Abstract
Purpose
To examine the changes in choroidal thickness (ChT) after 6 months of 1% or 0.01% atropine treatment and the independent factors associated with eye elongation.
Methods
A total of 207 myopic children aged 6 to 12 years were recruited and randomly assigned to groups A and B in a ratio of 1:1. Participants in group A received 1% atropine once a day for 1 week, and then once a week for 23 weeks. Participants in group B received 0.01% atropine once a day for 6 months. ChT and internal axial length (IAL) were measured at baseline, 1 week, 3 months, and 6 months.
Results
In group A, the ChT significantly increased after a 1-week loading dose of 1% atropine (26 ± 14 µm; P < 0.001) and the magnitude of increase stabilized throughout the following weekly treatment. The internal axial length did not significantly change at the 6-month visit (−0.01 ± 0.11 mm; P = 0.74). In contrast, a decreased ChT (−5 ± 17 µm; P < 0.001) and pronounced eye elongation (0.19 ± 0.12 mm; P < 0.001) were observed in group B after 6 months. Multivariable regression analysis showed that less increase in ChT at the 1-week visit (P = 0.03), younger age (P < 0.001), and presence of peripapillary atrophy (P = 0.001) were significantly associated with greater internal axial length increase over 6 months in group A.
Conclusions
One percent atropine could increase the ChT, whereas 0.01% atropine caused a decrease in ChT after 6 months of treatment. For participants receiving 1% atropine, the short-term increase in ChT was negatively associated with long-term eye elongation. Younger age and the presence of peripapillary atrophy were found to be risk factors for greater eye elongation.
Keywords: atropine, choroidal thickness, peripapillary atrophy, myopia control
Over the past few decades, the prevalence of myopia has substantially increased, thus becoming a public health concern.1,2 High myopia can cause irreversible damage to visual acuity (VA) as myopic maculopathy develops and progresses.3,4 Therefore, it is essential to find an effective and safe therapy that may prevent the progression of myopia, especially among children and adolescents. At present, 1% atropine has been demonstrated to have the strongest clinical effect on slowing myopia progression in children.5–8 Yet, severe and long-lasting side effects as well as rebound after drop cessation have limited its clinical application.9,10 This, in turn, has led to a surge in the popularity of using low-concentration atropine owing to fewer side effects and less rebound following atropine cessation.11–14
Decreasing the frequency of 1% atropine to once a week was associated with fewer side effects (photophobia, 0%–29%; near blurred vision, 0.6%)15–17 compared with daily drug use (52.4% and 5.5%, respectively)10 in the treatment of myopia or amblyopia. However, the safety of this treatment regimen (e.g., distance and near VA, pupil size, and accommodation amplitude) has not yet been evaluated in detail.
The choroid has a role in modulating eye growth by regulating the oxygen levels of the sclera18 and by secreting or transmitting retina-derived signals to sclera.19–22 Previous studies have reported a negative association between the changes in choroidal thickness (ChT) and axial length (AL) in animals wearing defocus lens23,24 and in longitudinal studies of myopic children.25 An increase in ChT was observed among myopic children treated with orthokeratology,26,27 and the short-term increase in ChT was negatively associated with long-term eye elongation.27 Choroidal thickening was also observed in healthy children treated with 1% atropine for 1 week28 and in myopic children administered with 0.01% atropine for less than 8 weeks.29–31 However, the long-term effects of atropine on ChT in myopic children and its association with eye elongation remain unknown.
This study was conducted to explore the changes in ChT in Chinese myopic children aged 6 to 12 years treated with 1% atropine once per week or with 0.01% atropine once per day, as well as to investigate the possible association of eye elongation with the changes in ChT and other factors during the treatment period. Additional objectives included a safety evaluation of the treatment regime.
Methods
Study Participants
The present study is a part of the Atropine for Children and Adolescent Myopia Progression study, which is a randomized clinical trial evaluating the efficacy and safety of atropine treatment for myopia control. The study design has been previously described.30 In brief, 207 children aged 6 to 12 years with myopic refraction of at least −0.5 diopter (D) and astigmatism of less than −2.0 D in both eyes were enrolled in this randomized clinical trial. After excluding those with ocular diseases or severe systemic diseases, previous use of myopia interventions (such as atropine, pirenzepine, or orthokeratology lens), or allergy to atropine and cyclopentolate, children were randomly assigned to one of two groups in a ratio of 1:1: group A received 1% atropine (Dishan, Shenyang Xingqi Pharmaceutical Co., LTD., Shenyang, China), and group B received 0.01% atropine (Myopine, Shenyang Xingqi Eye Hospital Co., LTD., Shenyang, China), in both eyes. Participants in group A received 1% atropine once per day during the first week (to explore the effect of atropine cycloplegia), and then once per week for the next 23 weeks. Participants in group B received 0.01% atropine once per day over 6 months. Written informed consent forms were obtained from the participants and their parents or guardians. The study protocol was approved by the Ethics Committee of Shanghai General People's Hospital, Shanghai, China (Approval number: 201939), and registered at the Clinical Trials.gov PRS (Registration No. NCT03949101). All procedures were conducted in accordance with the tenets of the Declaration of Helsinki.
Study Procedures
To decrease the influence of diurnal variation of choroid,32 the measurement was conducted from 10:00 am to 3:00 pm each day, and follow-ups were prearranged within 2 hours based on the time of the baseline visit. Examination procedures were performed as previously described.30 Briefly, each participant underwent a series of ophthalmic examinations, including measurement of intraocular pressure using a noncontact tonometry (NT-1000; Nidek, Tokyo, Japan), pupil size using an IOL-Master 700 (Carl Zeiss Meditec AG, Jena, Germany), best-corrected distance VA in the logMAR, near VA under best-corrected distance spectacle correction at 40 cm, and near point of accommodation with best-corrected distance spectacle correction. The accommodation amplitude was calculated as the inverse of the near point of accommodation. After excluding the contradictions of cycloplegia, one drop of topical 0.5% proparacaine (Alcaine; Alcon, Fort Worth, TX) was administered in both eyes, followed by two drops of 1% cyclopentolate (Cyclogyl; Alcon) at a 5-minute interval. A third drop of cyclopentolate was given 45 minutes later if the pupillary light reflex was still present or the pupil size was less than 6.0 mm. Further drops of cyclopentolate were administered if necessary. Cycloplegia was not performed at the 1-week visit. Autorefraction was performed using an autorefractor (Topcon KR 800; Optical Corp., Guangdong, China). The AL was measured using an IOL-Master 700 (Carl Zeiss Meditec AG).
