Abstract
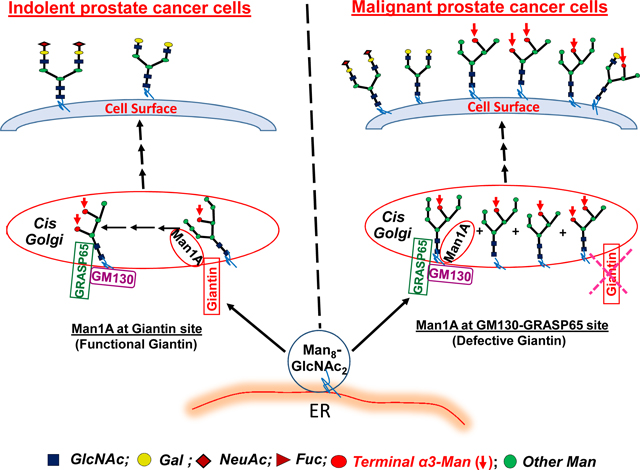
The ability to distinguish malignant from indolent prostate cancer cells is critically important for identification of clinically significant prostate cancer to minimize unnecessary overtreatment and sufferings endured by patients who have indolent cancer. Recently, we discovered that loss of giantin function as the primary Golgi targeting site for endoplasmic reticulum-derived transport vesicles in aggressive prostate cancer cells caused a shift of the Golgi localization site of α-mannosidase 1A to 130 KDa Golgi matrix protein (GM130)-65 KDa Golgi reassembly-stacking protein (GRASP65) site resulting in emergence of high mannose N-glycans on trans-Golgi enzymes and cell surface glycoproteins. To extend this observation, we isolated two cell clones (Clone 1 and Clone 2) from high passage LNCaP cells, which exhibited androgen refractory property missing in low passage LNCaP cells, and characterized their malignant property. We have found that comparing to Clone 2, which does not have cell surface high mannose N-glycans and exhibits localization of α-mannosidase 1A at giantin site, Clone 1 displays cell surface high mannose N-glycans, exhibits localization of α-mannosidase 1A at GM130-GRASP65 site, and shows a faster rate of closing the wound in a wound healing assay. The results indicate that Golgi localization of α-mannosidase 1A at GM130-GRASP65 site and appearance of cell surface high mannose N-glycans may serve as markers of malignant prostate cancer cells.
Keywords: Advanced prostate cancer markers, Golgi α-mannosidase 1A, GM130-GRASP65, high mannose N-glycans, Galanthus nivalis lectin
Graphical abstract
Introduction
Prostate-specific antigen (PSA) level in the blood alone is not useful for diagnosis of clinically significant prostate cancer because it overestimates indolent prostate cancer as aggressive [1,2]. This shortcoming has led to over-use of invasive diagnostic procedures and over-treatment of patients with indolent prostate cancer, causing needless long-term sufferings of these patients [1,2]. Therefore, development of biomarkers that can aid the diagnosis of clinically significant prostate cancer is needed.
Altered glycosylation is known to accompany malignant transformation of cancer [3–5]. Therefore, lots of effort have been devoted to the identification of these altered glycans in cancer with the goal of using them for identification and targeted therapy of advanced cancer [6,7]. However, lack of understanding of the fundamental mechanisms of altered glycosylation in cancer especially advanced cancer has limited the progress. Our recent effort to characterize the mechanism of glycosylation in the Golgi led us to the discovery that loss of function of giantin, the primary Golgi targeting site for endoplasmic reticulum-derived transport vesicles [8–10], in aggressive prostate cancer cells causes these vesicles to use GM130-GRASP65 site for docking (9,10). As a result, some high mannose N-glycans fail to be fully processed at this site and are left untouched through the subsequent glycosylation steps, which leaves trans-Golgi and cell surface glycoproteins decorated with high mannose N-glycans [10]. In this communication, we have extended this observation by showing that after extended culture, some androgen-dependent prostate cancer cells acquired malignant property in wound healing assay in addition to shifted Golgi localization of α-Mannosidase 1A (Man1A) and appearance of high mannose N-glycans on cell surface glycoproteins. Thus, Golgi localization of Man1A at GM130-GRASP65 site and appearance of high mannose N-glycans at the cell surface may serve as biomarkers for identification of advanced prostate cancer.
