Abstract
Whereas in ovo exposure of genetically male (ZZ) chicken embryos to exogenous estrogens temporarily feminizes gonads at the time of hatching, the morphologically ovarian ZZ-gonads (FemZZs for feminized ZZ gonads) are masculinized back to testes within 1 year. To identify the feminization-resistant “memory” of genetic male sex, FemZZs showing varying degrees of feminization were subjected to transcriptomic, DNA methylome, and immunofluorescence analyses. Protein-coding genes were classified based on their relative mRNA expression across normal ZZ-testes, genetically female (ZW) ovaries, and FemZZs. We identified a group of 25 genes that were strongly expressed in both ZZ-testes and FemZZs but dramatically suppressed in ZW-ovaries. Interestingly, 84% (21/25) of these feminization-resistant testicular marker genes, including the DMRT1 master masculinizing gene, were located in chromosome Z. Expression of representative marker genes of germline cells (eg, DAZL or DDX4/VASA) was stronger in FemZZs than normal ZZ-testes or ZW-ovaries. We also identified 231 repetitive sequences (RSs) that were strongly expressed in both ZZ-testes and FemZZs, but these RSs were not enriched in chromosome Z. Although 94% (165/176) of RSs exclusively expressed in ZW-ovaries were located in chromosome W, no feminization-inducible RS was detected in FemZZs. DNA methylome analysis distinguished FemZZs from normal ZZ- and ZW-gonads. Immunofluorescence analysis of FemZZ gonads revealed expression of DMRT1 protein in medullary SOX9+ somatic cells and apparent germline cell populations in both medulla and cortex. Taken together, our study provides evidence that both somatic and germline cell populations in morphologically feminized FemZZs maintain significant transcriptomic and epigenetic memories of genetic sex.
Keywords: chicken embryonic gonad, sex conversion, ethynylestradiol, transcriptome, DNA methylome, repetitive sequence expression
Gonadal sex differentiation in vertebrate embryos provides unique opportunities to study how genetic and environmental factors affect organogenesis independently or in coordinated manners. The process in which a common somatic gonadal anlage differentiate into 2 distinct organs—testes and ovaries—initiates at early stages of embryonic development and continues beyond birth. These developmental outcomes may deviate from the genetic sex under natural or artificial influences of the environment (1–8).
Birds have heteromorphic sex chromosomes Z and W, whose heterogametic combination ZW makes a female and homogametic ZZ a male (9–11). Although the genetic sex of birds is determined at fertilization, their gonadal anlagen remains sexually undifferentiated and bipotential until the advanced stages of organogenesis (12). In chicken, the onset of gonadal sex differentiation becomes morphologically evident at day 6 to 6.5 (13). In ZZ-gonads, medullary cords thicken and incorporate primordial germ cells to form seminiferous tubules. In ZW-gonads, the entire right gonad shrinks, and the medullary cords in the left gonads become vacuolated while medullary somatic cells, as well as somatic and germ cells in the cortex, proliferate. As the 2 Z chromosomes in avian ZZ-males are not subjected to global dosage compensation (9, 14–21), recent studies support the notion that stronger expression of the Z-linked DMRT gene in ZZ-gonads plays pivotal roles in masculinizing birds, while significance of the W chromosome in feminization is yet to be demonstrated (9–11, 22). This is in contrast to sex determination in therian mammals, in which the Y-linked SRY gene switches the undifferentiated genital ridge towards testicular development, whereas 1 of the 2 X chromosomes in a female cell is inactivated (23–26).
Phenotypic gonadal sex of avian embryos at birth can be experimentally reversed by in ovo exposure to sex hormones or their inhibitors. For example, in ovo exposure of ZZ-male chicken or quail embryos to estrogenic agents such as 17α-ethynylestradiol or 17β-estradiol causes testis-to-ovary gonadal sex conversion (27–30). Conversely, in ovo exposure of ZW-embryos to fadrozole (an aromatase inhibitor) or tamoxifen (an antiestrogen) induces ovary-to-testis gonadal sex conversion (22, 29–35). The critical importance of estrogens in the sex differentiation of avian gonads has been demonstrated further by male-to-female gonadal sex reversal in ZZ-male chicken embryos overexpressing aromatase after blastodermal injection of a retroviral expression vector (36). A recent study also showed that both ZW and ZZ chicken gonadal cells can form ovarian cortex regardless of the phenotypic sex of the medulla as long as estrogen is provided (29).
Importantly, hormonally reverted sex of chicken gonads or whole body has been shown to be unstable. Whereas in ovo exposure of ZZ-males to estrogens can strongly feminize their gonads at hatching, this effect is transient, and the sex-reversed ZZ birds revert back to the masculine gonadal and body phenotypes within a year after hatching (37). Even when transgenic ZZ-chickens stably overexpressing aromatase maintain a blood estrogen level higher than wild type ZW-females, their feminized gonadal and body phenotypes observed at hatching are largely masculinized at 5 to 6 weeks of age (38). Conversely, studies of ZW-female masculinization by in ovo exposure to fadrozole reported varying outcomes—namely, some ZW birds may have testis-like gonads at hatching but form normal ovarian follicles as they age (39), while other birds can maintain masculine phenotypes in their body and gonads, including spermatogenesis as adults (30, 40). Thus, the reversible aspects of the hormonally induced chicken sex reversal suggest the existence of “memory” of genetic sex in chicken cells.
Studies of gynandromorphic chickens, which are rare bilateral chimeras whose 1 side of the body shows male phenotypes while the other side is female, revealed that the male side body predominantly consists of ZZ cells, whereas the female side contains significant amounts of ZW cells (41, 42). Reported cases of sexually matured gynandromorphic chickens had varying testis- or ovary-like morphologies, reflecting their cellular composition of ZZ- and ZW-cells (41, 42). The male and female sides of gynandromorphic chickens as well as their gonads share a common blood circulation system and, hence, are exposed to the same levels of sex hormones. These observations support the notion that chicken cells “remember” their genetic sex, which is conceptualized as cell autonomous sex identity (CASI) (30, 43).
To obtain insights into genomic basis of the genetic sex memory and CASI, in the current study we examined transcriptomes and DNA methylomes of estrogen-feminized ZZ-chicken gonads (FemZZs for feminized ZZ gonads), with varying degrees of feminization. Our study revealed persistent expression of a group of protein-coding genes enriched in Z-chromosome (including DMRT1) even in strongly feminized FemZZs but not in wild type ZW-ovaries. We also identified over 200 repetitive sequences (RSs) strongly expressed in both ZZ-testes and FemZZs, whereas no feminization-inducible RS was detected. Expression of several germline genes and antiapoptotic genes was stronger in FemZZs than in normal ZZ-testes or ZW-ovaries, suggesting survival of overproliferated germline cells in FemZZs. DNA methylome distinguished FemZZs from normal gonads with feminization-induced hypermethylation at the transcription start sites of meiosis gene. Collectively, our study provides evidence that morphologically feminized FemZZs maintain transcriptomic and epigenetic memories of genetic sex.