The ChT was measured using swept-source optical coherence tomography (Topcon Corp., Tokyo, Japan). The parameters were previously described.30 The segmentation of each layer was automatically obtained using the built-in software, and manual segmentation was performed where the software misjudged the borderline of each layer. To determine the reproducibility of manual correction, 20 images that needed manual segmentation were randomly selected and corrected twice by a specific technician. A Bland–Altman plot showed high reproducibility of the ChT segmentation (Supplementary Fig. S1). The mean difference between two corrections was −0.1 µm, and the 95% limits of agreement ranged from −5 to 5 µm. The intraobserver correlation coefficient was 0.998 (P < 0.001). The ChT was measured as the vertical distance between the Bruch membrane and the choroid–sclera interface (Fig. 1A). The Early Treatment Diabetic Retinopathy Study grid was adopted to calculate the averaged ChT in each grid sector using the built-in software. The diameters of the subfoveal, parafoveal, and perifoveal circles were 1 mm, 3 mm, and 6 mm, respectively, and they were further divided into superior, inferior, temporal, and nasal quadrants (Fig. 1B). The retinal photographs by swept-source optical coherence tomography were adopted to identify the presence of peripapillary atrophy (PPA; an inner crescent of chorioretinal atrophy with good visibility of the large choroidal vessels and the sclera; Fig. 1C) by two independent, well-trained examiners (LYY and YS). In cases of disagreement, adjudication was made by a senior ophthalmologist (JFZ).
Figure 1.
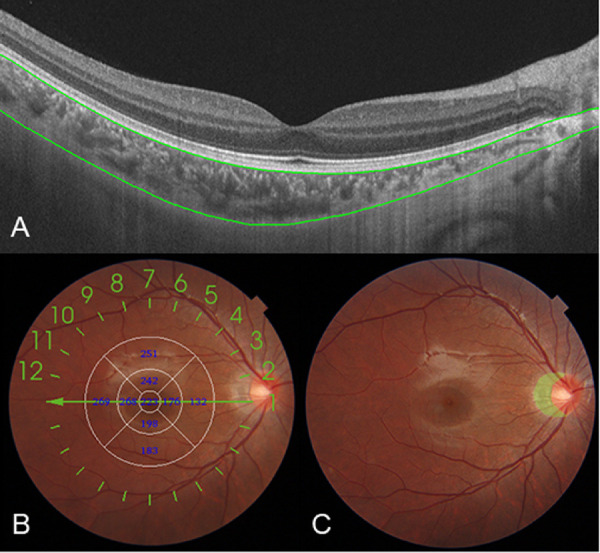
A map showing the ChT and PPA in a healthy 9-year-old girl (right eye) obtained by swept-source optical coherence tomography. ChT, measured as the distance between the Bruch membrane (upper green line) and the choroid-sclera interface (lower green line) (A). The Early Treatment Diabetic Retinopathy Study grid was applied to the map, and the mean ChT was obtained for each sector (B). A retinal photograph centered on the optic disc in the same girl with the area of the PPA manually outlined (green shade area; C).
Parents or guardians were in charge of drug administration and were required to keep medication diaries. The compliance level was classified according to the mean number of atropine use per month or week over 6 months. Participants with a medication adherence percentage of 80% or greater (i.e., 3.2 d/mo for group A or 5.6 d/wk for group B) were considered to have good compliance33,34 and were included in the analysis. Photochromatic glasses and presbyopic glasses (reading add) were provided if children experienced glare or had difficulty with near vision. Children and parents were free to report any other side effects, illness, and hospitalization during treatment. Any adverse events, regardless of whether they were related to the use of atropine, were documented.
Outcomes
The primary outcome was the change in the average ChT over 6 months. The secondary outcomes included the changes in AL, internal AL (IAL), spherical equivalent, accommodation amplitude, pupil size, distance VA, and near VA over 6 months. IAL was defined as the distance from the anterior cornea to the anterior sclera and it was calculated by adding the subfoveal ChT as determined by swept-source optical coherence tomography to the AL measured using the IOL-Master.26 The spherical equivalent was calculated as the spherical power plus one-half of the cylindrical power.
Statistical Analysis
According to the protocol of this clinical trial, participants in group A received 1% atropine once per week for 6 months, followed by 0.01% atropine once per day for the next 6 months. The estimated myopia progression rate for group A was assumed to be −0.37 D/y, which was averaged from the myopia progression rate for daily 0.1% atropine in the Atropine for the Treatment of Myopia 2 (ATOM2) study11 (which was probably close to the effect of 1% atropine of weekly use, −0.31 ± 0.50 D/y) and 75% of the myopia progression rate for daily 0.01% atropine in the Low-concentration Atropine for Myopia Progression (LAMP) study (−0.59 ± 0.61 D/y).13 Participants in group B were administered with 0.01% atropine daily throughout 12 months, and the estimated myopia progression rate was set to −0.59 D/y based on the result of daily 0.01% atropine in the LAMP study.13 The within-group standard deviation was assumed to be 0.6 D.13 To detect a mean difference of at least 0.5 D between treatment groups,13 158 participants (79 per group) could achieve 90% power at a significance level of 0.05. By factoring in an attrition rate of 20%, this study required 198 subjects (99 per group).
The baseline characteristics were described as mean ± standard deviation or proportion. Correlation analysis between two ocular parameters was analyzed using Pearson correlation coefficients. A χ2 test was used to test the group differences of categorical data. The intergroup differences of continuous data were tested using the Student t-test or ANOVA with post hoc tests (Bonferroni). The differences in ocular parameters between the baseline and the follow-ups and the differences in ChT changes between two macular sectors were analyzed using the paired t-test. A generalized estimating equation model with robust standard errors for longitudinal data analysis35 was used to compare the different changes in ocular parameters over time with adjustment for age and sex, accounting for repeated measurements. P values were adjusted for multiple comparisons with sequential Bonferroni adjustment. Multiple linear regression models were established using the IAL change over 6 months as the independent variable to assess the possible association with other ocular parameters. Statistical analysis was performed using SPSS v. 22 software (IBM, Armonk, NY, USA). A P value of less than 0.05 was considered statistically significant. Because the spherical equivalent (r = 0.89; P < 0.001), IAL (r = 0.97; P < 0.001), and ChT (r = 0.91; P < 0.001) of the right and left eyes at baseline were highly correlated, only the right eyes were involved in the analysis.
