Abstract
Purpose
To compare the treatment effects and tolerability of a topical application of mizoribine (MZR) and cyclosporine A (CsA) eye drops (Restasis; Allergan, Inc., Irvine, CA, USA) in a mouse dry eye model.
Methods
C57BL/6 mice subjected to desiccating stress (DS) were treated with 0.05% MZR in phosphate-buffered saline (PBS) or Restasis eye drops four times a day for 5 days. Untreated mice served as control. Tear secretion, Oregon green dextran staining, and the conjunctival goblet cell quantity were evaluated. The apoptosis and matrix metalloproteinase 9 (MMP-9) in the ocular surface, conjunctival CD4, and T helper–related cytokines were verified. The ocular tolerance of these two drugs was evaluated by observing the mice's behavioral changes.
Results
Topical administrations of MZR or Restasis both increased tear production, maintained goblet cell density, and improved corneal barrier function. Both MZR and Restasis suppressed the expression of MMP-9 and apoptosis in the ocular surface. Meanwhile, both MZR and Restasis decreased the infiltration of CD4+ T cells, reversed the production of interferon-γ, interleukin (IL)–17A, and IL-13 in conjunctiva under DS. The abovementioned efficacies between these two eye drops were not statistically significant. However, the number of scratching and wiping behaviors in the MZR-treated group was significantly less than in the Restasis-treated group.
Conclusions
MZR (0.05% in PBS) could be a good competitive product for Restasis because of the comparable treatment effect in dry eye diseases and better ocular tolerability in ocular itch and pain.
Translational Relevance
This study provided an immunosuppressive agent comparable to Restasis for the treatment of dry eye disease.
Keywords: mizoribine, cyclosporine A, dry eye, ocular tolerance
Introduction
Dry eye disease (DED) is an inflammatory disease that can induce innate and adaptive immune responses and activation of cytokines produced by recruited CD4+ T cells, which are key contributors to the pathophysiology of chronic dry eye, including Th1 and Th17 cells.1 Anti-inflammatory treatment is essential to improve the therapeutic effects of DED.
Many anti-inflammatory agents are used currently in DED treatment, including nonsteroidal anti-inflammatory drugs, topical corticosteroids, and immunomodulatory agents. Cyclosporine A (CsA) is an anti-inflammatory immunomodulatory drug that was approved by the US Food and Drug Administration (FDA) in 2003 for the treatment of DED. The treatment with 0.05% CsA can significantly decrease punctate corneal epithelial fluorescein staining,2 increase goblet cell density,3,4 reduce conjunctival epithelial apoptosis,4 and suppress ocular surface inflammation.5 However, CsA is a hydrophobic molecule, which is difficult to formulate into conventional topical ophthalmic delivery systems. To overcome these obstacles due to the vehicles used, researchers have made great efforts in the past few years to develop safer and more effective ocular delivery systems for CsA, including vegetable oils, hydrogel, collagen shields, and colloidal carriers (liposomes, nanoparticles, emulsions).6,7 Restasis (Allergan, Inc., Irvine, CA), the main active ingredient is CsA, is widely used by the ophthalmologist. It is an anionic oil-in-water nanoemulsion with antiseptic effect, containing castor oil dissolved in polysorbate 80 as emulsifier.6 Despite this, patients' complaints are often heard during Restasis clinical use, including burning, pain, itching, and redness, which turned out to be from the side effects of delivery systems.6,8–10 Until now, no perfect solution has been found to solve simultaneously the solubility and tolerability of CsA. Therefore, we shift the research direction to the development of water-soluble immunosuppressants, which can solve the dilemma encountered by CsA and other liposoluble eye drops.
Mizoribine (MZR) is widely used as a primary immunosuppressive agent in human renal transplantation for oral administration. MZR, an imidazole nucleoside, is a water-soluble hydrophilic compound with very low lipophilicity (log P is −2.87).11 It can be prepared easily in aqueous formulations for ophthalmic use. Our previous study has shown that topical application of MZR eye drops in phosphate-buffered saline (PBS) significantly increased tear production, decreased goblet cell loss, reduced conjunctival CD4+ T-cell infiltration, suppressed apoptosis of ocular surface cells, and improved corneal barrier function in the mouse dry eye model.12
Since both CsA and MZR are immunomodulatory drugs that have therapeutic efficacy in the treatment of dry eye, this study aimed to further compare the therapeutic effects and ocular tolerability of Restasis and MZR eye drops in PBS using the experimental mouse model of dry eye and evaluated the value of further development of MZR eye drops.
Materials and Methods
Animal Model of Dry Eye
This research program was approved by the experimental animal ethics committee of Xiamen University. Ten to 12-week-old female C57BL/6 (B6) mice (Shanghai SLAC Laboratory Animal Center, Shanghai, China) were used according to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Desiccating stress (DS) was induced by subcutaneous injection of scopolamine hydrobromide (cat. MB5860; meilubio, Dalian, China) and dry environment. Scopolamine hydrobromide (0.5 mg/0.2 mL) was injected four times daily (8 AM, 11 AM, 2 PM, and 5 PM), and the mouse was kept in environments with an air draft where air humidity was 30% ± 5% for 5 days. The sex- and age-matched mice, which served as nonstressed (NS) controls, were kept in a normal environment (50%–75% relative humidity, not exposed to forced air) without scopolamine hydrobromide injection.