Materials and Methods
Feminization of chicken embryos by in ovo exposure to 17α-ethynylestradiol
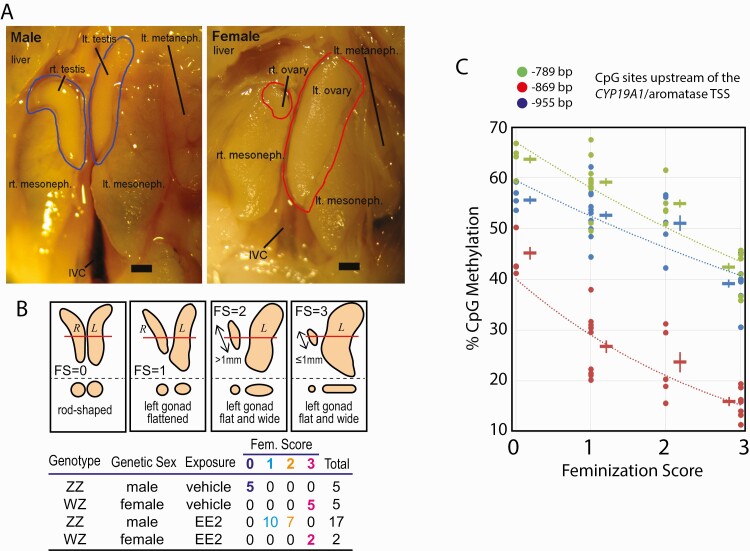
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC). Fertile, research-grade Specific Pathogen-Free eggs of White Leghorn chickens (Gallus gallus domesticus) were purchased from Charles River Laboratories (Wilmington, MA, USA) and incubated at 37.8°C under 70% to 80% humidity, with intermittent rotations in research-grade avian egg incubators. Incubation was initiated on day 1, and eggs were inspected by candling on day 3 to remove unfertile or dead eggs. Greater than 95% of fertile eggs that were alive on day 3 survived until day 19. Gonadal feminization was induced, as we previously described (27). Briefly, 50 µL oil emulsion containing 17α-ethynylestradiol (EE2, 20 µg/egg; >98% purity; Sigma-Aldrich, St. Louis, MO, USA) was injected directly into the yolk on day 6. Briefly, 20 g lecithin (Thermo-Fisher, Waltham, MA, USA) was dissolved in 30 mL dichloromethane (Sigma-Aldrich), and the solubilized lecithin was slowly mixed with 200 mL research-grade peanut oil (Spectrum, New Brunswick, NJ, USA) under heat (40°C –60°C) until homogeneity. After dichloromethane was completely removed by evaporation under nitrogen gas flow, the lecithin/peanut oil mixture was autoclaved and aseptically divided to 10 mL aliquots in sterile 30-mL thick glass bottles. The oil mixture can be stored at -80°C for 12 months. Powder of 17α-ethynylestradiol (EE2; Sigma-Aldrich) was added to the oil mixture (10 mg per aliquot) and dissolved by mild heating and sonication. Sterile water was added to the EE2-containing oil mixture (15 mL water per 10 mL oil aliquot) and sonicated to form homogenous emulsion, which can be stored at -80°C until use for 3 months). For injection, a small window (~5 mm in diameter) was opened on the shell of a fertilized day-6 egg using an electric engraver with fine tips (Dremel model 290-01, Dremel, Racine, WI, USA). The window was opened off the side of the embryo, avoiding major blood vessels or damaging the shell membrane. A drop of light mineral oil was applied to the shell membrane in the window to make it transparent, and 50 uL of prewarmed emulsion (50 µL, containing 20 µg EE2) was injected directly into yolk. The embryo, which is located at the top of yolk, was surrounded by the injected emulsion, which has adequate buoyancy. The window was sealed with a paper sticker, and the egg was returned to the incubator for further development. Gonads were inspected for their degrees of gross morphological characteristics on day 19 and assigned to feminization scores (FSs), as we defined in our previous study (27). Both the right and left sides of normally developed ZZ-male gonads (FS = 0) showed rod-shaped morphology, with no apparent signs of flattening. The left side of normally developed ZW-female gonads (FS = 3) were strongly flattened and widened compared with the FS = 0 gonads, while the right gonads shrank to less than 1 mm in length. Weakly feminized FS = 1 gonads were characterized with significantly flattened left gonads, while right gonads were still maintaining a rod-like shape. More strongly feminized FS = 2 gonads showed strongly flattened and widened left gonads; right FS = 2 gonads were significantly diminished but still greater than 1 mm in length.
Isolation of genomic DNA and total RNA, genetic sex typing, and determination of CpG methylation in the promoter of the CYP19A1 gene
Embryonic liver and gonadal tissues were collected by dissection on day 19. Because of the known asymmetry of chicken embryonic gonads (11), only the left gonads were collected from all embryos for genomic analyses. Adrenal glands or mesonephros adjacent to the gonads were carefully removed under dissecting microscopes. Genomic DNA (gDNA) and total RNA were simultaneously isolated using AllPrep Micro kit (Qiagen, Germantown, MD, USA) and quantified using the Qubit fluorometer (Thermo Fisher). The quality of total RNA was evaluated using Agilent Tapestation with RNA screen tapes and confirmed the absence of significant degradation or gDNA contamination (RINe > 8.0). The genetic sex of embryos was determined by TaqMan quantitative polymerase chain reaction (qPCR) detection of Z and W sex chromosomes in liver gDNA, as we previously described (27, 44). Cytosine methylation at 3 CpG sites in the promoter of the CYP19A1/aromatase gene (789, 869, and 955 bp upstream from the transcription start site [TSS]) was quantitatively determined by bisulfite pyrosequencing using EZ DNA methylation kit (Zymo Research, Irvine, CA, USA) and PyroMark Q96 MD pyrosequencer (Qiagen), as we previously described (27). Complete bisulfite conversion of unmethylated cytosines (>99.8%) was confirmed using a polymerase chain reaction (PCR) amplicon of human mitochondrial DNA fragment spike-in, as we described (45).
RNA-seq library construction, deep sequencing, and data analysis
RNA-seq libraries with barcode indexes were synthesized using the ABI SOLiD 5500 Fragment Library Core Kit (Applied Biosystems, Waltham, MA, USA) and subjected to 50-nucleotide, single-read multiplex sequencing with ABI SOLiD 5500XL deep sequencers, as we previously described (46). The color-space raw sequence reads in the CSfasta format were aligned to the galGal3 chicken reference genome sequence using the RNA-seq pipeline of the ABI Lifescope software (Applied Biosystems, Waltham, MA, USA). Numbers of mapped reads were at least 18.5 million for each gonad, as shown in Suppl. Table 1 (47). All supplementary tables and figures are deposited in NIH Figureshare Archive Collection https://doi.org/10.35092/yhjc.c.5000516.v1, and each individual material is accessible as a reference. Library construction and sequencing were performed in several batches, each of which included similar numbers of ZZ-testes, ZW-ovaries, FS = 1 feminized ZZ-gonads, and FS = 2 feminized ZZ-gonads to suppress batch effects.