Results
General Characteristics
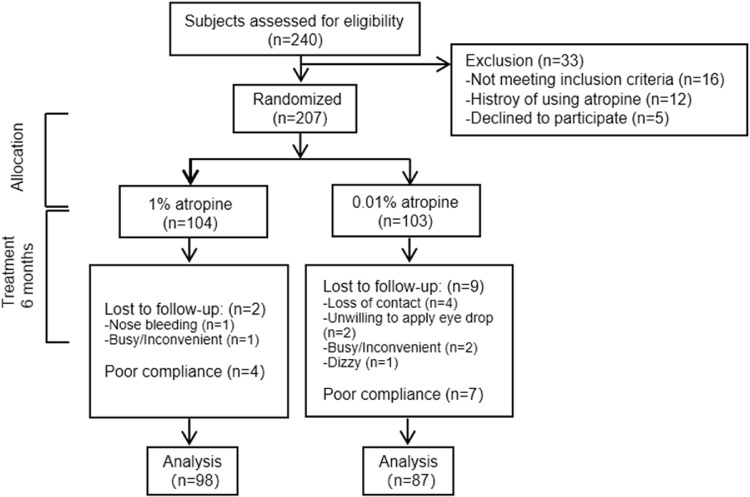
A total of 240 children were assessed for eligibility. Ultimately, 207 children were recruited into the study, 104 in group A and 103 in group B (Fig. 2). The baseline characteristics of the participants are described in Table 1. There was no significant difference between the two groups (P = 0.06 to 0.92). There were 102 (98.1%) participants in group A and 94 (91.2%) in group B who completed the 6 months of treatment; 98 participants (94.2%) in group A and 87 participants (84.5%) in group B had good compliance. The baseline characteristics of the 185 children included in the analysis were similar to those of the 22 children who were excluded (P = 0.07 to 0.91), except for a thinner average ChT (P = 0.03) and a higher proportion of PPA (P = 0.01).
Figure 2.
A flowchart showing participants enrolled in the Atropine for Children and Adolescent Myopia Progression study.
Table 1.
Baseline Characteristics for All Participants and for Those Included and Excluded From the Analysis
| Total | Included | Excluded | ||||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | |||
| Variables | (n = 104) | (n = 103) | P Value* | (n = 98) | (n = 87) | (n = 6) | (n = 16) | P Value† |
| Age, y | 8.98 (1.57) | 8.88 (1.71) | 0.67 | 8.94 (1.55) | 8.84 (1.65) | 9.50 (1.64) | 9.00 (1.83) | 0.50 |
| Male gender, n (%) | 49 (47.1) | 49 (47.6) | 0.84 | 49 (50.0) | 44 (50.6) | 0 (0.0) | 10 (62.5) | 0.07 |
| Body mass index, kg/m2 | 17.03 (2.71) | 16.75 (2.63) | 0.53 | 17.09 (2.77) | 16.87 (2.75) | 16.15 (1.21) | 16.12 (1.73) | 0.14 |
| Average ChT, µm | 214 (45) | 222 (41) | 0.17 | 214 (45) | 218 (39) | 214 (49) | 245 (47) | 0.03 |
| AL, mm | 24.32 (0.81) | 24.25 (0.72) | 0.45 | 24.34 (0.82) | 24.27 (0.74) | 23.98 (0.55) | 24.14 (0.61) | 0.22 |
| IAL, mm | 24.54 (0.79) | 24.48 (0.71) | 0.55 | 24.57 (0.81) | 24.50 (0.73) | 24.20 (0.52) | 24.40 (0.60) | 0.27 |
| Spherical equivalent, D | −2.11 (1.10) | −2.13 (1.10) | 0.92 | −2.12 (1.09) | −2.16 (1.10) | −1.85 (0.69) | −1.96 (1.07) | 0.39 |
| Presence of PPA, n (%) | 65 (62.5) | 73 (70.9) | 0.20 | 62 (63.3) | 67 (77.0) | 3 (50.0) | 6 (37.5) | 0.01 |
| Intraocular pressure, mm Hg | 14.92 (2.60) | 14.26 (2.25 | 0.06 | 14.90 (2.66) | 14.21 (2.23) | 15.33 (1.37) | 14.38 (2.45) | 0.91 |
| Distance VA, logMAR | −0.02 (0.04) | −0.03 (0.05) | 0.10 | −0.02 (0.04) | −0.03 (0.05) | −0.03 (0.04) | −0.01 (0.03) | 0.60 |
| Near VA, logMAR | 0.01 (0.04) | 0.02 (0.04) | 0.13 | 0.01 (0.04) | 0.02 (0.04) | 0.01 (0.02) | 0.01 (0.02) | 0.36 |
| Pupil size, mm | 5.08 (1.12) | 5.21 (0.85) | 0.68 | 5.05 (1.10) | 5.20 (0.82) | 5.50 (0.99) | 5.47 (1.13) | 0.11 |
| Accommodation amplitude, D | 12.52 (3.21) | 12.42 (3.36) | 0.75 | 12.43 (3.05) | 12.59 (3.49) | 14.00 (4.94) | 11.51 (2.41) | 0.86 |
| Parental myopia, n (%) | 92 (88.5) | 91 (88.3) | 0.58 | 87 (88.8) | 77 (88.5) | 5 (83.3) | 11 (84.5) | 0.84 |
Data are presented as mean (standard deviation) unless otherwise indicated.
Comparison between the two groups enrolled in the study using the Student t-test for continuous data or the χ2 test for categorical data.
Comparison between participants included the analysis and those excluded using the Student t-test for continuous data or the χ2 test for categorical data.
Changes in Ocular Parameters
The ocular parameters in each visit are summarized in Supplementary Table S1, and the changes in ocular parameters over time are shown in Table 2. In group A, there was an overall increase in ChT (26 ± 14 µm; P < 0.001) after a 1-week loading dose of 1% atropine, and the magnitude of choroidal thickening remained unchanged at the 3-month visit (29 ± 22 µm; P = 0.47) and the 6-month visit (27 ± 23 µm; P = 1.00). The ChT in the perifoveal nasal quadrant exhibited the least increase among the horizontal sectors (all P < 0.05), whereas the vertical changes in ChT did not significantly differ (Figs. 3A–C). There was a decrease in the AL over time (−0.04 ± 0.03 mm, −0.06 ± 0.09 mm, and −0.03 ± 0.12 mm for the 1-week, 3-month, and 6-month visits, respectively; all P < 0.001). The IAL did not significantly change at the 1-week and 6-month visits but presented a significant reduction at the 3-month visit (−0.04 ± 0.08 mm; P < 0.001). There was a significant hyperopic shift of 0.38 ± 0.21 D during the first week (P < 0.001), and the magnitude of change reduced to 0.26 ± 0.33 D and 0.28 ± 0.37 D at the end of 3 and 6 months, respectively (both P < 0.001).
Table 2.