Topical Eye Drops Application
To compare the therapeutic effect of MZR eye drops and CsA eye drops on ocular surface damage of dry eye, we choose Restasis as a positive control because of its high market share and recognition. According to our previous study, 0.05% MZR in PBS has the best therapeutic effect,12 so the final plan was to compare the therapeutic effect of 0.05% MZR in PBS and 0.05% CsA in nanoemulsion (Restasis). Then, 5 µL of eye drops was administered four times daily followed by manual blinking two to three times for 5 days under DS.
Ocular Irritation Study
We judge ocular irritation by the behavior of mice. According to the findings of Shimada and LaMotte,13 scratching of the hindlimbs is used as an index of itching, and wiping of the forelimbs is an indicator of pain. The number of scratching or wiping 5 minutes after PBS, MZR, or Restasis eye drops was recorded by video recording to quantify the irritation behavior. This experiment was performed three times by three independent laboratory technicians, and the average value was the mean ± SD.
Conjunctival Hyperemia Observation
To evaluate the irritation of the drug, a slit-lamp microscope was used to observe the congestion of bulbar conjunctival vessels after PBS, MZR, or Restasis administration. Without anesthesia, the superior conjunctiva of mice was photographed within 3 minutes after eye dropping. All slit-lamp photos were magnified 60 times.
Measurement of Tear Production
At the same time point (8 AM), the tear production was measured without topical anesthesia using phenol red cotton threads (Zone-Quick; Yokota, Tokyo, Japan). The thread was put under the lower conjunctival fornix for 15 seconds. The threads became red when soaked in tears. The amount of tear production was assessed by measuring the length of the wet red thread under the microscope in millimeters.
Oregon Green Dextran Staining
Oregon green dextran (OGD; cat. D7173; 70,000 molecular weight (MW), anionic, lysine fixable; Invitrogen, Eugene, OR, USA) staining was used to evaluate the corneal epithelium integrity.12 Briefly, after the mice were sacrificed, 0.5 µL 50 mg/mL OGD was instilled into the conjunctival sac. After three times of manual blinks, the OGD stayed in the conjunctival sac for 1 minute, and then the cornea was rinsed with 5 mL saline and photographed (AZ100; Nikon, Tokyo, Japan) under fluorescence excitation at 470 nm. The fluorescence intensity within the 3-mm diameter of the central cornea was calculated using NIS Elements software (version 4.1; Nikon, Melville, NY, USA).
Measurement of Goblet Cells
The eyes and adnexa of mice were removed, fixed in 4% formaldehyde, dehydrated in gradient concentrations of ethanol, and infiltrated with paraffin (cat. P3683; Sigma-Aldrich, St. Louis, MO, USA). Periodic acid–Schiff (PAS) staining was performed using a commercially available kit (cat. 395B-1KT; Sigma-Aldrich). Each group contained five specimens, and the left eye was examined uniformly. Each sample was observed at three sections, which were at least 300 µm apart. All stained photos were taken with an HD digital camera (Eclipse E400 with a DS-Fi1; Nikon). NIS Elements software was used to count the number of goblet cells, including the upper and lower conjunctiva, and expressed as the number of goblet cells per millimeter.
Immunofluorescent Staining
The eyes and adnexa of mice were excised and embedded in cryopreservation medium optimal cutting temperature compound (cat. 4583; SAKURA Tissue-Tek, Torrance, CA, USA) and saved at –80°C. Frozen sections were used to detect the expression of matrix metalloproteinase 9 (MMP-9) in the central corneal epithelium and the expression of activated caspase-8 in the central conjunctiva by immunofluorescence. The tissue sections were fixed in acetone at –20°C for 10 minutes and then blocked with 2% bovine serum albumin for 60 minutes at room temperature. Thereafter, samples were incubated at 4°C overnight with the primary antibodies: anti–MMP-9 (1:500; cat. ab38898; Abcam, Cambridge, MA, USA) and anti–AC caspase-8 (1 µg/mL; cat. ab25901; Abcam). After rinsing in PBS, samples were incubated with Alexa-Fluor 594 anti-goat IgG (1:300; cat. A11055; Invitrogen, Eugene, OR, USA) or anti-rabbit IgG (1:300; cat. A21206; Invitrogen) at room temperature in the dark for 1 hour, followed by counterstaining with DAPI (cat. H-1200; Vector, Burlingame, CA, USA) for 5 minutes. A Leica microscope (DM2500; Leica Microsystems, Wetzlar, Germany) was used to take representative images.
Immunohistochemistry
Acetone-fixed cryostat sections were stained with rabbit anti-mouse CD4 (1:50; cat. 553647; BD Pharmingen, San Diego, CA, USA) primary antibody and appropriate biotinylated secondary antibodies (1:25; cat. 559286; BD Pharmingen). Avidin-biotin peroxidase method (Vectastain Elite ABC kit; cat. PK-6100; Vector) was performed according to the instructions. Representative pictures were photographed with a digital microscope camera (Eclipse E400 with a DS-Fi1; Nikon). From the limbal to the tarsal conjunctiva and the depth of 75 µm below the basement membrane, the positive cells were counted. Data are reported as the average number of cells per millimeter.14
TdT-dUTP Terminal Nick-End Labeling Assay
Paraffin sections were used for TdT-dUTP terminal nick-end labeling (TUNEL) staining. TUNEL assay was performed using a commercialized test kit (DeadEnd Fluorometric TUNEL System; cat. G3250; Promega, Madison, WI, USA). Representative images were captured with an HD Leica microscope (DM2500; Leica Microsystems). The results were expressed as the number of positively stained cells.
Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from the corneal epithelium and conjunctiva using a commercially available PicoPure RNA isolation kit (cat. KIT0204; Arcturus, San Diego, CA, USA) according to the instructions. Complementary DNA was synthesized using a reverse transcription kit (cat. RR047A; TaKaRa, Shiga, Japan). Real-time polymerase chain reaction (PCR) was performed on a StepOne Real-Time PCR System (Applied Biosystems, Alameda, CA, USA), and the parameters were set as previously reported.12 The primers are provided in the Table.
Table.
Primers Used in This Study
| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| MMP-9 | CAATCCTTGCAATGTGGATG | AGTAAGGAAGGGGCCCTGTA |
| IL-13 | GCAGCATGGTATGGAGTGT | TATCCTCTGGGTCCTGTAGATG |
| IL-17A | CGCAATGAAGACCCTGATAGAT | CTCTTGCTGGATGAGAACAGAA |
| IFN-γ | AAATCCTGCAGAGCCAGATTAT | GCTGTTGCTGAAGAAGGTAGTA |
| β-actin | CCTAAGGCCAACCGTGAAAAG | AGGCATACAGGGACAGCACAG |
Enzyme-Linked Immunosorbent Assay
The conjunctiva was collected in cold RIPA buffer (cat. R0278; Sigma-Aldrich) containing a protease inhibitor cocktail (cat. 78440; Thermo Fisher Scientific, Waltham, MA, USA), lysed on ice for 15 minutes, and subjected to total protein assay kit (cat. 23225; Thermo Fisher Scientific). The concentrations of interleukin (IL)–17A (cat. BMS6001; eBioscience, San Diego, CA, USA), IL-13 (cat. BMS6015; eBioscience), and interferon-γ (IFN-γ) (cat. BMS606; eBioscience) were determined by enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions.
Western Blotting
Total protein concentrations of conjunctival epithelia were determined with a protein assay kit described above. The equivalent protein (30 µg) was separated by electrophoresis on a 12% sodium lauryl sulfate–polyacrylamide gel electrophoresis gel, transferred to a polyvinylidene fluoride membrane (cat. IPVH00010; Millipore, Billerica, MA, USA), and probed with the following specific antibodies: anti–caspase-8 (cat. ab25901; Abcam) and β-actin (1:10,000; Sigma-Aldrich). Chemiluminescence assays (cat. ECL-500; ECL, Lulong, Xiamen, China) were used for the detection of proteins. Quantitative Western blot analysis was calculated by the transilluminator (Image Lab software 6.0; Bio-Rad, Philadelphia, PA, USA).
Statistical Analysis
Data were represented as mean ± SD for each group. Based on the normality of the data distribution, comparison among groups was evaluated by one-way analysis of variance with Tukey's post hoc test. GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA) was used for data analysis. P < 0.05 was considered statistically significant.
Results
MZR (0.05% in PBS) and Restasis Had Comparable Effects in Improving Tear Secretion
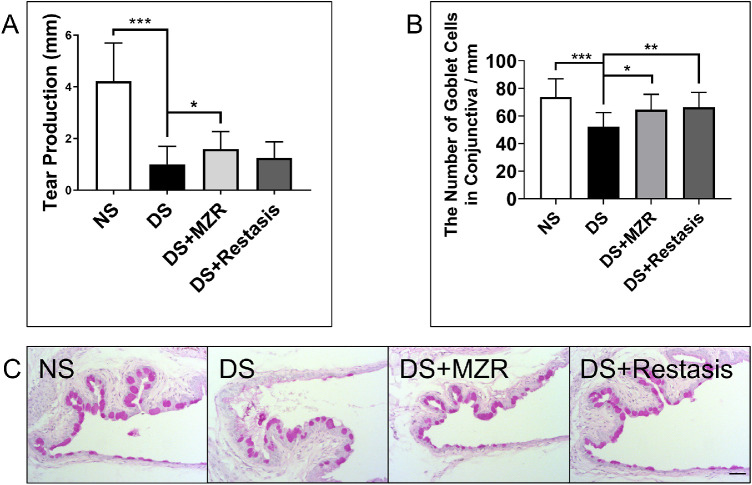
We first measured the tear production of mice using the phenol red thread test. It was demonstrated that the tear production was decreased in the DS group compared with the NS group (4.2 ± 1.5 mm vs. 1.0 ± 0.7 mm; P < 0.001). Topical application of 0.05% MZR significantly improved tear secretion in mice under DS conditions (1.0 ± 0.7 mm vs. 1.6 ± 0.7 mm; P < 0.05). Topical application of Restasis also improved tear secretion, but there was no statistical difference between the Restasis group and the DS group (1.0 ± 0.7 mm vs. 1.3 ± 0.6 mm; P = 0.6786). In addition, there was no statistical difference of aqueous tear production between 0.05% MZR- and Restasis-treated groups (Fig. 1A), n = 5 for each group.
Figure 1.
The effects of topical application of 0.05% MZR in PBS and Restasis on tear production and goblet cell loss of mice under DS. (A) Tear production was measured by the phenol red thread test. (B) Statistical analysis of the number of goblet cells per millimeter. (C) Representative images of PAS staining in the conjunctiva (scale bar: 200 µm). *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as mean ± SD (n = 5 for each group).