Materials and Methods
Cell lines
LNCaP cells [11] obtained from ATCC were cultured in RPMI 1640 medium (Sigma) supplemented with 10% FBS and 100 units/mL Penicillin-100 μg/mL Streptomycin. DU145 cells obtained from ATCC were cultured in Dulbecco modified Eagle Medium (Sigma) supplemented with 10% FBS and 100 units/mL Penicillin-100 μg/mL Streptomycin. Both cells were incubated at 37 °C under 5% CO2 and water saturated environment.
In situ Proximity Ligation Assay (PLA) of co-localization of Man1A with either giantin or GM130-GRASP65
PLA assay was performed using the Duolink in situ PLA kit (Sigma) according to the manufacturer’s protocol. Briefly, after fixation in 4% paraformaldehyde/PBS and removal of the fixative, the cells cultured on glass inserts in a 6-well plate were treated with two primary antibodies from different species (mouse and rabbit) against two targets of interest, which include mouse mAb to Man1A (Abcam, Cat#ab140613) and rabbit pAb to Giantin (Abcam, Cat#ab24586), rabbit mAb to GM130 (Abcam, Cat#ab52649) or rabbit mAb to GRASP65 (Abcam, Cat#ab174834). Following primary Ab treatment, the cells were incubated with oligonucleotide-conjugated anti-mouse minus and anti-rabbit plus PLA secondary probes. Ligation oligonucleotides were added to generate red fluorescence dye-tagged DNA. Then, these cells were covered with antifade containing DAPI and then examined by fluorescence microscopy. The red fluorescent spots (signal) captured under a Zeiss 710 fluorescence microscope represent interaction between the target molecules.
Live cell FITC-Galanthus nivalis lectin (GNL)[12] stain of cell surface high mannose N-glycans
Cells cultured overnight on cover slips in a 6-well plate were rinsed twice with PBS followed by incubation with FITC-GNL (E-Y labs)(20 μg/mL in serum-free medium) at 37 °C for 1 h before fixation in 4% paraformaldehyde/PBS at room temperature for 30 min. The fluorescence signal was examined under a fluorescence microscope after covered with antifade containing DAPI.
Wound healing assay
Prostate cancer cells were cultured on a 6-well plate until around 70–80% confluent. Then, a wound was created in the middle with a pipette tip. The width of the opening was measured at 0, 4, 10, and 22 h. Each cell line was analyzed in duplicate and the average rate of migration (nm/h) was obtained.
Results and Discussions
During our routine screening of Golgi localization of Man1A at giantin versus GM130-GRASP65 site in prostate cancer cells, we found that high-passage LNCaP-P117 cells contained two populations of cells based on localization of Man1A at giantin versus GM130/GRASP65 in the Golgi (10). We proceeded to isolate these two cell clones, clone 1 and clone 2, using cloning cylinders and characterize their biochemical and malignant properties under cultured conditions.