Changes in Ocular Biometry Over 6 Months in Different Treatment Groups
| Group A (n = 98) | Group B (n = 87) | ||||
|---|---|---|---|---|---|
| Variables | Mean (SD) | Range | Mean (SD) | Range | P Value† |
| Average ChT, µm | |||||
| Baseline to 1 week | 26 (14)* | −15 to 82 | 8 (9)* | −13 to 31 | <0.001 |
| Baseline to 3 months | 29 (22)* | −19 to 106 | 6 (12)* | −22 to 38 | <0.001 |
| Baseline to 6 months | 27 (23)* | −12 to 93 | −5 (17)* | −68 to 58 | <0.001 |
| P value‡ | 0.47, >0.99, >0.99 | >0.99, <0.001, <0.001 | |||
| AL, mm | |||||
| Baseline to 1 week | −0.04 (0.03)* | −0.12 to 0.01 | −0.01 (0.02)* | −0.08 to 0.04 | <0.001 |
| Baseline to 3 months | −0.06 (0.09)* | −0.30 to 0.19 | 0.08 (0.07)* | −0.09 to 0.20 | <0.001 |
| Baseline to 6 months | −0.03 (0.12)* | −0.37 to 0.30 | 0.19 (0.12)* | −0.10 to 0.49 | <0.001 |
| P value‡ | 0.03, <0.001, 0.71 | <0.001, <0.001, <0.001 | |||
| IAL, mm | |||||
| Baseline to 1 week | −0.01 (0.02) | −0.07 to 0.04 | 0.00 (0.02) | -0.05 to 0.05 | 0.001 |
| Baseline to 3 months | −0.04 (0.08)* | −0.24 to 0.24 | 0.08 (0.07)* | −0.11 to 0.21 | <0.001 |
| Baseline to 6 months | −0.01 (0.11) | −0.26 to 0.34 | 0.19 (0.12)* | −0.10 to 0.48 | <0.001 |
| P value‡ | <0.001, <0.001, 0.74 | <0.001, <0.001, <0.001 | |||
| Spherical equivalent, D | |||||
| Baseline to 1 week | 0.38 (0.21)* | −0.13 to 0.88 | −0.09 (0.20)* | −0.50 to 0.50 | <0.001 |
| Baseline to 3 months | 0.26 (0.33)* | −0.50 to 1.13 | −0.08 (0.25)* | −0.63 to 0.88 | <0.001 |
| Baseline to 6 months | 0.28 (0.37)* | −0.50 to 1.25 | −0.27 (0.34)* | −1.00 to 0.63 | <0.001 |
| P value‡ | <0.001, 0.66, 0.03 | >0.99, <0.001, <0.001 | |||
| Distance VA, logMAR | |||||
| Baseline to 1 week | 0.00 (0.05) | −0.18 to 0.18 | 0.00 (0.05) | −0.10 to 0.10 | 0.94 |
| Baseline to 3 months | −0.00 (0.05) | −0.10 to 0.10 | 0.00 (0.05) | −0.10 to 0.10 | 0.83 |
| Baseline to 6 months | −0.01 (0.05) | −0.12 to 0.12 | −0.01 (0.07) | −0.18 to 0.18 | 0.52 |
| P value‡ | 0.28, 0.11, 0.27 | 0.13, 0.46, 0.32 | |||
| Near VA, logMAR | |||||
| Baseline to 1 week | 0.68 (0.23)* | 0.00 to 1.00 | 0.01 (0.08) | −0.16 to 0.40 | <0.001 |
| Baseline to 3 months | 0.04 (0.13)* | −0.18 to 0.70 | −0.01 (0.04) | −0.18 to 0.11 | <0.001 |
| Baseline to 6 months | 0.02 (0.12) | −0.15 to 1.00 | −0.02 (0.05) | −0.22 to 0.10 | 0.11 |
| P value‡ | <0.001, 0.002, <0.001 | 0.09, 0.23, 0.11 | |||
| Pupil size, mm | |||||
| Baseline to 1 week | 2.48 (1.20)* | 0.12 to 4.90 | 0.83 (0.95)* | −1.60 to 3.90 | <0.001 |
| Baseline to 3 months | 1.87 (1.75)* | −0.04 to 4.40 | 0.85 (0.70)* | −1.30 to 3.80 | <0.001 |
| Baseline to 6 months | 1.59 (1.24)* | −0.10 to 4.00 | 0.20 (1.20) | −1.40 to 2.80 | <0.001 |
| P value‡ | <0.001, <0.001, <0.001 | 0.22, <0.001, <0.001 | |||
| Accommodation amplitude, D | |||||
| Baseline to 1 week | −11.55 (3.73)* | −19.50 to −4.75 | −2.88 (3.42)* | −12.10 to 5.00 | <0.001 |
| Baseline to 3 months | −4.25 (2.98)* | −15.50 to 3.50 | −1.81 (2.97)* | −11.00 to 4.00 | <0.001 |
| Baseline to 6 months | −3.01 (3.85)* | −14.40 to 4.30 | −1.11 (3.71)* | −13.00 to 4.20 | 0.02 |
| P value‡ | <0.001, <0.001, <0.001 | <0.001, <0.001, <0.001 | |||
The data are the changes from baseline visits for each group.
P < 0.05 for comparisons between the baseline and the follow-ups using the paired t-test.
Comparisons between the two groups using the Student t-test.
A generalized estimating equation model with robust standard errors was used to compare the different changes in the ocular parameters over time with adjustment for age and sex, accounting for repeated measurements. Multiple comparisons with sequential Bonferroni adjustment were performed between baseline to 1 week and baseline to 3 months, baseline to 3 months and baseline to 6 months, and baseline to 1 week and baseline to 6 months, respectively.
Figure 3.
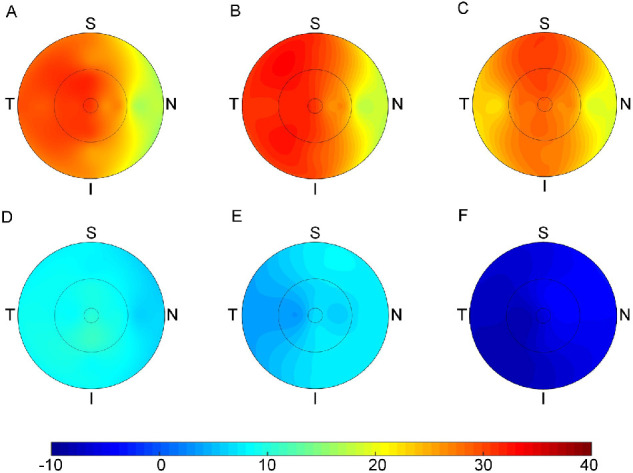
Topographic variation of the changes in ChT at the 1-week, 3-month, and 6-month visits for group A (A, B, and C, respectively) and group B (D, E, and F, respectively).