MZR (0.05% in PBS) and Restasis Had Similar Effects in Rescuing Conjunctival Goblet Cell Loss
The quantity of the conjunctival goblet cells was counted with PAS staining. Compared to the NS group, the DS group induced significant decreases of conjunctival goblet cells (NS versus DS, 74 ± 13 vs. 52 ± 10/mm; P < 0.001). Both 0.05% MZR and Restasis eye drops significantly increased the number of conjunctival goblet cells under the DS condition (DS versus DS + MZR, 52 ± 10 vs. 64 ± 11/mm, P < 0.05; DS versus DS + Restasis, 52 ± 10 vs. 66 ± 11/mm, P < 0.01). However, there was no significant difference between the 0.05% MZR-treated mice and Restasis-treated mice. (Figs. 1B, 1C), n = 5 for each group.
MZR (0.05% in PBS) and Restasis Had Comparable Effects in Recovering the Corneal Barrier Integrity
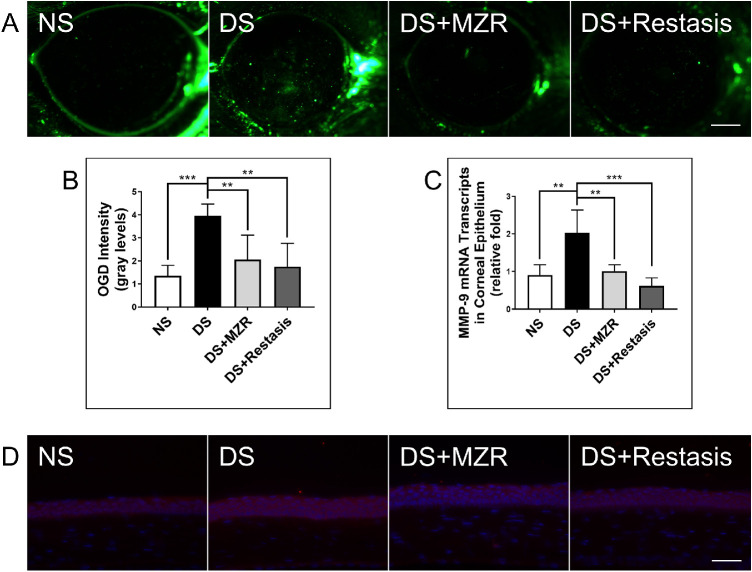
Corneal epithelial permeability to the high molecular weight fluorescent molecule OGD was assessed in all groups. As shown in Figures 2A, 2B, the corneal uptake of OGD was significantly higher in the DS group (3.96 ± 0.50) than in the NS group (1.36 ± 0.44, P < 0.01), with wider areas of punctate and intense confluent dye staining. It was revealed that both 0.05% MZR and Restasis eye drop treatment had a protective effect on the corneal barrier integrity under the DS condition (DS versus DS + MZR, 3.96 ± 0.50 vs. 2.06 ± 1.06; DS versus DS + Restasis, 3.96 ± 0.50 vs. 1.74 ± 1.02, both P < 0.01). OGD uptake in eyes treated with these two agents was not statistically different, n = 5 for each group.
Figure 2.
The effects of topical application of 0.05% MZR in PBS and Restasis on the corneal barrier integrity of mice under DS. (A) Representative images of OGD staining in the cornea (scale bar: 500 µm). (B) Statistical analysis of the mean intensity of OGD staining. (C) Statistical analysis of gene expression of MMP-9 in corneal epithelium. (D) Representative images of MMP-9 immunofluorescence staining in corneal epithelium (scale bar: 200 µm). Data are shown as mean ± SD. **P< 0.01, ***P < 0.001 (n = 5 for each group).
It is previously reported15 that MMP-9 is the key factor in corneal barrier disruption in DS-induced DED. As shown in Figure 2C, it was demonstrated that the gene expressions of MMP-9 were significantly increased in the DS group compared to the NS group (NS versus DS, 0.91 ± 0.27 vs. 2.03 ± 0.60, P < 0.01). Meanwhile, both 0.05% MZR and Restasis treatments significantly downregulated MMP-9 gene expression in corneal epithelium under the DS condition (DS versus DS + MZR, 2.03 ± 0.60 vs. 1.01 ± 0.17, P < 0.01; DS versus DS + Restasis, 2.03 ± 0.60 vs. 0.62 ± 0.21, P < 0.001). No significant difference was noted between MZR- and Restasis-treated groups. At the protein level of MMP-9, similar results were discovered (Fig. 2D), n = 5 for each group.