Detection of Golgi localization sites of α-mannosidase 1A in LNCaP-P8, LNCaP-P121-C1, LNCaP-P121-C2, and DU145 cells
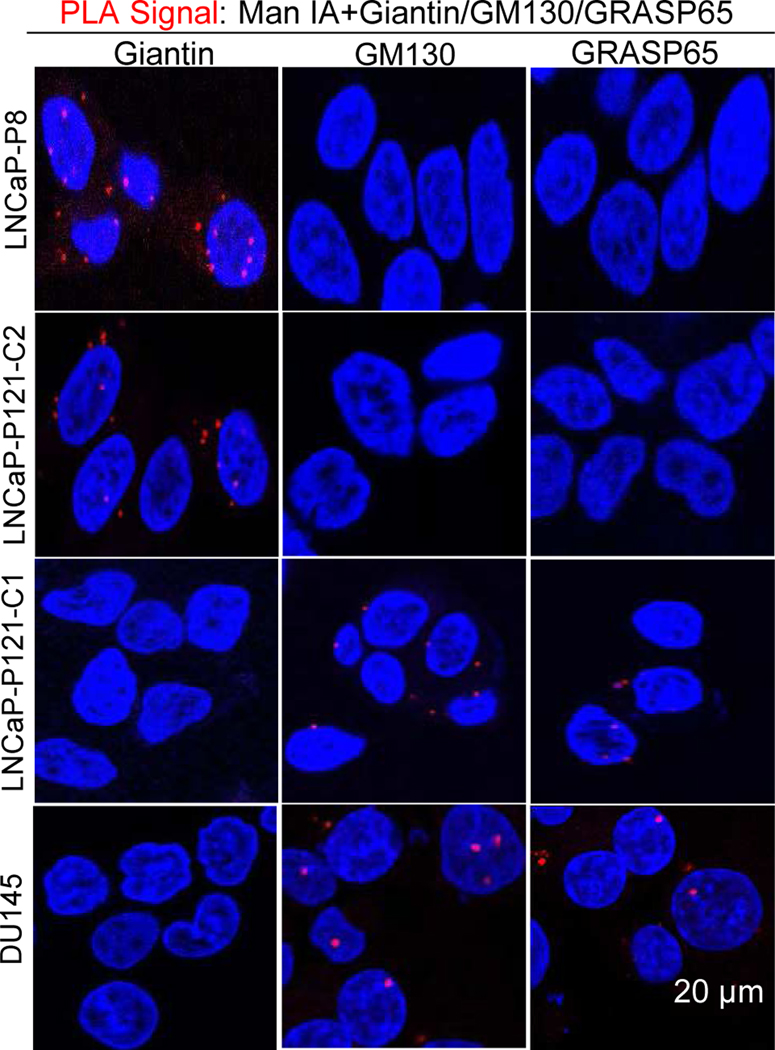
We employed in situ PLA to identify Golgi localization site of Man1A in low passage LNCaP cells, clone 1 and clone 2 of high passage LNCaP cells, and DU145 cells. We localized Man1A at giantin site in LNCaP-P8 and LNCaP-P121-C2 cells, and at GM130-GRASP65 site in LNCaP-P121-C1 and DU145 cells (Figure 1).
Figure 1. In situ Proximity Ligation Assay of co-localization of Man1A with giantin or GM130/ GRASP65.
LNCaP-P8, LNCaP-P121-C2 & C1, and DU145 cells were grown on glass insert, fixed, and analyzed by PLA. The red signals were examined under a fluorescence microscope after exposed to DAPI.
Detection of cell surface high mannose N-glycans in LNCaP-P121-C1 and DU145 cells but not LNCaP-P8 and LNCaP-P121-C2 cells
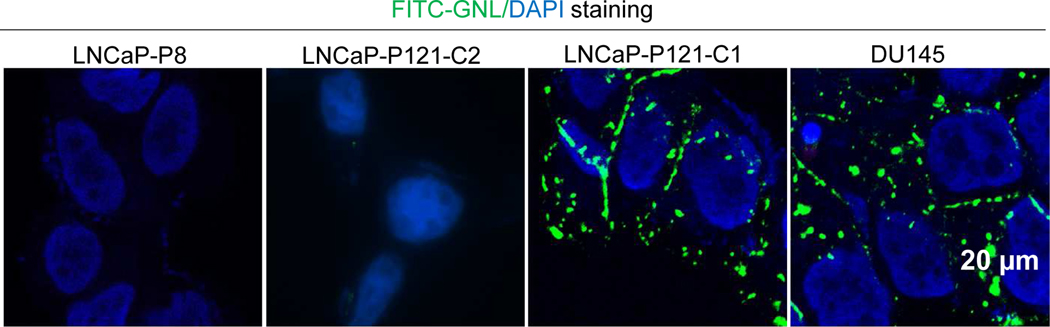
We performed live cell FITC-GNL staining to detect cell surface N-glycans before fixation on LNCaP-P121-C1, LNCaP-P121-C2, DU145, and LNCaP-P8 cells. We found high mannose N-glycans at the cell surface of LNCaP-P121-C1 and DU145 cells but not that of LNCaP-P8 and L NCaP-P121-C2 cells (Figure 2).