Group B presented significantly different ocular biometric changes compared with group A (all P < 0.001). ChT was found to be slightly increased at the 1-week and 3-month visits (8 ± 9 µm and 6 ± 12 µm, respectively; both P < 0.001), followed by a decrease at the 6-month visit (−5 ± 17 µm; P < 0.001). There was no significant difference in ChT change among the sectors, horizontally and vertically (Figs. 3D–F). Axial elongation and refraction progression were observed over time, with more significant changes during the second 3 months compared with the first 3 months (all P < 0.001).
Changes in Ocular Parameters With Baseline Characteristics
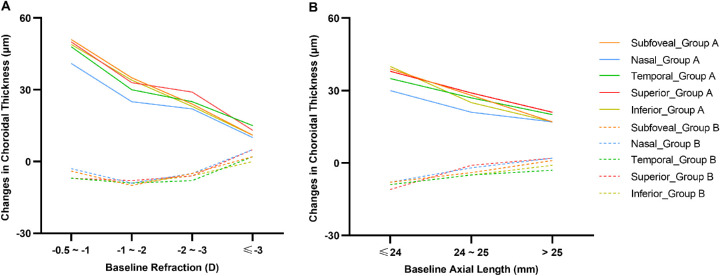
The relationship between the changes in ocular parameters over 6 months and baseline characteristics are presented in Table 3 and Figures 4 and 56. In group A, participants with higher myopic refraction at baseline, longer AL at baseline, or the presence of PPA exhibited less increase in ChT in all nine macular sectors than those with lower myopic refraction, shorter AL, or normal fundus, respectively (all P < 0.05). Significantly greater eye elongation was observed in participants with higher myopic refraction or the presence of PPA (all P < 0.05). There was an increasing trend of eye elongation in participants with a longer baseline AL, although the differences among AL categories were statistically insignificant. In group B, however, these changes in ocular parameters did not significantly differ across groups (P = 0.11 to 0.47).
Table 3.
Changes in Ocular Parameters Stratified by Baseline Characteristics
| Group A (n = 98) | Group B (n = 87) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Average ChT, µm | AL, mm | IAL, mm | N | Average ChT, µm | AL, mm | IAL, mm | |
| Baseline refraction | ||||||||
| −0.5 to <−1 D | 12 | 45 (33) | −0.09 (0.15) | −0.03 (0.13) | 10 | −6 (32) | 0.18 (0.20) | 0.17 (0.17) |
| −1 to <−2 D | 37 | 29 (21) | −0.06 (0.08) | −0.01 (0.07) | 31 | −9 (13) | 0.21 (0.12) | 0.21 (0.12) |
| −2 to <−3 D | 31 | 25 (18) | −0.03 (0.14) | 0.00 (0.12) | 27 | −7 (15) | 0.20 (0.11) | 0.19 (0.12) |
| ≤ −3 D | 18 | 13 (14) | 0.05 (0.14) | 0.06 (0.13) | 19 | 3 (15) | 0.14 (0.07) | 0.15 (0.07) |
| P value | 0.001 | 0.02 | 0.04 | 0.13 | 0.32 | 0.47 | ||
| Baseline AL | ||||||||
| ≤24 mm | 31 | 35 (25) | −0.06 (0.10) | −0.01 (0.09) | 32 | −9 (18) | 0.22 (0.11) | 0.22 (0.10) |
| 24< to 25 mm | 45 | 25 (23) | −0.03 (0.13) | 0.00 (0.11) | 41 | −3 (18) | 0.16 (0.14) | 0.16 (0.14) |
| >25 mm | 22 | 19 (14) | −0.00 (0.14) | 0.03 (0.13) | 14 | 0 (12) | 0.19 (0.07) | 0.18 (0.06) |
| P value | 0.04 | 0.31 | 0.41 | 0.16 | 0.11 | 0.13 | ||
| Presence of PPA | ||||||||
| Without | 36 | 42 (25) | −0.09 (0.12) | −0.04 (0.11) | 20 | −9 (19) | 0.22 (0.13) | 0.21 (0.15) |
| With | 62 | 18 (15) | 0.00 (0.12) | 0.03 (0.11) | 67 | −4 (17) | 0.18 (0.12) | 0.18 (0.11) |
| P value | <0.001 | <0.001 | 0.001 | 0.25 | 0.33 | 0.25 | ||
The data are the changes from baseline visits for each group, presented as mean (standard deviation).
P value for comparisons among refraction and AL groups using ANOVA, or between participants with and without PPA using the Student t-test.
Figure 4.
The changes in ChT over 6 months under the fovea and at four quadrants stratified by baseline refraction (A) and AL (B) for two treatment groups.
Figure 5.
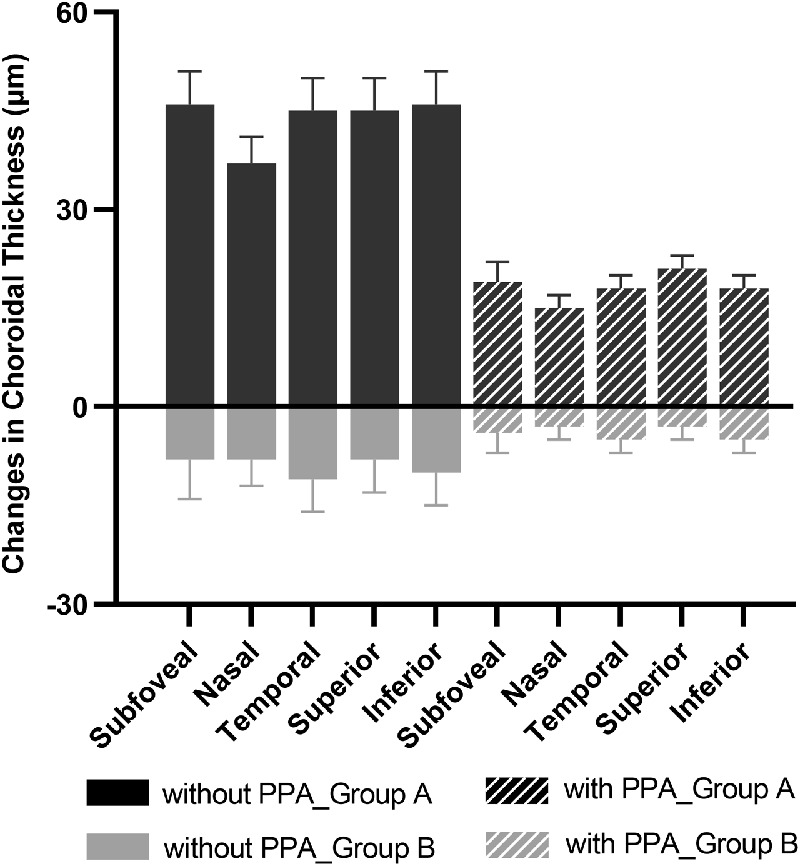
The changes in ChT over 6 months under the fovea and at four quadrants sectors stratified by the presence of PPA for two treatment groups.