Expression of mRNA transcripts from protein-coding genes annotated in the galGal3 reference genome was determined by assigning uniquely aligned reads (bam format) to exons of the galGal3 gene model using Bioconductor (version 3.8) packages Rsubread (version 2.0.1) (48) and edgeR (version 3.28.1) (49), as we previously described (50). The relative mRNA expression data were normalized using the negative binominal trimmed mean of M-values (TMM) method implemented by the edgeR package. Normalized counts-per-million reads (cpm) mRNA expression data are provided as Suppl. Table 2 (51). Box plot analysis of the normalized counts showed no evidence of batch effects (Suppl. Fig. 1 (52)). Principal component analysis (PCA) was performed using the prcomp function of R and visualized using the plot3D function and the RGL package of 3-D interactive graphics. Unsupervised hierarchical clustering (average linkage) and heatmap visualization were performed using Cluster (53) and Java TreeView (54), respectively, as we previously described (55). Gene ontology analysis was performed using the DAVID server (56).
RNA expression from chicken RSs was evaluated using the ERVmap pipeline (57) with modifications. Because ERVmap was developed for quantitative detection of RNA transcripts expressed from a hand-selected subset of human endogenous retroviruses, we replaced its list of human endogenous retroviruses with the RepeatMasker file (58, 59) of the chicken reference genome. We observed that applying the TMM normalization (49) to the RS count data generated more stable outcomes than the original approach of ERVmap, which normalizes the count data based on the number of uniquely mapped reads (57). Principal component analysis, clustering, and data visualization were performed as described above for protein-coding mRNA transcripts.
MBD-seq library construction, deep sequencing, and data analysis
Genome-wide DNA methylation profiles were determined by methyl-CpG binding domain protein-enriched genome sequencing (MBD-seq), as we previously described (50, 60, 61). Genomic DNA was fragmented to ~200 bp by sonication at 4°C using the Covaris S2 sonicator and subjected to enrichment of methylated DNA fragments using the MethylMiner kit (Life Technologies, Carlsbad, CA, USA). Deep sequencing libraries were synthesized using ABI SOLiD 5500 Fragment Library Core Kit and subjected to 50-nucleotide, single-read sequencing with ABI SOLiD 5500XL deep sequencers (61). The CSfasta raw reads were aligned to the galGal3 chicken reference genome sequence using the MethylMiner-seq pipeline of the ABI Lifescope software. At least 10 million reads were uniquely mapped for each library. Saturation plot analysis (62) confirmed that sufficient amounts of uniquely mapped reads were obtained for genome-wide DNA methylation analysis by MBD-seq. Library construction and sequencing were scheduled to suppress batch effects, as described for RNA-seq. Statistical evaluation of DNA methylome was performed using the Bioconductor package MEDIPS (version 1.38.0) (62). Principal component analysis, clustering, and heatmap computing and visualization were performed, as described earlier for RNA-seq data analysis. To visualize the MBD-seq read tracks, the raw aligned data in the bam format were converted into normalized bigWig format using the bamCoverage module of deepTools (63) and visualized using the integrated genomics viewer (64).
Histological examinations
Day-9 chicken embryonic gonads were collected by dissection and fixed in 4% formaldehyde (alcohol-free, electron microscope-grade diluted in phosphate-buffered saline [PBS]) at 4°C overnight. The fixed tissues were soaked in 30% sucrose/PBS overnight at 4°C and frozen in the optimal cutting temperature (OCT) compound. Cryosections (6-µm thick) were processed for hematoxylin and eosin (H&E) staining, and sections adjacent to the H&E slides were subjected to immunostaining. For immunofluorescence staining, slides were blocked with 5% donkey serum and incubated overnight at 4°C with primary antibodies: rabbit anti-DMRT1 (LS-C408137-100, 1:500 dilution; LSBio, Seattle, WA, USA (65)), goat anti-SOX9 (AF3075, 1:50 dilution; R&D Systems, Minneapolis, MN, USA (66)), and rabbit anti-SYCP3 (NB300-232, 1:200 dilution; Novus Biologicals, Littleton, CO, USA (67)). The secondary antibodies were donkey anti-rabbit-488 (ab150061, 1:500 dilution; Abcam (68)) and donkey anti-goat-568 (ab175704, 1:500 dilution; Abcam, Cambridge, MA, USA (69)). Nuclear staining was performed using Hoechst33342 (H21492; Thermo Fisher Scientific). Hematoxylin and eosin and fluorescence microscopic images were taken using a LSM710 confocal microscope (Zeiss, Oberkochen, Germany).
Statistics
Differences in average values were tested by Student’s t-test (unpaired, 2-tailed). Heteroscedastic data with significant F-test results (P < 0.05) were subjected to a t-test with the Welch correction (unpaired, 2-tailed). Differentially expressed genes were identified from the TMM-normalized RNA-seq data using the likelihood ratio test (70) implemented by edgeR based on the generalized linear model (49).
Results
Quantitative evaluation of EE2-induced partial gonadal feminization of day-19 ZZ-male chicken embryos
We previously described a chicken embryo model of quantitatively manageable gonadal feminization achieved by intrayolk injection of EE2 (27). Taking advantage of this model, we exposed ZZ-male embryos to 20 µg/egg EE2 from day 6 until day 19 and collected gonads by dissection. Viability or overall development of embryos was not affected by injection of the emulsion with or without EE2. Normal day-19 testes of unexposed control ZZ-male embryos showed a rod-shaped appearance with nearly an identical size of right and left gonads (Fig. 1A, left). In contrast, the right ovaries of day-19 unexposed ZW-females were highly atrophic, while the left ovaries were widened and flattened (Fig. 1A, right). Gross morphological degrees of feminization shown by the EE2-exposed ZZ-male gonads (FemZZs for feminized ZZ gonads) were scored using the FS, as described in our previous study (27, 31) and shown in Fig. 1B. Agreeing with our and other laboratories’ prior observations (27, 31), left FemZZs altered their gross morphology homogenously without forming mutually exclusive, clearly distinguishable regions that presented testicular or ovarian characteristics within a single gonad. This outcome was in contrast to the formation of ovotestes, in which testis-like and ovary-like regions co-exist in a single gonad after in ovo exposure of chicken embryos to tamoxifen (31). For genomic analyses, we collected a total of 29 gonads with various genetic sex, exposure and FS from day-19 embryos, as shown in the table of Fig. 1B and Suppl. Table 1 (47). Only the left gonads were subjected to analyses due to the known asymmetry of the right and the left chicken embryonic gonads (11).
Figure 1.