Figure 2. Live cell FITC-GNL staining of cell surface high mannose N-glycans.
LNCaP-P8, LNCaP-P121-C2 & C1, and DU145 cells grown on cover glass were treated with FITC-GNL and DAPI and then examined under a fluorescence microscope.
Wound healing properties of LNCaP-P8, LNCaP-P121-C2 & C1, and DU145 cells
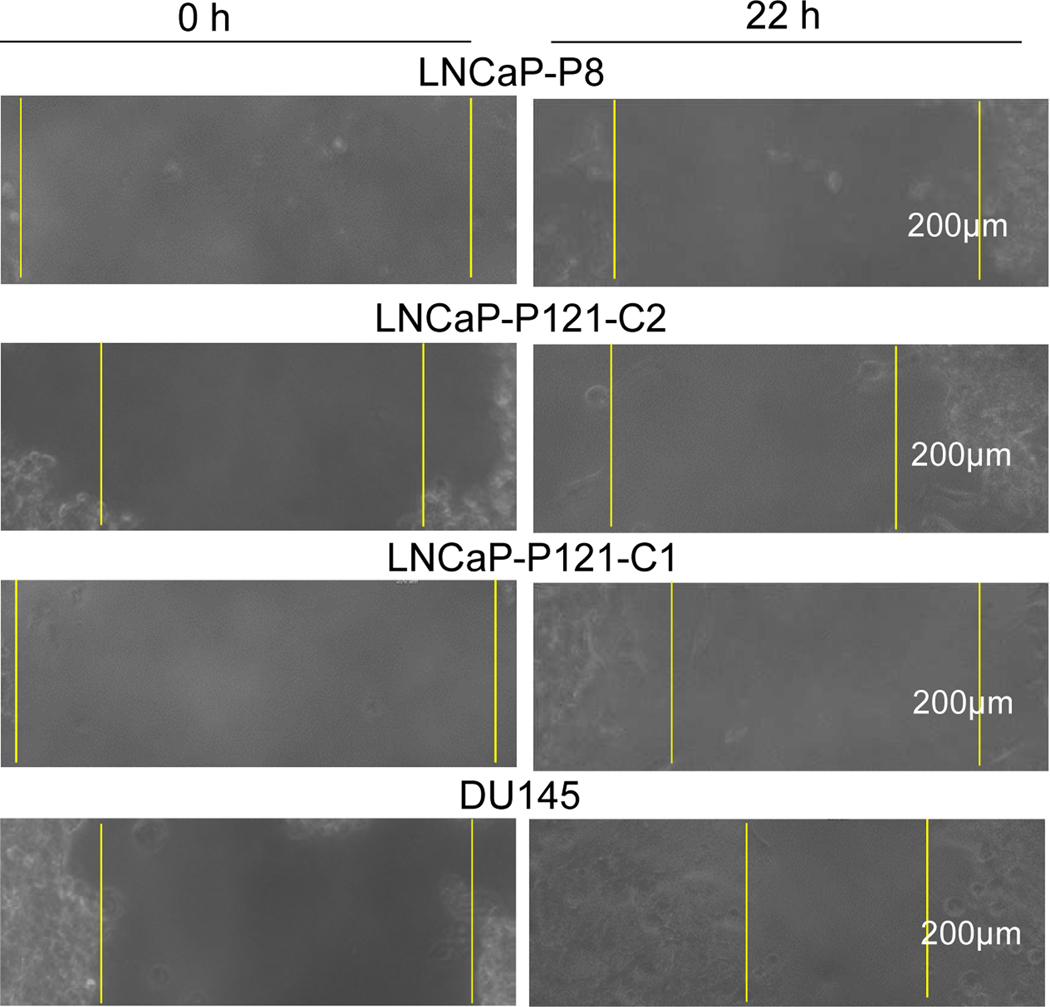
To characterize the in vitro malignant properties of these prostate cancer cells, we performed a scratch wound healing assay. We found that the rates of migration (μm/h) of LNCaP-P121-C1 and DU145 cells to heal the wound were greater than those of LNCaP-P8 and LNCaP-P121-C2 cells (Figure 3). Table 1 summarizes the results of the experiment.
