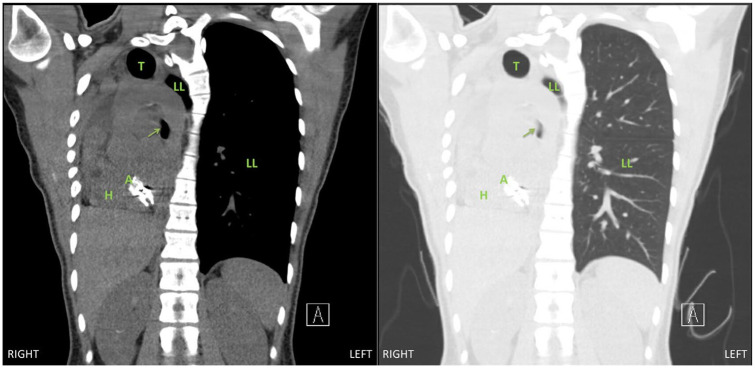
An 18-year-old female with a history of right pulmonary vein atresia who underwent a right pneumonectomy 2 years prior presented with a 6-month history of worsening dyspnea. Pulmonary function tests were notable for a 43% decline in her forced expiratory volume in 1 second from 1.34 L to 0.77 L. A CT scan of her chest revealed complete shift of the mediastinum to the right, severe narrowing of the left main bronchus between the descending thoracic aorta, thoracic spine, and the left pulmonary artery to 0.3 cm, hyperinflation of the left lung, and a dilated and right-shifted trachea (Figures 1 and 2). She was scheduled for a right thoracotomy with chest implantation of tissue expanders for medialization of her mediastinum.
Figure 1.
Chest computed tomography image, axial view, demonstrating severe narrowing of left main bronchus (arrow) between the descending thoracic aorta and left pulmonary artery with deviation into the right chest. Abbreviations: H, heart; LPA, left pulmonary artery; Ao, descending thoracic aorta; LL, left lung.
Figure 2.
Chest computed tomography image, coronal view, demonstrating extreme mediastinal shift and rotation into the right chest with narrowing of the left main bronchus (arrow). Abbreviations: T, trachea; LL, left lung; H, heart; A, atrial septal defect patch.
Prior to induction of anesthesia, a radial arterial line was placed with local anesthesia. An inhalational induction technique was performed with sevoflurane, with maintenance of spontaneous ventilation. Direct laryngoscopy revealed a grade 1 view and a 6.0 microlaryngeal ETT was passed through the vocal cords and into the trachea without issue. There were signs of obstruction on capnography; however, the patient was maintaining adequate oxygenation and ventilation despite her known left mainstem bronchial obstruction. Following intubation, fiber-optic bronchoscopy was used to confirm ETT placement and evaluate the lower airway. The right main bronchial stump was intact; the left main bronchus was significantly narrowed (Figure 3). Prior to the start of surgery, additional vascular access was obtained with a central venous catheter placed in the right internal jugular vein. A transesophageal echocardiography (TEE) probe was placed to aid intraoperative monitoring.
Figure 3.
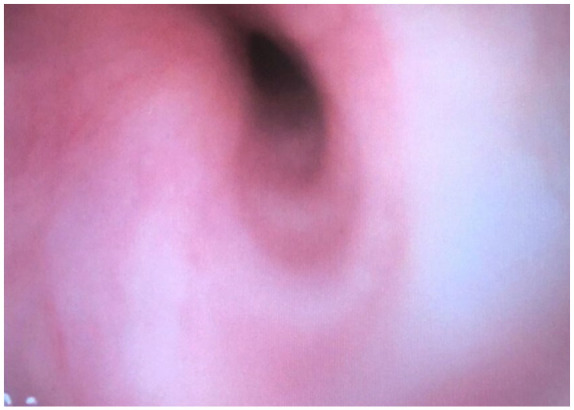
Left main bronchus narrowing prior to surgery on bronchoscopy.