Feminization of chicken embryonic gonads by exposure to 17α-ethynylestradiol (EE2). A: Morphological characteristics of normal chicken embryonic gonads at day-19 of incubation. Testes in males and ovaries in females are shown in blue and red contours, respectively. Scale bar = 1 mm. B: Feminization scores (FS) of embryonic gonads. Schematic drawings show day-19 embryonic gonads of wild type males (FS = 0), wild type females (FS = 3), and EE2-feminized genetic males (FS = 1 or 2). The bottom part of each panel shows cross sections at the level indicated by red line in the top part. Table shows 4 experimental groups with numbers and FSs of embryonic gonads involved in the present study. EE2-exposed ZW-ovaries (n = 2) were morphologically indistinguishable from nonexposed ovaries and so assigned to FS = 3. C: Demethylation of 3 sex-sensitive CpG sites in promoter of the CYP19A1/aromatase gene upon EE2-feminization of ZZ-males. Each datum point indicates bisulfite pyrosequencing determination of CpG methylation of individual gonad. Cross symbols indicate mean ± SEM of DNA methylation at each CpG site and experimental group. Abbreviations: CpG, 5’-C-phosphate-G-3’; DNA, deoxyribonucleic acid; IVC, inferior vena cava; SEM, standard error of mean.
In our preceding study, we reported feminization-dependent demethylation of 3 CpG sites in the promoter sequence of the CYP19A1/aromatase gene (27). Degrees of methylation at these CpG sites (955, 869, and 789 bp upstream of the TSS) were lowest in normal ZW-female ovaries, highest in normal ZZ-male testes, and intermediate in FemZZs. The feminization-dependent demethylation of these CpG sites was specific to gonads because these sites were hypermethylated in the hearts of both males and females (27). Confirming the quantitative reproducibility of the above observations, our present study further revealed that more strongly feminized FemZZs showed greater degrees of demethylation at each of these 3 CpG cites than the FemZZs with weaker feminization (Fig. 1C; compare FS = 1 and FS = 2). Thus, the morphological and epigenetic characteristics supported the quantitative reproducibility of our chicken embryo model of EE2-induced partial gonadal feminization.
RNA-seq transcriptomic analysis classifies gonadal sex-specific differentially expressed genes by responsiveness to EE2-induced feminization
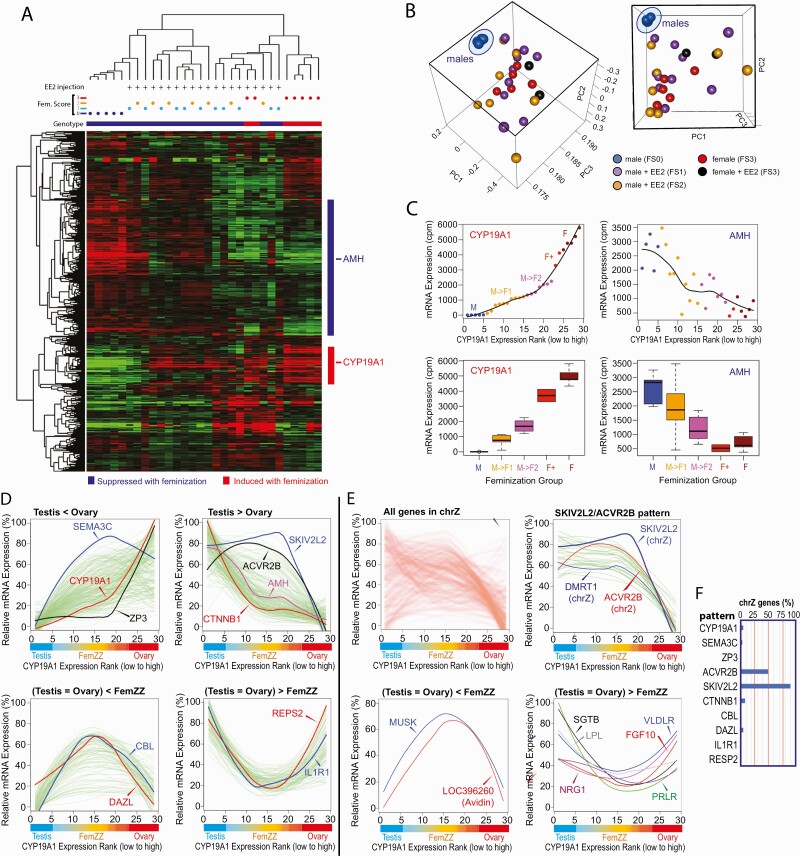
Our previous mRNA microarray study identified genes differentially expressed between normal ZZ-male testes and ZW-female ovaries of day-19 chicken embryos and demonstrated that some of the female-specific differentially expressed genes (DEGs), including CYP19A1, were strongly expressed in FemZZs to the levels comparable to normal ZW-ovaries (27). In the present study, we determined transcriptomic profiles of FemZZs and normal gonads (ZZ-testes and ZW-ovaries) in day-19 chicken embryos by RNA-seq. Heatmap representation of unsupervised hierarchical clustering showed a clear separation of FemZZs from ZZ-testes or ZW-ovaries, whereas FS = 1 and FS = 2 FemZZs were not separated (Fig. 2A). On the heatmap, clusters of genes whose expression was induced (red bar on the right) or suppressed (blue bar) were identified, and representative sex-specific gonadal marker genes CYP19A1 (ovarian marker) and AMH (testicular marker) were found in these clusters, respectively. List of genes in the feminization-inducible cluster (50 genes) and suppressible cluster (171 genes) are provided as Suppl. Table 3 (71).
Figure 2.