MZR (0.05% in PBS) and Restasis Had Similar Effects in Suppressing CD4+ T-Cell-Mediated Inflammation
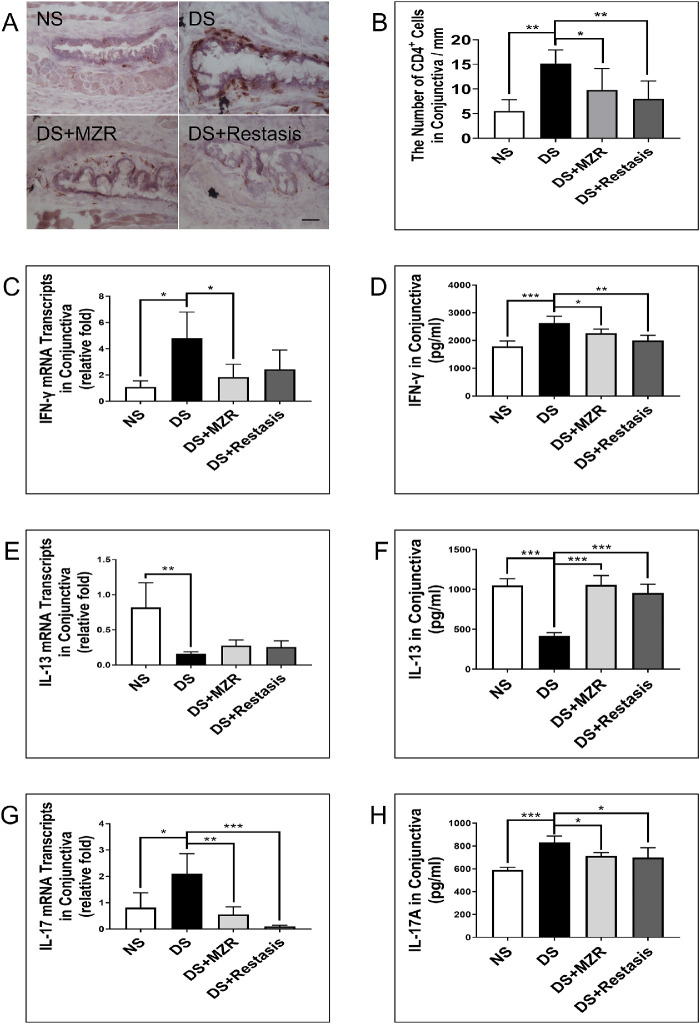
Dry eye is an inflammatory ocular surface disease characterized by infiltration of CD4+ T cells producing IFN-γ and IL-17A in the ocular surface. We then detected CD4+ T cells as well as the related inflammatory factors: IFN-γ, IL-13, and IL-17A in the conjunctiva of mice. It was shown that both 0.05% MZR and Restasis treatment suppressed DS-induced CD4+ T-cell infiltration in the conjunctiva (Figs. 3A, 3B; DS versus DS + MZR, 15.18 ± 2.76 vs. 9.79 ± 4.35/mm, P < 0.05; DS versus DS + Restasis, 15.18 ± 2.76 vs. 7.99 ± 3.65/mm, P < 0.01). However, there was no significant difference between the 0.05% MZR group and the Restasis group. It was demonstrated by quantitative reverse transcription PCR and ELISA assay that both 0.05% MZR and Restasis treatment decreased the gene expression and production of IL-17A and IFN-γ and increased the gene expression and production of IL-13 in conjunctiva under DS (Figs. 3C–H). No significant difference between these measurements was found between 0.05% MZR- and Restasis-treated mice, n = 5 for each group.
Figure 3.
The effects of topical application of 0.05% MZR in PBS and Restasis on CD4+ T-cell-based inflammation in the conjunctiva of mice under DS. (A) Representative images of CD4+ T-cell staining (scale bar: 500 µm). (B) Statistical analysis of the number of CD4+ T cells in conjunctiva per millimeter. Statistical; analysis of gene expression and protein levels of IFN-γ (C, D), IL-13 (E, F), and IL-17A (G, H) in the conjunctiva. Data are shown as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 (n = 5 for each group).
MZR (0.05% in PBS) and Restasis Had Comparable Effects in Suppressing the Apoptosis of Ocular Surface Cells
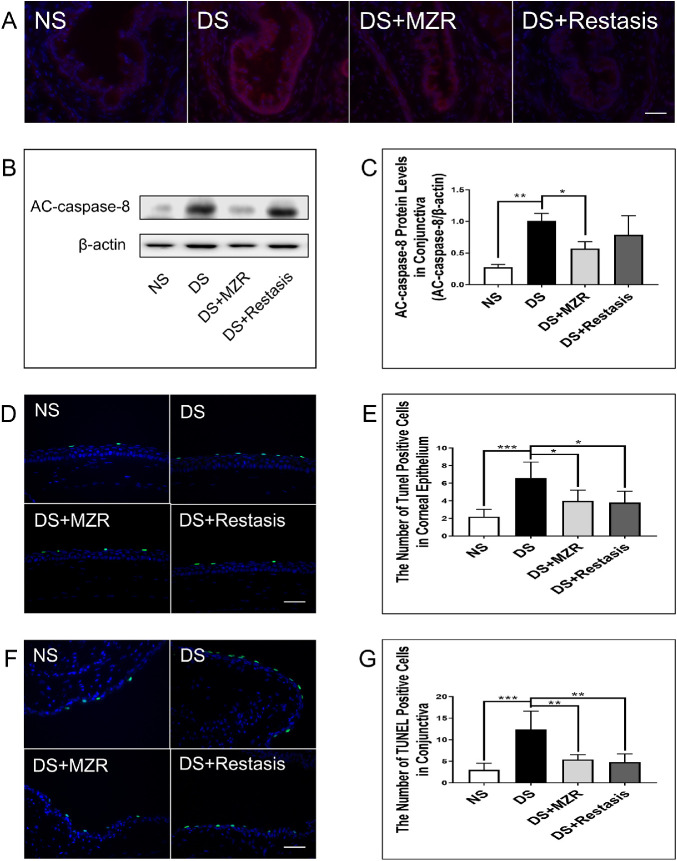
Evidence for the association between ocular surface inflammation and apoptosis has been investigated.16 Apoptosis plays an important role in the pathogenesis of DED. We finally measured the key factors of apoptosis: AC-caspase-8 and the levels of TUNEL staining positive cells. It was observed that topical application of 0.05% MZR or Restasis both effectively decreased the levels of caspase-8 (Figs. 4A–C; DS versus DS + MZR, 1.00 ± 0.11 vs. 0.57 ± 0.11, P < 0.05; DS versus DS + Restasis, 1.00 ± 0.11 vs. 0.78 ± 0.30, P = 0.3137) and the number of TUNEL-positive cells in the ocular surface (Figs. 4D–G; cornea epithelium, DS versus DS + MZR, 6.6 ± 1.8 vs. 4.0 ± 1.2, P < 0.05; DS versus DS + Restasis, 6.6 ± 1.8 vs. 3.8 ± 1.3, P < 0.05; conjunctival epithelium, DS versus DS + MZR, 12.4 ± 4 .3 vs. 5.4 ± 1.1, P < 0.01; DS versus DS + Restasis, 12.4 ± 4.3 vs. 4.8 ± 1.9, P < 0.01). No significant difference between AC-caspase-8 (Figs. 4A–C; P = 0.3371) and TUNEL staining positive cells (Figs. 4D–G; cornea epithelium, P = 0.9952; conjunctival epithelium, P = 0.9816) was found between 0.05% MZR- and Restasis-treated mice.