Figure 3. Scratch assay of wound healing.
After LNCaP-P8, LNCaP-P121-C2, LNCaP-P121-C1, and DU145 cells were cultured in a 6-well plate to 70–80% confluence, cells in the center area were removed with a pipette tip and allowed to refill the space. The migration rates, μm/h, of duplicate samples were measured at 0, 4, 10 and 22 h and the images of 0 and 22 h are shown. The migration rates are shown in Table 1.
Table 1.
Summary of Man 1A Golgi localization site, cell surface FITC-GNL stain, and wound healing migration rate of LNCaP-P8 and LNCaP-P121-C2 & C1 and DU145 cells
| Cell Lines | Man 1A Golgi Localization Site | FDIC-GNA Lectin Staining | Scratch Assay Migration Rate (μm/h) | ||
|---|---|---|---|---|---|
| Giantin | GM130 | GRASP65 | |||
| LNCaP-P8 | + | − | − | − | 3.8 |
| LNCaP-P121-C2 | + | − | − | − | 5.0 |
| LNCaP-P121-C1 | − | + | + | + | 10.9 |
| DU145 | − | + | + | + | 9.1 |
Given the limited utility of blood PSA levels for distinguishing benign from advanced prostate cancer, development of alternative biomarkers is needed to minimize the use of invasive diagnosis procedures and treatment that can cause needless long-term sufferings endured by the patients with benign cancer. Current report presents novel glycosylation-based biomarkers as potential candidates for identification of advanced prostate cancer to meet this need.
High mannose N-glycans have been reported in many cancers, including breast [13], pancreas [14], hepatocellular carcinoma [15], colon [16,17], rectum [18], and prostate cancer [19]. But, the mechanism was not known. Recently, we found high mannose N-glycans terminated with α3mannose in trans-Golgi and cell surface of advanced but not benign prostate cancer cells [10]. In this communication [10], we described a mechanism that can explain how high mannose N-glycans are generated in advanced prostate cancer. In this cancer, loss of giantin function as the docking site for endoplasmic reticulum-derived transport vesicles causes these vesicles to use GM130-GRASP65 for docking, resulting in reduced efficiency of Man1A to fully process Man8GlcNAc2 down to Man5GlcNAc2 to enable initiation of the formation of complex-type N-glycans. The Man6–8GlcNAc2 and Man5GlcNAc2 N-glycans that fail to be converted to Man5GlcNAc2 and GlcNAc-Man5GlcNAc2, respectively will remain untouched during their journey through the rest of the Golgi stacks. These high mannose N-glycans can be detected with GNL because they are terminated with α3mannose [12]. Since these high mannose N-glycans are detected on trans-Golgi and cell surface glycoproteins in aggressive cancer cells and not benign cancer and normal cells [10], they become candidate markers of aggressive cancer cells. We wish to point out that formation of high mannose N-glycans in advanced prostate cancer cells does not preclude synthesis of complex-type N-glycans as long as Man5GlcNAc2 are produced and the subsequent enzymes are available because this glycoform is required for initiation of the formation of complex-type N-glycans.