Protein coding gene transcriptomes of chicken embryonic gonads at day 19 of incubation. A: Heatmap of unsupervised hierarchical clustering. Vertical columns represent individual embryos, and horizontal rows show genes. At the top, genetic sex (genotype; black = ZZ males, white = ZW females), feminization scores, and EE2 injection are indicated for each gonad. At the right, genes induced or suppressed by feminization are shown with red and blue bars, respectively, and locations of the representative ovarian (CYP19A1) and testicular (AMH) marker genes are indicated. B: Principal component analysis. Values at axes indicate coefficients of each principal component. Both cubes show the same PCA plot viewed from different angles. Dots indicate gonadal transcriptomes of FS0 normal males (blue), FS1 FemZZs (purple), FS2 FemZZs (orange), FS3 normal females (red), and FS3 females exposed to EE2 (black). Note that 5 independent ZZ-males exposed to control vehicle (DMSO) formed a very tight transcriptomic cluster (blue oval). C: Expression of CYP19A1 and AMH in gonads with varying degrees of feminization. Normalized mRNA expression is shown with smooth fitted lines (top panels) and boxplots (bottom panels). Dots and boxes are color-coded based on embryonic genetic sex, exposure, and CYP19A1 expression: M (blue), ZZ males exposed to DMSO; M->F1 (orange), ZZ males exposed to EE2, weak CYP19A1 expression; M->F2 (purple), ZZ males exposed to EE2, strong CYP19A1 expression; F+ (red), ZW females exposed to EE2; F (brown), ZW females, exposed to DMSO. D–F: Differential mRNA expression between testes, ovaries, and FemZZs. Gonads were sorted for expression of CYP19A1/aromatase mRNA (left-to-right direction of x-axis corresponds to low-to-high expression), and their associated morphological characteristics (testes, FemZZs, and ovaries) are indicated. Each green line indicates smoothed expression of a gene across gonadal samples. D: Whole-genome analysis. Representative 4 expression profiles are presented in separate panels—namely, expression in ovaries is stronger than in testes (top left), expression in testes is stronger than in ovaries (top right), expression in testes and ovaries is weaker than FemZZs (bottom left), and expression in testes and ovaries is stronger than FemZZs (bottom right). Expression profiles of representative genes are shown with colored lines. E: Analysis of genes in the sex chromosome Z. Heatmap of all chrZ genes showing the overall tendency of differential gene expression between testis, ovary, and FemZZs (top left). Whereas the majority of genes show ACVR2B-like profile (top right), several genes show CBL/DAZL-like profile (bottom left) or ILFR1/RESP2-like profile (bottom right). F: Incidence rates of genes located in the Z chromosome and following the indicated patterns of gene expression profiles. Abbreviation: chrZ, chromosome Z; DMSO, dimethyl sulfoxide; EE2, 17α-ethynylestradiol; mRNA, messenger ribonucleic acid; PCA, principal component analysis.
Principal component analysis revealed very strong transcriptomic homogeneity among 5 normal ZZ-testes (Fig. 2B, blue dots). In contrast, normal ZW-females showed significantly heterogeneous transcriptomic profiles with (5 red dots) or without (2 black dots) exposure to EE2. Transcriptomes of FemZZs (10 FS = 1, purple dots; 7 FS = 2, yellow dots) also showed high degrees of heterogeneity comparable to those of normal ZW-females (Fig. 2B). These results indicate that tightly homogenous transcriptomes characterize day-19 ZZ-male gonads compared with ZW-females with highly heterogenous transcriptomes, and that FemZZs show significantly heterogenous transcriptomes comparable to ZW-females. Principal component (PC) 1 explained 90.6% of all variations, while PC2 and PC3 explained only 3.1% and 2.5%, respectively. PC1 was associated with EEFA1, PABPC1, and NPM1, and PC2 was associated with CYP19A1. EMB, TXN, and LOC422926 were associated with both PC1 and PC2. EEFA1 and NPM1 (72), and CYP19A1 and EMB (73), were previously reported as sexually dimorphic genes in chicken embryonic gonads.
To evaluate the quantitative responsiveness of chicken embryonic gonads to the EE2-induced feminization, we ranked all gonads by the strength of aromatase mRNA expression determined by RNA-seq and found that the strength of CYP19A1 mRNA expression closely aligned with their morphological feminization scores (Fig. 2C, left panels; Suppl. Table 1 (47)). Although there were only 2 ZW-female ovaries exposed to EE2, their expression of CYP19A1 mRNA tended to be lower than their expression in ZW-ovaries without exposure (Fig. 2C, compare F+ [EE2-exposed ZW-female ovaries] vs F [ZW-female ovaries]). When gonads were aligned along the CYP19A1 mRNA expression rank, expression of AMH mRNA showed a roughly mirror image profile, although its low expression in ZW-ovaries was not affected further by exposure to EE2 (Fig. 2C, right panels).
We next attempted to determine DEGs between various combinations of the 5 degrees of morphological feminization—namely, FS = 0 normal genetic ZZ-testes, FS = 1 weakly feminized FemZZs, FS = 2 more advanced FemZZs, FS = 3 normal genetic ZW-ovaries, and FS = 3 ZW-ovaries exposed to EE2. Note that both normal genetic ZW-ovaries and ZW-ovaries exposed to EE2 were not morphologically distinguishable and so were scored as FS = 3. Suppl. Table 4 (74) shows a list of DEGs classified into 10 groups. Each tab in Suppl. Table 5 (75) provides a nonoverlapping list of DEGs belonging to a group showing a specific expression profile represented by the gene shown as the tab name. We generated linear and box plots for each of these DEGs and manually verified their expression profiles (Suppl. Fig. 2 (76)). The verified DEGs and chromosomes they belong to are listed in the “verified” tabs of Suppl. Table 5 (75).
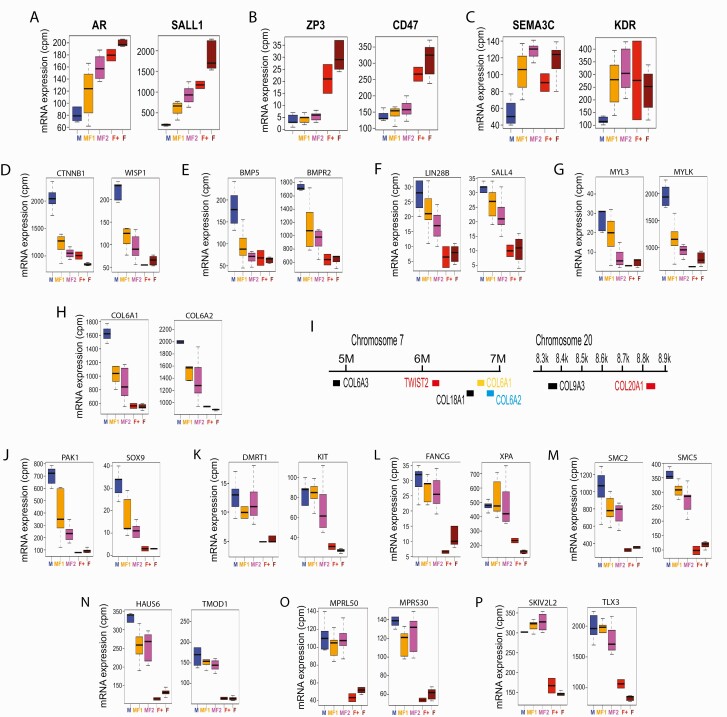
Taking the above approach, we were able to classify 114 verified, feminization-inducible DEGs into 3 distinct groups represented by CYP19A1, ZP3, and SEMA3C (Fig. 2D, “Testis < Ovary” panel; Fig. 3A–3C; and Suppl. Table 5 (75)). The CYP19A1 group consisted of 46 verified DEGs whose strength of expression correlated well with the feminization scores (Fig. 3A shows 2 representative genes in this group, AR and SALL1); whereas the CYP19A1 group DEGs showed significant expression in the FemZZs and expression of the ZP3 group DEGs (5 verified genes) remained suppressed in FemZZs, contrasting with their very strong expression in ZW-ovaries (Fig. 3B shows 2 representative genes CD47 and ZP3). On the other hand, the SEMA3C group DEGs (63 verified genes) were strongly induced even in the weakly feminized FS = 1 FemZZs to levels comparable with ZW-ovaries, whereas their expression in ZZ-testes was very low (Fig. 3C shows 2 representative genes, KDR and SEMA3C). Boxplots and line graphs of all DEGs belonging to the above 3 groups are shown in Suppl. Fig. 2. Gene ontology analysis did not detect significant enrichment for these 3 groups of DEGs to any terms. These observations demonstrate the highly heterogeneous aspects of responsiveness among ovary-specific genes to EE2-induced ZZ-gonad feminization. The ovary-specific expression of FOXL2 was previously reported by other laboratories (9, 22, 30, 36, 73, 77) as well as ours (27). In the current study, normalized RNA-seq counts of FOXL2 for FS = 1, 2, and 3 gonads were 3.1 ± 0.6, 3.5 ± 0.8, and 8.0 ± 1.2 (unexposed) or 8.2 ± 1.9 (EE2-exposed), respectively (mean ± standard error of mean [SEM] of counts per million reads, Suppl. Table 2 (51)). No RNA-seq reads were assigned to FOXL2 in any of the 5 normal ZZ-testes. Thus, feminization-inducible expression of FOXL2 is evident, but our unsupervised clustering analysis (Fig. 2A) did not pick up this gene due to its relatively low counts and our relatively strict filtering criteria.