Figure 6.
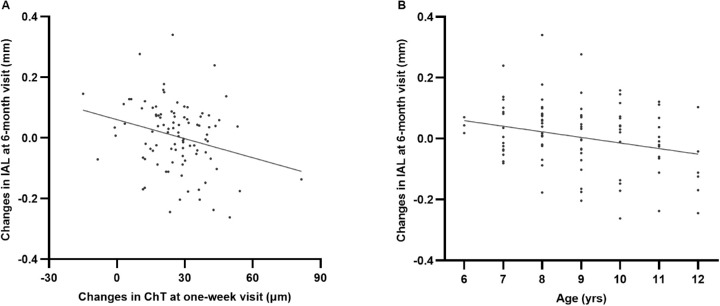
(A) Scatter plots showing the changes in IAL over 6 months of treatment and the changes in ChT at the 1-week visit in group A (y = −0.002*x + 0.06; P = 0.01). (B) Scatter plots showing the changes in IAL over 6 months of treatment and baseline age in group A (y = −0.02*x + 0.17; P = 0.01).
Factors Associated With the Eye Elongation Over 6 Months
A negative association was observed between the changes in IAL and ChT over 6 months in both groups (r = −0.48 and r = −0.27 for groups A and B, respectively; both P < 0.01). In group A, the IAL change at the 6-month visit was negatively associated with the change in ChT at the 1-week visit (r = −0.27, P = 0.01; Fig. 6A) and the baseline age (r = −0.26, P = 0.01; Fig. 6B). Multivariable regression analysis was established to identify independent factors associated with IAL changes over 6 months in each group. Because the presence of PPA was significantly associated with the baseline refraction and the baseline AL (both P < 0.001), only the presence of PPA was added into the model. The results showed that the IAL change over 6 months was negatively associated with age (P < 0.001) and the change in ChT at the 1-week visit (P = 0.03), and positively correlated with the presence of PPA (P = 0.001) adjusting for age and sex in group A (Table 4). In group B, the IAL change at the 6-month visit was negatively correlated with age (r = −0.50, P < 0.001), but was not associated with the change in ChT at the 1-week visit (P = 0.81).
Table 4.
Multiple Regression Analysis of Associated Factors With the Changes in IAL in Group A (n = 98)
| Variables | B (95% CI) | P Value |
|---|---|---|
| Age, y | −0.025 (−0.039 to −0.012) | <0.001 |
| Sex | −0.002 (−0.042 to 0.038) | 0.903 |
| One-week change in ChT, 10 µm | −0.016 (−0.031 to −0.002) | 0.030 |
| PPA, baseline | 0.078 (0.035 to 0.122) | 0.001 |
R 2 = 0.259.
Adverse Events and Changes in Associated Ocular Parameters
In group A, the changes in accommodation amplitude and pupil size at the 6-month visit were −3.01 ± 3.85 D (P < 0.001) and 1.59 ± 1.24 mm (P < 0.001), which were lower than the changes at the 1-week and 3-month visits (both P < 0.001). Fewer changes in accommodation amplitude and pupil size were observed in group B compared with group A (all P < 0.05). The distance VA and near VA did not significantly change over 6 months in both groups.
In group A, 80.6% and 66.3% of participants reported photophobia and near blurred vision with a mean duration of 3.4 days and 4.3 days, respectively. Also, 30.6% and 10.2% of participants needed photochromic glasses and presbyopic glasses, respectively. In group B, 6.9% and 1.1% of participants reported photophobia and near blurred vision, respectively, with no need for photochromic glasses or presbyopic glasses. Among all participants enrolled in the study, allergic conjunctivitis was reported in two participants from group B; dizziness occurred in two participants from group B; rubella was reported in one participant from group B; and nose bleeding occurred in two participants from group A and one participant from group B.
Discussion
In this study, we explored the changes in ChT over 6 months and the independent factors associated with eye elongation. Our results revealed that there was a significant increase in the ChT after a 1-week loading dose of 1% atropine, and the magnitude of increase stabilized throughout the following weekly treatments. The IAL did not significantly change over 6 months. Conversely, 0.01% atropine (administrated daily) led to a decrease in the ChT and pronounced eye elongation after 6 months. Moreover, among participants receiving 1% atropine, the short-term increase in ChT was negatively associated with long-term eye elongation. Younger age and the presence of PPA were risk factors for greater eye elongation. Also, weekly use of 1% atropine was evaluated to render endurable side effects.
The Changes in ChT
Our results revealed a significantly thickened ChT after a 1-week loading dose of 1% atropine, which was consistent with findings from Zhang et al (15 ± 16 µm).28 The magnitude of choroidal thickening was maintained throughout the following weekly treatments. Notably, the impact of weekly use of 1% atropine on ChT cannot be concluded from the present study owing to the influence of the loading dose during the first week. The underlying mechanisms of this choroidal thickening after atropine treatment remain unclear. Based on the previous animal studies,36,37 nitric oxide may participate in the choroidal thickening induced by atropine,38 possibly by influencing the blood flow and the stromal components of the choroid via relaxation of the choroidal vascular and nonvascular smooth muscles. Moreover, dopamine could also be responsible for the choroidal thickening induced by atropine. Intravitreal injection of atropine in chicks’ eyes can increase dopamine release from the retina,39 and D2 agonist has been found to increase the ChT in chicks wearing negative lenses.40 Previous studies have found a thinning of the ChT during accommodation,41 probably via the biomechanical force between ciliary muscle and choroid.42 Thus, the biomechanical effect on the ChT may go into the opposite direction when the ciliary muscle was relaxed by atropine. Further research is needed to demonstrate the underlying mechanisms for choroidal thickening.
A slight increase in ChT was observed in participants receiving 0.01% atropine for 3 months, which was consistent with other studies (5–6 µm).29,31 Yet, an opposite change in ChT was observed at the 6-month visit (−5 ± 17 µm). It could be that the effect of 0.01% atropine on choroid attenuated after the first 3 months of use and ChT decreased as AL elongates25 during the second 3 months. In contrast, participants may experience more significant myopia progression and axial elongation during the second 3 months (autumn and winter) compared with the first 3 months (summer and autumn),43 probably owing to the more time spent on near work and less time spent outdoors. Accordingly, increased choroidal thinning with greater ocular growth25 could be observed during the second 3 months, which may outweigh the slight choroidal thickening originally caused by 0.01% atropine. A longer observation period of at least 1 year is required to indicate the underlying mechanisms.