It is known that cancer progression proceeds through several steps, including benign, locally advanced, and metastasis. For prostate cancer, the steps can be classified as androgen-dependent and androgen-refractory, which correspond to benign and aggressive stages, respectively. These tumor progression stages have been reproduced in vitro in a LNCaP culture system [20]. These authors showed that as the passage numbers of LNCaP cells increased, their sensitivity to androgen shifts from androgen-dependent to androgen-refractory. The results of current report show that the high passage LNCaP cells contain two populations of cells, one is benign and one is aggressive. They exhibit different cell biological properties, i.e. Golgi localization of Man1A at giantin vs. GM130-GRASP65; biochemical properties, i.e. absence vs. presence of cell surface high mannose N-glycans; and malignant properties, i.e. slower vs. faster rate of wound healing, respectively. This phenomenon confirms the known concept that advanced tumors contain cancer cells at different stages of progression [21]. Another implication which is very important in the study of cancer cells is that cells derived from freshly isolated single cell clone should be used to carry out the study. Monitoring the localization site of Man1A and/or presence or absence of cell surface high mannose N-glycans can be used to aid characterization and isolation of these two populations of cancer cells.
Current finding may be used for identification of aggressive prostate cancer in three ways. First, it may be used in liquid biopsy to isolate and identify metastatic prostate cancer cells in circulation by employing FITC-GNL assay. Second, it may be used for identification of metastatic tumors and demarcation of locally advanced primary tumor to aid efficient surgical dissection of the tumor. Finally, it may be used for targeted therapy of prostate cancer especially the metastasized tumors. This is particularly important because currently there is no cure for metastatic cancers.
Supplementary Material
High passage LNCaP cells contain indolent and malignant cells
Indolent LNCaP cells exhibit Golgi localization of αmannosidase 1A at giantin
Indolent LNCaP cells do not display high mannose N-glycans at cell surface
Malignant LNCaP cells exhibit Golgi localization of αmannosidase1A at GM130-GRASP65
Malignant LNCaP cells display high mannose N-glycans at cell surface
Acknowledgements
The work is funded by the Peter Michael Foundation (to PWC), the NCI P30 CA036727 grant (to The Fred & Pamela Buffett Cancer Center), the NIGMS U54GM115458 (to the Great Plains IDeA-CTR Network), and UNMC medical student summer research fellowship (to SD). All authors contribute significantly to this manuscript: PWC formulates the idea and writes the manuscript, SD performed cell culture and collected the data on wound healing assay and GB performed live cell staining and proximity ligation assay. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- GM130
130 KDa Golgi matrix protein
- GRASP65
65 KDa Golgi reassembly-stacking protein
- PSA
Prostate-specific antigen
- PLA
Proximity Ligation Assay
- Man1A
α-Mannosidase 1A
- GNL
Galanthus nivalis lectin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hernandez J, Thompson IM, Prostate-specific antigen: A review of the validation of the most commonly used cancer marker, Cancer 1019 (2004) 894–904. 10.1002/cncr.20480 [DOI] [PubMed] [Google Scholar]
- [2].Payne H, Cornford P, Prostate-specific antigen: An evolving role in diagnosis, monitoring, and treatment evaluation in prostate cancer, Urologic Oncology: Seminars and Original Investigations 29 (2011) 593–601. 10.1016/j.urolonc.2009.11.003 [DOI] [PubMed] [Google Scholar]
- [3].Pinho SS, Reis CA, Glycosylation in cancer: Mechanisms and clinical implications, Nat. Rev. Cancer 15 (2015) 540–555. doi: 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- [4].Ruhaak LR, Miyamoto S, Lebrilla CB, Developments in the identification of glycan biomarkers for the detection of cancer, Mol. Cell Proteomics 12 (2013) 46–55. doi: 10.1074/mcp.R112.026799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Drake RR, Jones EE, Powers TW, Nyalwidhe JO. Altered glycosylation in prostate cancer, Adv. in Cancer Res 126 (2015) 345–382. doi: 10.1016/bs.acr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- [6].Krasnova L, Wong C-H, Exploring human glycosylation for better therapies, Mol. Asp. Med 51 (2016) 125–143. doi: 10.1016/j.mam.2016.05.003. [DOI] [PubMed] [Google Scholar]