The patient was ventilated with tidal volumes of 6 mL/kg of ideal body weight and a positive end-expiratory pressure of 4 cm H2O. Peak airway pressures were kept below 20 cm H2O. The surgery proceeded without issue. Careful attention was paid to hemodynamics and ventilator parameters during the filling of the tissue expanders to ensure that there was no cardiac or pulmonary compression from the medialization of her mediastinum. The patient tolerated this well.
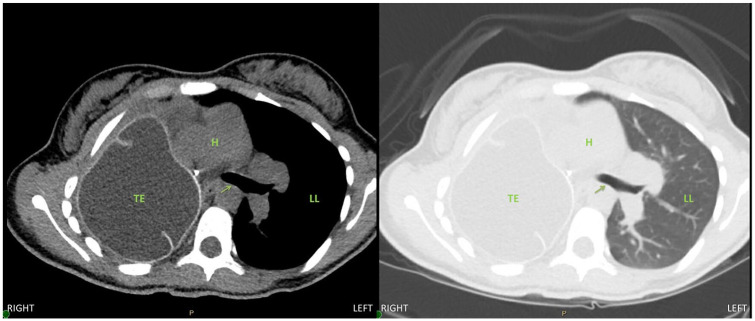
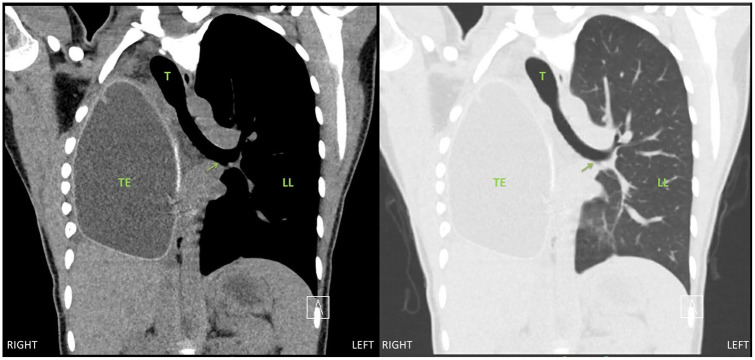
At the conclusion of the surgery, repeat fiber-optic bronchoscopy was performed and confirmed complete resolution of the left main bronchus obstruction (Figure 4). The patient was extubated in the operating room and taken to the postanesthesia recovery unit. Her postoperative pain control was managed with a thoracic epidural that was placed preoperatively. She had significant improvement in her dyspnea and she was discharged from the hospital on postoperative day 4. Her postoperative forced expiratory volume in one second was 1.35 L, which is her baseline. Repeat CT scan was performed 6 months after the surgery showed evidence of mild mediastinal rightward shift but no left main bronchus obstruction (Figures 5 and 6).
Figure 4.
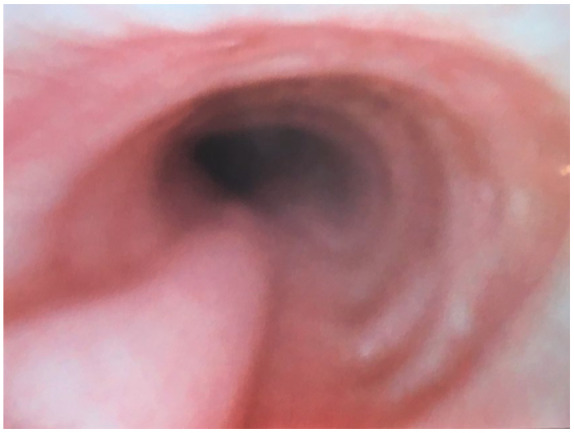
Left main bronchus in the location of previous narrowing following tissue expander implantation on bronchoscopy.
Figure 5.
Six-month postoperative chest computed tomography image, axial view, demonstrating a patent left main bronchus (arrow) in the left chest. Abbreviations: TE, tissue expander; H, heart; LL, left lung.
Figure 6.
Six-month postoperative chest computed tomography image, coronal view, demonstrating a patent left main bronchus (arrow) in the left chest. Abbreviations: TE, tissue expander; T, trachea; H, heart; LL, left lung.