Figure 4.
The effects of topical application of 0.05% MZR in PBS and Restasis on apoptosis in the ocular surface of mice under DS. (A) Representative images of AC-caspase-8 immunofluorescence staining in the conjunctiva (scale bar: 300 µm). (B) Representative images of protein level of AC-caspase-8 in the conjunctiva. (C) Statistical analysis of the protein level of AC-caspase-8 in the conjunctiva. (D, F) Representative images of TUNEL staining in the cornea and the conjunctiva (scale bar: 50 µm). (E, G) Statistical analysis of the number of TUNEL-positive cells in the cornea and the conjunctiva. Data are shown as mean ± SD. *P < 0.05, **P <0.01, ***P < 0.001 (n = 5 for each group).
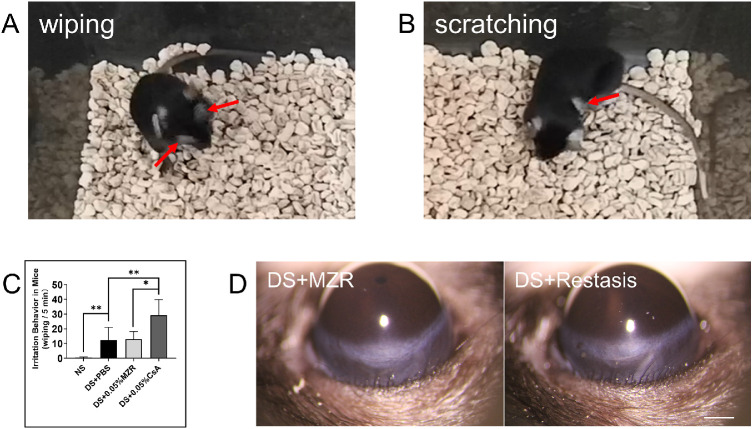
MZR (0.05% in PBS) Had Less Ocular Irritation Compared to Restasis after Topical Administration
Assessment of ocular irritation was performed after topical administration of the NS group and PBS alone or 0.05% MZR in PBS or Restasis eye drops in the mouse dry eye model. All groups of treated mice exhibited wiping behavior using the forelimbs, which is an indicator of pain (Fig. 5A). However, after 5 minutes of observation, we found that the number of wiping behaviors in the PBS- or MZR-treated group was significantly less than in the Restasis-treated group (Fig. 5C; DS + PBS versus DS + Restasis, 12.44 ± 8.58 vs. 29.2 ± 10.7, P < 0.01; DS + 0.05% MZR versus DS + Restasis, 13.0 ± 5.3 vs. 29.2 ± 10.7, P < 0.05). Interestingly, Restasis-treated mice also displayed scratching of the hindlimbs, an index of itching, but not in MZR- or PBS-treated mice (Fig. 5B). There was no significant conjunctival hyperemia in both treated groups (Fig. 5D), n = 5 for each group.
Figure 5.
Ocular irritation after topical application of PBS, 0.05% MZR in PBS and Restasis. (A) Representative images of wiping of the forelimbs (arrow). (B) Representative images of scratching of the hindlimbs (arrow). (C) Statistical analysis of the number of wiping behaviors 5 minutes after topical application of PBS alone, 0.05% MZR in PBS or Restasis. Data are shown as mean ± SD. **P <0.01, *P < 0.05 (n = 5 for each group). (D) Representative images of hyperemia of superior bulbar conjunctiva (scale bar: 150 µm).
Discussion
DED is an inflammatory disease, and clinical evidence suggests that topical anti-inflammatory treatment of dry eye is effective. Our study demonstrated that topical application of MZR could rescue goblet cell loss, improve corneal barrier functions, suppress ocular surface epithelial cell apoptosis, and decrease conjunctival CD4+ T-cell infiltration, which shows the same therapeutic effect as that of CsA during DS.