Figure 3.
Representative mRNA transcripts differentially expressed between ovaries and testes. Boxplots show median, upper, and lower quartiles, and the highest and lowest values of normalized mRNA expression in 29 gonads (y-axis shows normalized RNA-seq read counts per million mapped reads; cpm). Each panel shows 2 prototypical genes showing expression profiles represented by CYP19A1: modest but significant induction in FemZZs (A); ZP3: minimal induction in FemZZs (B); SEMA3C: robust induction in minimally converted FemZZs (C); D–J: CTNNB1: robust suppression in minimally converted FemZZs and involved in Wnt signaling (D), BMP signaling (E), pluripotency (F), myosin light chain expression and regulation (G), and collagen synthesis and regulation (H) (chromosomal locations shown in (I)) and miscellaneous pathways (J); and SKIV2L2: minimal suppression in genetic males and involved in gonadal sex differentiation and germline development (K), DNA repair (L), (maintenance of chromosomal structure (M), cytoskeleton (N), mitochondrial ribosomal formation (O), and miscellaneous pathways (P). Abbreviation: BMP, bone morphogenetic protein; DNA, deoxyribonucleic acid; mRNA, messenger ribonucleic acid.
We took a similar approach to characterize 172 verified, feminization-suppressible DEGs, which were classified into groups represented by CTNNB1, SKIV2L2, and ACVR2B (Fig. 2D, “Testis > Ovary” panel; Fig. 3D–3P; and Suppl. Table 5 (75)). The CTNNB1 group consisted of 146 verified DEGs, including the testicular marker gene AMH (Fig. 2D). Their expression was strongest in the normal ZZ-testes, weaker in FemZZs, and strongly suppressed in ZW-ovaries (Figs. 2D, 3D–3J). Boxplots and line graphs of all DEGs belonging to the above 3 groups are shown in Suppl. Fig. 2. In the CTNNB1 group DEGs, we identified genes representing several functional pathways—namely, Wnt signaling (CTNNB1, WNT5A, and WISP2; Fig. 3D and Suppl. Fig. 2), signaling of bone morphogenetic proteins (BMP5 and BMPR2; Fig. 3E), pluripotency (LIN28B, SALL4, and TBX3; Fig. 3F and Suppl. Fig. 2), myosin light chain expression and regulation (MYL2, MYL3, and MYLK; Fig. 3G and Suppl. Fig. 2), and collagen synthesis and regulation (COL6A1, COL6A2, COL6A3, COL9A2, COL9A3, COL18A1, COL20A1, LUM, and TWIST2; Fig. 3H and Suppl. Fig. 2). Several DEGs involved in collagen synthesis and regulation were closely localized in chromosomes 7 and 20 (Fig. 3I). Gene ontology analysis revealed strong enrichment of the CTNNB1 group DEGs to GO terms “cell adhesion” and “male genitalia development” (Suppl. Table 6 (78)). These results imply possible involvement of the above pathways in maintenance of the testicular gonadal phenotypes. The CTNNB1 group included several other genes whose roles in testicular development are known or suggested, including CXCR7, GHR, ITGA9, LIFR, MAP7, MiR202, NES, NIM1, NRSA2, PAK1, PDGFRA, PTCH1, RPS6, SOX9, SRC, STRBP, TJP2, and TYRO3; Fig. 3J and Suppl. Fig. 2).
DEGs belonging to the SKIV2L2 and ACVR2B groups were strongly expressed in both ZZ-testes and FemZZs, whereas their expression was remarkably suppressed in ZW-ovaries (Fig. 2D and Suppl. Table 5 (75)). The strong suppression of the SKIV2L2 group DEGs (22 verified genes) was not affected by exposure to EE2 (Fig. 3K–3P). We noted 4 DEGs whose expression seemed stronger in EE2-exposed ZW-ovaries than in nonexposed ZW-ovaries—namely, ACVR2B, CAMK4, DCTN3, and NTM—although the limited number of the EE2-exposed ZW-ovary specimens (n = 2) was insufficient to confirm its statistical significance. We presented these 4 DEGs as the ACVR2B group (Suppl. Table 5 (75)). The SKIV2L2 group contained several important genes involved in gonadal sex differentiation and germline cell development (DMRT1 and KIT; Fig. 3K), DNA repair (FANCG and XPA; Fig. 3L), maintenance of chromosomal structure (SMC2 and SMC5; Fig. 3M), regulation of cytoskeleton (HAUS6 and TMOD1; Fig. 3N), and mitochondrial ribosome formation (MPRL50 and MPRS30; Fig. 3O), although gene ontology analysis did not support their statistically significant enrichment due to the small queue size. Other members of this group included SKIV2L2 itself, which is an RNA helicase required for mitotic progression (79), and TLX3 encoding an orphan homeobox protein that may be involved in T cell leukemia formation in humans (80) (SKIV2L2 and TLX3; Fig. 3P).
Among the 3 groups of feminization-suppressible DEGs, the FemZZ-expressed groups represented by SKIV2L2 and ACVR2B showed a stark contrast in their chromosomal localization compared with the FemZZ-suppressed CTNNB1 group—namely, the great majority of the SKIV2L2 group DEGs (19/21, 91%) and the ACVR2B-group DEGs (2/4, 50%) were on sex chromosome Z, while the CTNNB1 group DEGs did not show significant enrichment in any chromosomes (Fig. 2E and 2F and Suppl. Table 5 (75)). We then asked the reverse question: How many of the 202 genes encoded in chromosome Z (Suppl. Table 5 (75), “chrZ all genes” tab) follow expression profiles similar to the SKIV2L2/ACVR2B groups? To address this question, we examined the expression of all Z-encoded genes (Fig. 2E, “all genes in chrZ” panel) and found that 43 of them (21%) indeed followed the SKIV2L2/ACVR2B profiles of differential expression (Fig. 2E, “SKIV2L2/ACVR2B pattern” and Suppl. Table 5 (75), “chrZ SKIV2L2 ACVR2B pattern” tab). In contrast, we did not detect any significant evidence of Z chromosome-encoded, feminization-inducible DEGs (Fig. 2E, “all genes in chrZ” panel).