Discussion
The challenges faced by anesthesiologists during the care of a patient with postpneumonectomy syndrome are potential difficult airway, ventilation, and hemodynamic instability. The dynamic airway compression caused by the rotated mediastinum can lead to complete collapse of the airway following muscle relaxation, and thus, the airway should be secured prior to loss of spontaneous ventilation. Potential induction techniques include inhalational induction with maintenance of spontaneous ventilation until the airway is secured or awake, fiber-optic-assisted intubation. The decision to perform either technique should be based on provider level of comfort and patient compliance.
Intubation can be complicated by tracheal deviation and/or tracheal or bronchial obstruction and it is important to recognize that there may be a need to bypass a narrowed segment in order to adequately ventilate the patient. Options for management of patients with severe bronchial obstruction include microlaryngeal ETT, airway exchange catheter with the ability to ventilate, bronchial dilation, and in cases of severe airway narrowing, veno-venous or venoarterial extracorporeal membrane oxygenation (ECMO). In our case, we opted for a 6.0 microlaryngeal ETT and positioned the ETT proximal to the obstruction as we had little difficulty with ventilation. Our secondary plan, if ventilation proved to be inadequate, was veno-venous ECMO. In a previously published case report on this topic, Evans et al were unable to adequately ventilate with a standard 8.0 ETT that was proximal to the narrowed bronchus. They described multiple failed attempts to bypass the obstruction despite using fiber-optic bronchoscopy and a bougie, ultimately having to dilate the stenotic segment.9
Should there be a need to bypass the bronchial obstruction, extreme caution must be taken to not injure the narrowed bronchus. One must also consider the possibility that even once the narrowed segment is bypassed, there may be still be difficulty with ventilation. The small diameter of microlaryngeal ETT causes higher resistance to gas flow so higher airway pressures are needed to achieve similar tidal volumes and longer expiration times are needed to prevent breath-stacking. Lung protective ventilation should be utilized with target tidal volumes of 4 to 6 mL/kg of ideal body weight as higher tidal volumes can lead to lung trauma.11 Peak airway pressures and positive end-expiratory pressure should be limited to prevent barotrauma and decreased blood flow to the lung.
The hemodynamic status of these patients may be tenuous due to the hyperinflated lung and potential for air trapping, which can cause decreased venous return. During filling of the tissue expanders, there can be cardiac compression and the heart can rotate on its axis, which can further impair venous return. In our case, we were fortunate as our patient tolerated the filling of the tissue expanders without hemodynamic compromise. TEE monitoring may be helpful, but image acquisition may be quite challenging in the setting of a rotated heart and severely hyperinflated lung. TEE was used in this case, but the image quality was poor for the aforementioned reasons. Caution must be exercised when placing a TEE probe in these patients as esophageal compression and deviation can increase the risk of perforation. Available imaging, such as endoscopy and CT, should be reviewed prior to the decision to proceed with TEE monitoring. If any resistance is met, the TEE should be aborted immediately. Invasive blood pressure monitoring served as a critical monitor for rapid detection of hemodynamic compromise, which was anticipated during surgical manipulation of the heart and potentially during the inflation of the tissue expanders. Pulmonary artery catheter monitoring was not considered for this case, as likelihood of ischemic injury to the right heart or torsion of the pulmonary vasculature during slow and gentle inflation of the tissue expanders was low. Furthermore, the patient had normal biventricular function and did not require pulmonary artery pressure monitoring in the postoperative period.
Important considerations in the postoperative management of these patients include aggressive pain control, early extubation, and maintenance of euvolemia. We opted for thoracic epidural analgesia in our case as this has been consistently shown to provide excellent pain control in open thoracic surgeries, which decreases the risk of splinting, thus decreasing the risk of atelectasis with consequent risk of pneumonia.12 Paravertebral blocks offer similar analgesia to thoracic epidurals and one study found that it is associated with less short-term complications, such as hypotension and nausea/vomiting when used for pneumonectomies13; currently, there is little data on the use of erector spinae plane blocks for thoracic surgeries but a double-blinded study performed by Fang et al showed promising results in which erector spinae plane blocks were shown to be comparable with paravertebral blocks for analgesia after thoracotomies.14 Intravenous opioids along with a multimodal pain medication regimen is an option for postoperative pain management; however, the respiratory depression associated with opioids may be detrimental to the recovery process and should be reserved for patients with contraindications to neuraxial or regional anesthetic techniques.