Although both drugs were effective, their mechanism of action was completely different. CsA selectively acts on the early stage of T lymphocyte activation and inhibits the production of interferon by lymphocytes. It blocks calmodulin/calcineurin-induced phosphorylation of the nuclear factor of activated T cells (NFAT) that is involved in early T-cell gene expression in the cytoplasm. Dephosphorylated NFAT is then transported to the nucleus to initiate transcription of T-cell-stimulated inflammatory cytokines, notably IL-2 and IFN-γ.2 MZR does not block early events in T-cell activation such as IL-2 or IL-2R expression, but its specific mechanism is the antiproliferation of lymphocytes by inhibiting DNA synthesis. It can block the movement of T and B cells from the G1 to S phase, as well as inhibit the synthesis of inosine monophosphate dehydrogenase and guanosine monophosphate synthetase, both enzymes believed to be required for the classical de novo pathway.17 Although these two drugs had different mechanisms of action, there was no significant difference in therapeutic effect in rescuing goblet cell loss, improving corneal barrier integrity, suppressing ocular surface epithelial cell apoptosis, and decreasing CD4+ T-cell infiltration-mediated inflammation in the ocular surface during DS. We believed that lymphocyte infiltration and/or activation were decreased by MZR and/or CsA on the ocular surface, which may be responsible for the comparable therapeutic effect between MZR and CsA in alleviating ocular surface damage induced by DS.
Reduced tear volume is one of the significant features of dry eye disease that causes ocular dryness, foreign body sensation, and ocular burning. Tear secretary function can be disrupted by disease of the afferent, efferent, or glandular components of the lacrimal functional unit, as well as from ocular surface or glandular inflammation.18,19 The release of inflammatory cytokines from lymphocytes and ocular resident cells can obstruct the tearing reflex by interfering with neurotransmitter release and the response of the tear-secreting machinery to the neurotransmitters.18,19 Evidence suggests that CsA treatment affects reflex tearing in patients with dry eye2,20 and significantly increases tear production at week 6 in murine dry eye.21 Topical application of MZR also significantly increases tear production during DS.12 Both may be improving tear secretion by suppressing T lymphocytes and inflammatory cytokines. From this study, we found that both MZR and CsA had a comparable effect on improving tear secretion, which is of great significance for aqueous deficient dry eye.
The sensory nerves on the ocular surface show morphologic and functional changes in DED and are the basis for the development of symptoms of discomfort.22 Inflammation plays an important role in the pathogenesis of dry eye and is the main cause of sensitization and damage to peripheral sensory neurons.23,24 Peripheral sensory nerve injury leads to the release of inflammatory mediators, such as substance P and calcitonin gene-related peptide (the main cytokines related to ocular pain).25 Together, these mediators activate resident mast cells and macrophages to release signal transduction mediators that directly activate or sensitize nociceptive and pruriceptive units, including prostaglandin, serotonin, tryptase, and histamine (the main function of the latter two is the transmission of itching).26 Therefore, the ultimate goal of dry eye treatment is to target the root cause of dry eye and to alleviate painful or itchy eyes without worsening existing irritation. However, the usage of CsA for ocular application represents a challenge due to its pharmaceutical formulation. Restasis formulation contains castor oil, which is the culprit that causes ocular burning and irritation.27 In addition, conjunctival hyperemia, discharge, epiphora, eye pain, foreign body sensation, pruritus, stinging, and visual disturbance are also the major drawbacks reported with Restasis.2,28–31 There was no symptom change over time by Ocular Surface Disease Index after topical administration of CsA, and 29% of subjects still experienced discomfort on instillation compared with 9% of subjects using vehicle.32 To date, more than 50 different approaches for the ocular delivery of CsA have been described,6 and it remains the most challenging compound to formulate in a suitable dosage form. Therefore, water-soluble ophthalmic immunosuppressants are the future direction for dry eye treatment. MZR is a water-soluble imidazole nucleoside with immunosuppressive activity. It is suitable for ophthalmic applications because of its inherent physical and chemical properties: hydrophilicity (the approximate solubility in water of MZR is 20 mg/mL compared to 0.0095 mg/mL of CsA). Therefore, the MZR eye drop is attractive because it can be prepared into a homogeneous solution that is clear and stable, has a long shelf life, and provides comfort and better compliance from the patient. In our study, from the behavioral experiments of mice with dry eye after topical application of these two drugs, we found that the ocular irritation of MZR was significantly less than that of CsA. Meanwhile, no obvious conjunctival hyperemia was observed during treatment. Therefore, we suggest that MZR may have application prospects in clinical ophthalmology.
In this experiment, we did not set up a vehicle control group because we did include a vehicle control group in our previous work12 and confirmed the efficiency of MZR in the treatment of dry eye. Similarly, the use of Restasis in DED has been described for several decades, and its effectiveness is widely recognized. While CsA used in this experiment (Restasis) is also a commercial product, it was somewhat difficult for us to get the exact formulation of its solvent and to establish a vehicle control group of Restasis.
In conclusion, we provided a repeatable efficacy of MZR as an eye drop to inhibit ocular inflammation of DED and demonstrated for the first time that 0.05% MZR in PBS eye drops has a comparable effect to Restasis in alleviating epithelial damage and CD4+ T-cell-mediated immunity in the ocular surface of DED. At the same time, MZR has less ocular irritation. The improvement in the ocular surface demonstrated by MZR meets the management standard for ocular surface disease, and MZR may be a promising potential drug suitable for dry eye therapy or other ocular surface inflammatory diseases.
Acknowledgments
Supported by grants from the National Key R&D Program of China (No. 2018YFA0107304, ZL), National Natural Science Foundation of China (No. 81330022, ZL; No. 81870627, ZL; No. 81900825, CH; No. 81570818, YC), and Natural Science Foundation of Fujian (No. 2017J01149, CH).