- [7].Fuster MM, Esko JD, The sweet and sour of cancer: glycans as novel therapeutic targets, Nat. Rev. Cancer 5 (2005) 526–542. DOI: 10.1038/nrc1649 [DOI] [PubMed] [Google Scholar]
- [8].Petrosyan A, Ali M, Cheng P-W, Glycosyltransferase-specific Golgi Targeting Mechanism, J. Biol. Chem 287 (2012) 37621–37627. doi: 10.1074/jbc.C112.403006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Petrosyan A, Holzapfel MS, Muirhead DE, Cheng P-W, Restoration of compact Golgi morphology in advanced prostate cancer enhances susceptibility to galectin 1-induced apoptosis by modifying mucin O-glycan synthesis, Mol. Cancer Res 12 (2014) 1704–1716. doi: 10.1158/1541-7786.MCR-14-0291-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bhat G, Hothpet V-R, Lin M-F, Cheng P-W, Shifted Golgi targeting of glycosyltransferases and α-mannosidase IA from giantin to GM130-GRASP65 results in formation of high mannose N-glycans in aggressive prostate cancer cells, B.B.A. - General Subjects 1861 (2017) 2891–2901. doi: 10.1016/j.bbagen.2017.08.006. [DOI] [PubMed] [Google Scholar]
- [11].Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rowenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma, Cancer Res. 43 (1983)1809–1818. PMID: 6831420 [PubMed] [Google Scholar]
- [12].Shibuya N N, J Goldstein I, Van Damme EJM, Peumans WJ, Binding properties of a mannose-specific lectin from snowdrop (Galanhus nivalis) bulb, J. Biol. Chem 263 (1988) 728–734. PMID:3335522 [PubMed] [Google Scholar]
- [13].de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB, High-mannose glycans are elevated during breast cancer progression, Mol. Cellular Proteomics 10 (2011) 1–9. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Powers TW, Neely BA, Shao Y, Tang H, Troyer DA, Mehta AS, Haab BB, Drake RR, 2014. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLos ONE 9, e106255. doi: 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nie H, Liu X, Zhang Y, Li T, Zhan C, Huo W, Anshun H, Yao Y, Jin Y, Qu Y, Sun X-L X-L, Li Y, Specific N-glycans of hepatocellular carcinoma cell surface and the abnormal increase of core-α−1, 6-fucosylated triantennary glycan via N-acetylglucosaminyltransferases-IVa regulation, Sci. Rep 5 (2015) 16007 10.1038/srep16007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Balog CI, Stavenhagen K, Fung WL, Koeleman CA, McDonnell LA, Verhoeven A, Mesker WE, Tollenaar RA, Deelder AM, Wuhrer M, N-glycosylation of colorectal cancer tissue, Mol. Cellular Proteomics 11 (2012) 571–585. doi: 10.1074/mcp.M111.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang D, Xi Q, Wang Q, Wang Y, Mia J, Li L, Zhang T, Cao X, Li Y, Mass spectrometry analysis reveals aberrant N-glycans in colorectal cancer tissues, Glycobiology 29 (2019) 372–384. doi: 10.1093/glycob/cwz005. [DOI] [PubMed] [Google Scholar]
- [18].Kaprio T, Satomaa T, Heiskanen A, Hokke CH, Deelder AM, Mustonen H, Hagstro J, Carpen O, Saarinen J, Haglund C, N-glycomic profiling as a tool to separate rectal adenomas from carcinoma,. Mol. Cellular Proteomics, 14 (2015) 277–288. doi: 10.1074/mcp.M114.041632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang D, N-glycan cryptic antigens as active immunological targets in prostate cancer patient, J. Proteomics and Bioinformatics 5 (2012) 90–95. doi: 10.4172/jpb.1000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin M-F, Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model, The Prostate 50 (2001) 222–235. DOI: 10.1002/pros.10054 [DOI] [PubMed] [Google Scholar]
- [21].Frame FM, Noble AR, Klein S, Walker H, Suman R, Kasproewicz R, Mann VM, Simms MS, Maitland NJ, Tumor heterogeneity and therapy resistance-implications for future treatments of prostate cancer, J. Cancer Metastasis Treat 3 (2017) 302–314. DOI: 10.20517/2394-4722.2017.34 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.