The Changes in IAL and Its Association With ChT Changes
Because the changes in ChT can influence AL, the IAL was adopted in the present study, revealing the true nature of the ocular growth.26 No significant change in IAL was observed in group A after 6 months of treatment; conversely, participants receiving 0.01% atropine presented pronounced eye elongation. Increasing evidence has revealed the role of the choroid in modulating eye growth.18–22 In this study, the changes in ChT at the 6-month visit were negatively associated with the IAL changes over 6 months in both groups. Similar results were found in myopic children treated with orthokeratology.26,27 These findings suggest that the choroidal thickening may participate in ocular growth retardation, probably by influencing the synthesis activity of molecular signals and the transmission ability of choroid,19–22 or by increasing the ChT and choroidal blood flow44–46 and mitigating scleral hypoxia.18
We found that the ChT change at the 1-week visit was negatively associated with the IAL change over 6 months in group A, which suggests that the short-term increase in ChT after a loading dose of 1% atropine may predict long-term eye elongation with weekly treatment. However, daily use of 1% atropine for 1 week caused severe side effects; thus, a shorter duration of the loading dose, which can indicate long-term eye elongation, should be further explored by future studies.
Factors Associated With the Eye Elongation in Group A
Previous studies have indicated that younger age14,47 and higher myopic refraction at baseline47,48 were risk factors for myopia progression in myopic children treated with atropine. As seen in group A, the ocular growth over 6 months was negatively correlated with the baseline age and myopic refraction. Furthermore, the presence of PPA resulted as another independent factor for eye elongation in participants receiving 1% atropine. The relatively small difference in IAL change between patients with and without PPA could be attributed to the limited course of observation. PPA is an early optic deformation associated with axial elongation-induced optic disc rotation found in myopes.49 The development and enlargement of PPA are risk factors for pathologic myopia.50,51 The true nature of the more pronounced eye elongation in patients with the presence of PPA remains unclear. We speculate that the optic deformation and scleral stretching may influence the function of the retina, retinal pigment epithelium, and choroid, eventually resulting in less choroidal thickening and more eye growth. The insignificant association between IAL change and the baseline AL indicated that the AL, a two-dimensional parameter, could not comprehensively reflect the ocular deformation.
The Safety of Atropine Treatment
Ocular parameters related to side effects were fully evaluated in the current study. The changes in accommodation amplitude and pupil size with weekly use of 1% atropine over 6 months were comparable with those of the 0.1% atropine group in the ATOM2 study (administrated daily, −2.77 ± 1.03 D and 2.42 ± 0.91 mm over a year, respectively)11 and of the 0.05% atropine group in the LAMP study (administrated daily, −2.38 ± 2.70 D and 1.06 ± 1.07 mm over 4 months, respectively).13 Higher incidences of photophobia and near blurred vision were reported in the current study when compared with the previous studies with weekly or monthly use of 1% atropine (photophobia, 0%–62.1%; near blurred vision, 0.6%–19.7%).15–17,52 However, these side effects were endurable considering the short duration and the low need for photochromic or presbyopic glasses. Much lower incidences of photophobia and near blurred vision were found in participants treated with 0.01% atropine daily, which were similar to those in the LAMP study (2.1% and 1.8%, respectively).13
Limitations
The present study has some limitations. First, the present study did not include a placebo control group. The findings from the ATOM study5,11 and the LAMP study13 clearly showed the efficacy of atropine treatment compared with a placebo, rendering a placebo arm unethical. Second, a 6-month follow-up period may not be enough for providing sufficient information on the efficacy of an atropine treatment regime and the suitable course, as well as the rebound after cessation. Third, although all parents or guardians were required to keep medication diaries, it was not possible to evaluate the accuracy with which this was done and thus and may influence the results. Fourth, the pupil size was measured under normal circumstances (estimated to 150 lux); thus, the baseline pupil size was larger than the reported one in previous studies with 300 lux.11,13 Fifth, time for outdoor activities and time spent on near work, which have been identified as risk factors for high myopia,53 were not taken into consideration in the present study.
Conclusions
Our data suggested that 1% atropine could increase ChT, whereas 0.01% atropine caused a decrease in ChT after 6 months of treatment. For participants receiving 1% atropine, the short-term increase in the ChT may predict long-term eye elongation. Myopic children with the presence of PPA should be closely monitored because they have a relatively poor response to 1% atropine and are at a high risk of developing high myopia and pathologic myopia.
Supplementary Material
Acknowledgments
Supported by the National Natural Science Foundation of China (Grant No: 81703287), Shanghai Health Committee, Clinical Research (Project No.2019240241), Shanghai Shenkang Hospital Clinical Research Program (Project No. SHDC12019X18), National Key R&D Program of China (Project No. 2016YFC0904800, 2019YFC0840607), National Science and Technology Major Project of China (Project No. 2017ZX09304010).