Groups of protein-coding DEGs and their characteristics—including numbers of DEGs, response to varying degrees of feminization, and link to chrZ—are summarized in Table 1.
Table 1.
Groups of DEGs in day-19 chicken embryonic gonads
| Representing Gene | Response to Feminization | Number of DEGs | Messenger RNA Expression | Link to chrZ | ||||
|---|---|---|---|---|---|---|---|---|
| FS = 0 ZZ-testes |
FS = 1 Weak FemZZ |
FS = 2 Strong FemZZ |
FS = 3 EE2-expopsed ZW-ovary |
FS = 3 Non-exposed ZW-ovary |
||||
| SEMA3C | Induced (high response) |
63 | - | ++/+++ | +++ | +++ | +++ | 3% |
| CYP19A1 | Induced (modest response) |
46 | - | -/+ | + | ++ | +++ | 4% |
| ZP3 | Induced (low response) |
5 | - | -- | - | +++ | +++ | 0% |
| CTNNB1/AMH | Suppressed (high response) |
146 | +++ | + | -/+ | - | - | 5% |
| SKIV2L2/DMRT1 | Suppressed (low response) |
21 | +++ | ++/+++ | ++/+++ | - | - | 90% |
| ACVR2B | Suppressed (low response) |
4 | +++ | ++/+++ | ++/+++ | + | - | 50% |
| CBL | FemZZ-specific induction |
8 | - | ++/+++ | ++/+++ | ++ | + | 0% |
| DMRTB1 | FemZZ-specific induction |
9 | -/+ | ++/+++ | ++/+++ | +/++ | -/+ | 0% |
| DAZL | FemZZ-specific induction |
21 | -/+ | ++/+++ | ++/+++ | -/+ | -/+ | 5% |
| IL1R1 | FemZZ-specific suppression | 19 | +++ | + | -/+ | -/+ | +++ | 0% |
| REPS2 | FemZZ-specific suppression | 9 | +++ | + | -/+ | ++/+++ | +++ | 0% |
Symbols indicate relative expression (-, no expression; -/+ less than 20%; +, 20-50%; ++, 51-80%; and +++, 81-100% expression).
Abbreviations: chrZ, chromosome Z; DEGs, differentially expressed genes; EE2, ethynylestradiol; FemZZ, feminized genetic male testes; FS, feminization score; ZZ-tested, genetic male testes; ZW-ovary, genetic female ovary.
Identification of protein-coding genes whose mRNA expression is specifically induced or suppressed in FemZZs
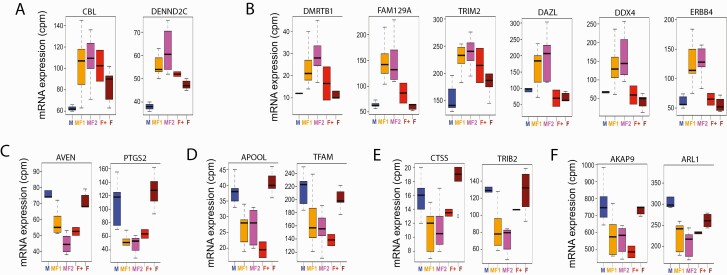
We next identified DEGs specifically inducible or suppressible in FemZZs (Figs. 2D and 4; Suppl. Tables 4 (74) and 5 (75)). The FemZZ-inducible DEGs were classified into 2 groups represented by CBL (8 DEGs whose expression in ZW-ovaries is slightly but significantly greater than in ZZ-testes) and DMRTB1/DAZL (30 DEGs whose expression in ZW-ovaries and ZZ-testes is statistically indistinguishable) (Fig. 2D, “(Testis = Ovary) < FemZZ” panel). Within the DMRTB1/DAZL group, expression of 9 genes seemed augmented by EE2 in ZW-ovaries (DMRTB1 subgroup), whereas expression of 21 other genes was not (DAZL subgroup) (Suppl. Table 5 (75); boxplots and line graphs of all DEGs belonging to the above 3 groups are shown in Suppl. Fig. 2). Note that the separation of these 2 subgroups of DEGs is based on their expression in EE2-exposed ZW-ovaries (n = 2) and so are not statistically supported. Expression profiles of representative DEGs are shown in Figs. 4A and 4B. Similarly, we classified the FemZZ-suppressible DEGs into 2 groups represented by IL1R1 and REPS2 (Fig. 2D, “(Testis = Ovary) > FemZZ” panel), which consisted of 19 and 9 verified DEGs, respectively (Suppl. Table 5 (75); boxplots and line graphs of all DEGs belonging to the above 3 groups are shown in Suppl. Fig. 2). Expression of the IL1R1 group DEGs in ZW-ovaries tended to be suppressed by EE2 (Fig. 4C–4F and Suppl. Fig. 2), whereas expression of REPS2 DEGs was affected more modestly (Suppl. Fig. 2); again, separation of these 2 groups was not statistically supported due to the small number of EE2-exposed ZW-ovarian specimens. The IL1R1 group DEGs contained several important genes involved in apoptosis resistance (AVEN, BCL6, PDCD10, PTGS2, and RAP18; Fig. 4C and Suppl. Fig. 2), mitochondria formation (APOOL, AVEN, BCL6, PPIF, PTGS2, and TFAM; Fig. 4D, and Suppl. Fig. 2), and development of cumulus cells, which are granulosa cells surrounding oocytes (CTSS and TRIB2; Fig. 4E and Suppl. Fig. 2). This group also contained several gene-encoding proteins involved in intracellular signal transduction (AKAP9, ARL1, C13H5orf15, COLEC12, DNAJB14, IL1R1, PALD1, and SPCS3; Fig. 4F and Suppl. Fig. 2). Gene ontology analysis did not find any significant enrichment for the FemZZ-specific DEG groups due to the small sizes of the queues. Taken together, the existence of DEGs specifically inducible or suppressible in FemZZs supports the notion that FemZZs are not simple mixtures of normally differentiated components of testes and ovaries.
Figure 4.
Representative mRNA transcripts specifically inducible or suppressible in FemZZs. Boxplots show median, upper, and lower quartiles, and the highest and lowest values of normalized mRNA expression in 29 gonadal tissue specimens (y-axis shows normalized RNA-seq counts per million mapped reads; cpm). A, B: Transcripts expressed more strongly in FemZZs than testes or ovaries with (A) or without (B) significant levels of expression in ZW-ovaries. C–G: Transcripts expressed more weakly in FemZZs than testes or ovaries and involved in resistance to apoptosis (C), mitochondria formation (D), cumulus cell differentiation (E), and miscellaneous genes (F). Abbreviation: mRNA, messenger ribonucleic acid.