Postpneumonectomy syndrome is a rare complication for patients who undergo pneumonectomy. It is even rarer for the anesthesiologist to encounter these patients in the operating room; however, as patients with congenital cardiac anomalies live longer and present to the operating room for repeat surgery related to their congenital illness or prior surgery, clinical syndromes, such as postpneumonectomy syndrome, will likely become less rare. Currently, there is only one case report and an interesting case image report in the anesthesiology literature about patients with this postpneumonectomy syndrome.9,10 Our case report further outlines the complexities involved in their care with regard to airway management due to airway obstruction, ventilation concerns for air trapping, and hemodynamic compromise, and offers modern-day solutions to oxygenation and ventilation (ie, consideration for ECMO).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Vivian Doan  https://orcid.org/0000-0003-1642-437X
https://orcid.org/0000-0003-1642-437X
References
- 1. Soll C, Hahnloser D, Frauenfelder T, Russi EW, Weder W, Kestenholz PB. The postpneumonectomy syndrome: clinical presentation and treatment. Eur J Cardiothorac Surg. 2009;35:319-324. [DOI] [PubMed] [Google Scholar]
- 2. Jansen JP, de la Rivière AB, Alting MP, Westermann CJ, Bergstein PG, Duurkens VA. Postpneumonectomy syndrome in adulthood. Surgical correction using an expandable prosthesis. Chest. 1992;101:1167-1170. [DOI] [PubMed] [Google Scholar]
- 3. Kopec SE, Irwin RS, Umali-Torres CB, Balikian JP, Conlan AA. The postpneumonectomy state. Chest. 1998;114:1158-1184. [DOI] [PubMed] [Google Scholar]
- 4. Smulders SA, Holverda S, Vonk-Noordegraaf A, et al. Cardiac function and position more than 5 years after pneumonectomy. Ann Thorac Surg. 2007;83:1986-1992. [DOI] [PubMed] [Google Scholar]
- 5. Shen KR, Wain JC, Wright CD, Grillo HC, Mathisen DJ. Postpneumonectomy syndrome: surgical management and long-term results. J Thorac Cardiovasc Surg. 2008;135:1210-1216. [DOI] [PubMed] [Google Scholar]
- 6. Grillo HC, Shepard JA, Mathisen DJ, Kanarek DJ. Postpneumonectomy syndrome: diagnosis, management, and results. Ann Thorac Surg. 1992;54:638-650. [DOI] [PubMed] [Google Scholar]
- 7. Jung JJ, Cho JH, Kim HK, et al. Management of post-pneumonectomy syndrome using tissue expanders. Thorac Cancer. 2016;7:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest. 2007;132:609-616. [DOI] [PubMed] [Google Scholar]
- 9. Evans GH, Clark RJ. Management of life threatening adult postpneumonectomy syndrome. Anaesthesia. 1995;50:148-150. [DOI] [PubMed] [Google Scholar]
- 10. Sharifpour M, Bittner EA. Postpneumonectomy syndrome: a case of shifting priorities. Anesthesiology. 2014;121:1334. [DOI] [PubMed] [Google Scholar]
- 11. Marret E, Cinotti R, Berard L, et al. Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery: a double-blind randomised controlled trial. Eur J Anaesthesiol. 2018;35:727-735. [DOI] [PubMed] [Google Scholar]
- 12. Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011;115:181-188. [DOI] [PubMed] [Google Scholar]
- 13. Powell ES, Cook D, Pearce AC, et al. A prospective, multicentre, observational cohort study of analgesia and outcome after pneumonectomy. Br J Anaesth. 2011;106:364-370. [DOI] [PubMed] [Google Scholar]
- 14. Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med. 2019;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]