Disclosure: L. Ye, None; Y. Shi, None; Y. Yin, None; S. Li, None; J. He, None; J. Zhu, None; X. Xu, None
References
- 1. Dolgin E. The myopia boom. Nature. 2015; 519: 276–278. [DOI] [PubMed] [Google Scholar]
- 2. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 3. Fricke TR, Jong M, Naidoo KS, et al.. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018; 102: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong YL, Sabanayagam C, Ding Y, et al.. Prevalence, risk factors, and impact of myopic macular degeneration on visual impairment and functioning among adults in Singapore. Invest Ophthalmol Vis Sci. 2018; 59: 4603–4613. [DOI] [PubMed] [Google Scholar]
- 5. Chua WH, Balakrishnan V, Chan YH, et al.. Atropine for the treatment of childhood myopia. Ophthalmology. 2006; 113: 2285–2291. [DOI] [PubMed] [Google Scholar]
- 6. Yi S, Huang Y, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. J AAPOS. 2015; 19: 426–429. [DOI] [PubMed] [Google Scholar]
- 7. Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn J Ophthalmol. 2007; 51: 27–33. [DOI] [PubMed] [Google Scholar]
- 8. Huang J, Wen D, Wang Q, et al.. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016; 123: 697–708. [DOI] [PubMed] [Google Scholar]
- 9. Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009; 116: 572–579. [DOI] [PubMed] [Google Scholar]
- 10. Gong Q, Janowski M, Luo M, et al.. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017; 135: 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chia A, Chua WH, Cheung YB, et al.. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012; 119: 347–354. [DOI] [PubMed] [Google Scholar]
- 12. Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014; 157: 451–457.e451. [DOI] [PubMed] [Google Scholar]
- 13. Yam JC, Jiang Y, Tang SM, et al.. Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019; 126: 113–124. [DOI] [PubMed] [Google Scholar]
- 14. Wei S, Li SM, An W, et al.. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020; 138: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foo LL, Htoon H, Farooqui SZ, Chia A. Part-time use of 1% atropine eye drops for prevention of myopia progression in children. Int Ophthalmol. 2020; 40: 1857–1862. [DOI] [PubMed] [Google Scholar]
- 16. Repka MX, Cotter SA, Beck RW, et al.. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004; 111: 2076–2085. [DOI] [PubMed] [Google Scholar]
- 17. Scheiman MM, Hertle RW, Kraker RT, et al.. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Arch Ophthalmol. 2008; 126: 1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu H, Chen W, Zhao F, et al.. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci USA. 2018; 115: E7091–E7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000; 70: 519–527. [DOI] [PubMed] [Google Scholar]
- 21. Rada JA, Huang Y, Rada KG. Identification of choroidal ovotransferrin as a potential ocular growth regulator. Curr Eye Res. 2001; 22: 121–132. [DOI] [PubMed] [Google Scholar]
- 22. Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004; 10: 588–597. [PubMed] [Google Scholar]
- 23. Hung LF, Wallman J, Smith EL 3rd. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000; 41: 1259–1269. [PubMed] [Google Scholar]
- 24. Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010; 51: 6262–6269. [DOI] [PubMed] [Google Scholar]
- 25. Xiong S, He X, Zhang B, et al.. Changes in choroidal thickness varied by age and refraction in children and adolescents: a 1-year longitudinal study. Am J Ophthalmol. 2020; 213: 46–56. [DOI] [PubMed] [Google Scholar]
- 26. Li Z, Cui D, Hu Y, Ao S, Zeng J, Yang X. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye. 2017; 40: 417–423. [DOI] [PubMed] [Google Scholar]
- 27. Li Z, Hu Y, Cui D, Long W, He M, Yang X. Change in subfoveal choroidal thickness secondary to orthokeratology and its cessation: a predictor for the change in axial length. Acta Ophthalmol. 2019; 97: e454–e459. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z, Zhou Y, Xie Z, et al.. The effect of topical atropine on the choroidal thickness of healthy children. Sci Rep. 2016; 6: 34936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao W, Li Z, Hu Y, et al.. Short-term effects of atropine combined with orthokeratology (ACO) on choroidal thickness. Cont Lens Anterior Eye. 2020 Jun 30. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Ye L, Li S, Shi Y, et al.. Comparisons of atropine versus cyclopentolate cycloplegia in myopic children. Clinical and Experimental Optometry. 2020 Aug 25. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31. Li W, Jiang R, Zhu Y, Zhou J, Cui C. Effect of 0.01% atropine eye drops on choroidal thickness in myopic children. J Fr Ophtalmol. 2020 Aug 19. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32. Burfield HJ, Patel NB, Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2018; 59: 5176–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 34. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011; 86: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duenas M, Salazar A, Ojeda B, Arana R, Failde I. Generalized estimating equations (GEE) to handle missing data and time-dependent variables in longitudinal studies: an application to assess the evolution of health related quality of life in coronary patients. Epidemiol Prev. 2016; 40: 116–123. [DOI] [PubMed] [Google Scholar]
- 36. Nickla DL, Wilken E, Lytle G, Yom S, Mertz J. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor L-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp Eye Res. 2006; 83: 456–464. [DOI] [PubMed] [Google Scholar]
- 37. Nickla DL, Wildsoet CF.. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004; 81: 111–118. [DOI] [PubMed] [Google Scholar]
- 38. Carr BJ, Stell WK. Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep. 2016; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci. 2000; 17: 165–176. [DOI] [PubMed] [Google Scholar]
- 40. Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010; 91: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woodman-Pieterse EC, Read SA, Collins MJ, Alonso-Caneiro D. Regional changes in choroidal thickness associated with accommodation. Invest Ophthalmol Vis Sci. 2015; 56: 6414–6422. [DOI] [PubMed] [Google Scholar]
- 42. Croft MA, Nork TM, McDonald JP, Katz A, Lutjen-Drecoll E, Kaufman PL. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci. 2013; 54: 5049–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donovan L, Sankaridurg P, Ho A, et al.. Myopia progression in Chinese children is slower in summer than in winter. Optometry Vision Sci. 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang S, Zhang G, Zhou X, et al.. Changes in choroidal thickness and choroidal blood perfusion in guinea pig myopia. Invest Ophthalmol Vis Sci. 2019; 60: 3074–3083. [DOI] [PubMed] [Google Scholar]
- 45. Li Z, Long W, Hu Y, Zhao W, Zhang W, Yang X. Features of the choroidal structures in myopic children based on image binarization of optical coherence tomography. Invest Ophthalmol Vis Sci. 2020; 61: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta P, Thakku SG, Saw SM, et al.. Characterization of choroidal morphologic and vascular features in young men with high myopia using spectral-domain optical coherence tomography. Am J Ophthalmol. 2017; 177: 27–33. [DOI] [PubMed] [Google Scholar]
- 47. Loh KL, Lu Q, Tan D, Chia A. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol. 2015; 159: 945–949. [DOI] [PubMed] [Google Scholar]
- 48. Wu PC, Yang YH, Fang PC. The long-term results of using low-concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther. 2011; 27: 461–466. [DOI] [PubMed] [Google Scholar]
- 49. Jonas JB, Wang YX, Zhang Q, et al.. Parapapillary gamma zone and axial elongation-associated optic disc rotation: the Beijing Eye Study. Invest Ophthalmol Vis Sci. 2016; 57: 396–402. [DOI] [PubMed] [Google Scholar]
- 50. Fang Y, Yokoi T, Nagaoka N, et al.. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology. 2018; 125: 863–877. [DOI] [PubMed] [Google Scholar]
- 51. Yan YN, Wang YX, Yang Y, et al.. Ten-year progression of myopic maculopathy: the Beijing Eye Study 2001-2011. Ophthalmology. 2018; 125: 1253–1263. [DOI] [PubMed] [Google Scholar]
- 52. Zhu Q, Tang Y, Guo L, et al.. Efficacy and safety of 1% atropine on retardation of moderate myopia progression in Chinese school children. Int J Med Sci. 2020; 17: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parssinen O, Kauppinen M.. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019; 97: 510–518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.