Disclosure: X. Lin, None; Y. Wu, None; L. Tang, None; W. Ouyang, None; Y. Yang, None; Z. Liu, None; J. Wu, None; X. Zheng, None; C. Huang, None; Y. Zhou, None; X. Zhang, None; Y. Chen, None; W. Li, None; J. Hu, None; Z. Liu, None
References
- 1. Pflugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res. 2013; 117: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000; 107: 631–639. [DOI] [PubMed] [Google Scholar]
- 3. Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008; 27: 64–69. [DOI] [PubMed] [Google Scholar]
- 4. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005; 24: 80–85. [DOI] [PubMed] [Google Scholar]
- 5. Kacmaz RO, Kempen JH, Newcomb C, et al.. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010; 117: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017; 117: 14–28. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal P, Rupenthal ID.. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov Today. 2016; 21: 977–988. [DOI] [PubMed] [Google Scholar]
- 8. Benitez del Castillo JM, del Aguila C, Duran S, Hernandez J, Garcia Sanchez J. Influence of topically applied cyclosporine A in olive oil on corneal epithelium permeability. Cornea. 1994; 13: 136–140. [DOI] [PubMed] [Google Scholar]
- 9. Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmology and Eye Diseases. 2014; 6: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byun Y-S, Rho CR, Cho K, Choi JA, Na KS, Joo C-K. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: a prospective, multicenter, open-label, surveillance study. Korean J Ophthalmol. 2011; 25: 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murakami T, Mori N.. Involvement of multiple transporters-mediated transports in mizoribine and methotrexate pharmacokinetics. Pharmaceuticals (Basel, Switzerland). 2012; 5: 802–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Lin X, Liu Z, et al.. Topical application of mizoribine suppresses CD4+ T-cell-mediated pathogenesis in murine dry eye. Invest Ophthalmol Vis Sci. 2017; 58: 6056–6064. [DOI] [PubMed] [Google Scholar]
- 13. Shimada SG, LaMotte RH.. Behavioral differentiation between itch and pain in mouse. Pain. 2008; 139: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schaumburg CS, Siemasko KF, De Paiva CS, et al.. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011; 187: 3653–3662. [DOI] [PubMed] [Google Scholar]
- 15. Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004; 45: 4293–4301. [DOI] [PubMed] [Google Scholar]
- 16. Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003; 44: 124–129. [DOI] [PubMed] [Google Scholar]
- 17. Dayton JS, Turka LA, Thompson CB, Mitchell BS. Comparison of the effects of mizoribine with those of azathioprine, 6-mercaptopurine, and mycophenolic-acid on lymphocyte-t proliferation and purine ribonucleotide metabolism. Mol Pharmacol. 1992; 41: 671–676. [PubMed] [Google Scholar]
- 18. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17: 584–589. [DOI] [PubMed] [Google Scholar]
- 19. Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004; 78: 409–416. [DOI] [PubMed] [Google Scholar]
- 20. Chen M, Gong L, Sun X, et al.. A comparison of cyclosporine 0.05% ophthalmic emulsion versus vehicle in Chinese patients with moderate to severe dry eye disease: an eight-week, multicenter, randomized, double-blind, parallel-group trial. J Ocul Pharmacol Ther. 2010; 26: 361–366. [DOI] [PubMed] [Google Scholar]
- 21. Kilic S, Kulualp K.. Efficacy of several therapeutic agents in a murine model of dry eye syndrome. Comp Med. 2016; 66: 112–118. [PMC free article] [PubMed] [Google Scholar]
- 22. Belmonte C, Nichols JJ, Cox SM, et al.. TFOS DEWS II pain and sensation report. Ocul Surface. 2017; 15: 404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devor M. Neuropathic pain: pathophysiological response of nerves to injury. In: McMahon S, Koltzenburg M, Tracey I, Turk DC, eds. Wall and Melzack's Textbook of Pain. Philadelphia, PA: Elsevier Ltd.; 2013: 861–888. [Google Scholar]
- 24. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen FY, Lee A, Ge S, Nathan S, Knox SM, McNamara NA. Aire-deficient mice provide a model of corneal and lacrimal gland neuropathy in Sjögren's syndrome. PLoS One. 2017; 12: e0184916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen HH, Yosipovitch G, Galor A. Neuropathic symptoms of the ocular surface: dryness, pain, and itch. Curr Opin Allergy Clin Immunol. 2017; 17: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang H, Cha K-H, Cho W, et al.. Cyclosporine amicellar delivery system for dry eyes. Int J Nanomed. 2016; 11: 2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allergan. RESTASIS (cyclosporine ophthalmic emulsion) 0.05%: highlights of prescribing information. Irvine, CA: Allergan; 2013. [Google Scholar]
- 29. Wan KH, Chen LJ, Young AL. Efficacy and safety of topical 0.05% cyclosporine eye drops in the treatment of dry eye syndrome: a systematic review and meta-analysis. Ocul Surface. 2015; 13: 213–225. [DOI] [PubMed] [Google Scholar]
- 30. Karn PR, Cho W, Park H-J, Park J-S, Hwang S-J. Characterization and stability studies of a novel liposomal cyclosporin A prepared using the supercritical fluid method: comparison with the modified conventional Bangham method. Int J Nanomed. 2013; 8: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta C, Chauhan A.. Ophthalmic delivery of cyclosporine A by punctal plugs. J Controlled Release. 2011; 150: 70–76. [DOI] [PubMed] [Google Scholar]
- 32. Leonardi A, Van Setten G, Amrane M, et al.. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol. 2016; 26: 287–296. [DOI] [PubMed] [Google Scholar]