Locations of DMRT1-expressing cells in normal and feminized gonads
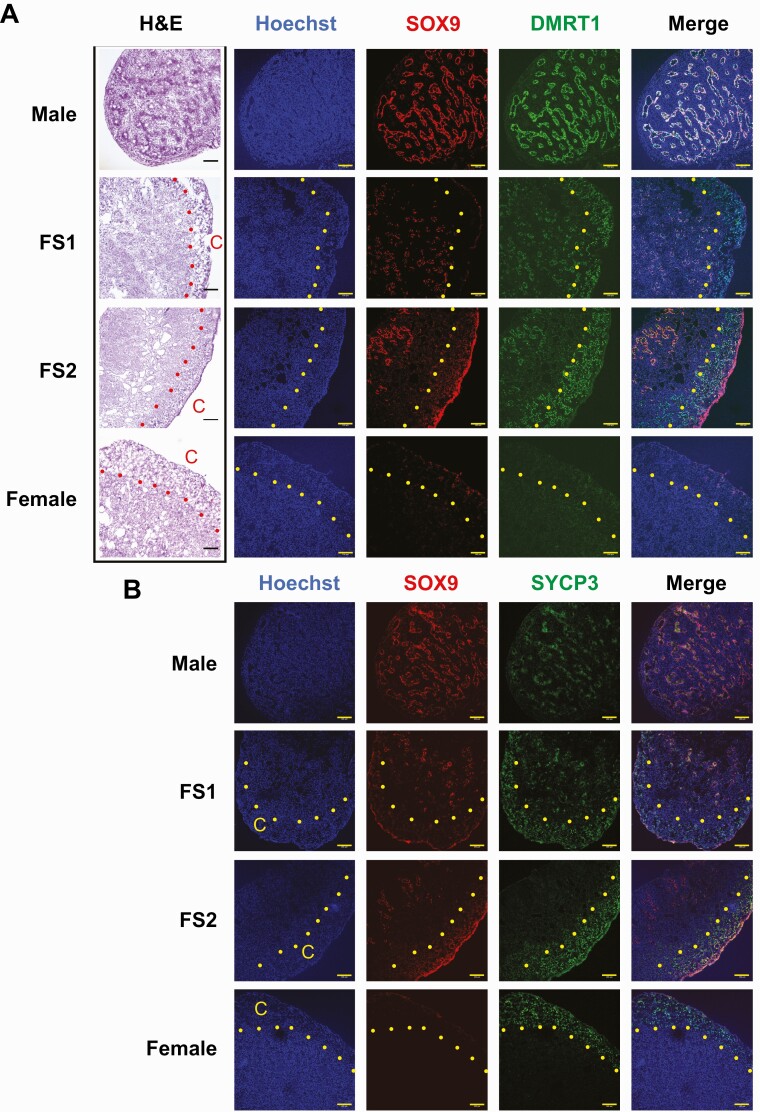
To obtain initial clues to the cell types expressing the feminization-resistant testicular genes in FemZZs, we attempted to detect DMRT1 protein in the normal and EE2-feminized chicken embryonic gonads at day 19. The histological data shown in Fig. 5 were obtained with a new repeat of the EE2-exposure experiment that produced normal and feminized chicken embryonic gonads (n ≥ 5 for each FS group); these embryonic specimens were thus generated independently from those used for the deep sequencing analyses in the current study.
Figure 5.
Histological examinations of day-19 chicken embryonic gonads for expression of SOX9 and DMRT1 (A) and SOX9 and SYCP3 (B). Serial slides of cryosections were subjected to H&E staining and immunofluorescence. All fluorescence images in each row were taken from the same slide. Scale bars indicate 100 µm, and dots indicate borders of cortex (C). Abbreviation: H&E, hematoxylin and eosin.
Hematoxylin and eosin images of cryosections showed well-developed seminiferous tubules in the unexposed FS = 0 ZZ-male gonads, but not in the medulla of FS = 2 FemZZs or FS = 3 ZW-female ovaries (Fig. 5A). The FS = 1 FemZZs often showed apparently shortened or fragmented tubular structures widely distributed throughout the medullary area. Distinctive cortex regions were observed with FemZZs (FS = 1 and FS = 2) and ZW-ovaries.
We detected SOX9 protein at the well-developed tubules in the FS = 0 ZZ-gonads and the fragmented tubules in the FS = 1 FemZZs. Smaller numbers of SOX9-positive cells were also detected only at the central region of the medullary area in the FS = 2 FemZZs, suggesting formation of incomplete seminiferous tubule-like structures there. The number of SOX9-positive cells decreased along with the increasing degrees of feminization; this observation was consistent with the feminization-suppressible nature of SOX9 mRNA expression shown in Fig. 3J.
DMRT1 protein, which is encoded by a representative feminization-resistant testicular gene encoded in the Z chromosome (Figs. 2E and 3K), was strongly expressed in the SOX9-positive seminiferous tubules somatic cells and associating germline cells in the FS = 0 ZZ-male gonads. DMRT1 was also clearly detected in the SOX9-positive somatic cells in the medulla of FS = 1 and FS = 2 FemZZs. In FemZZs, a small number of DMRT1-positive but apparently SOX9-negative cells were also detected adjacent to the SOX9-positive cells in the medullary area; these DMRT1-positive, SOX9-negative cells seemed to represent medullary germline cells. A large number of DMRT-1 positive cells were observed in the cortex of FS = 1 and FS = 2 FemZZs, with FS = 2 cortex containing greater numbers of DMRT1-positive cells. In contrast, the cortex of ZW-ovaries was completely devoid of DMRT1-positive cells.
The meiosis marker protein SYCP3 was detected in the tubule-associated germline cells in the FS = 0 ZZ-gonads and in the medulla of FS = 1 FemZZs adjacent to the SOX9-positive cells; in contrast, no SYCP3-positive cells were found in the medulla of FS = 2 FemZZs or ZW-ovaries. On the other hand, the cortex of the FS = 1 and FS = 2 FemZZs as well as the ZW-ovaries contained large numbers of SYCP3-positive germline cells. Due to technical limitations, in the current study we were unable to determine whether the DMRT1-positive cortex cells in the FemZZs are identical to the SYCP3-positive germline cells.
Taken together, our data show that the DMRT1-positive cells are located in both the medulla and the cortex of FemZZs. The medullary DMRT1-positive cells consist of SOX9-positive somatic cells forming tubule-like structures and SOX9-negative cells that are likely germline cells. The DMRT1-positive cells in the FemZZ cortex cells are SOX9-negative and appear to be germline cells.
Genetic sex-specific RS expression profile is largely maintained in EE2-induced FemZZs
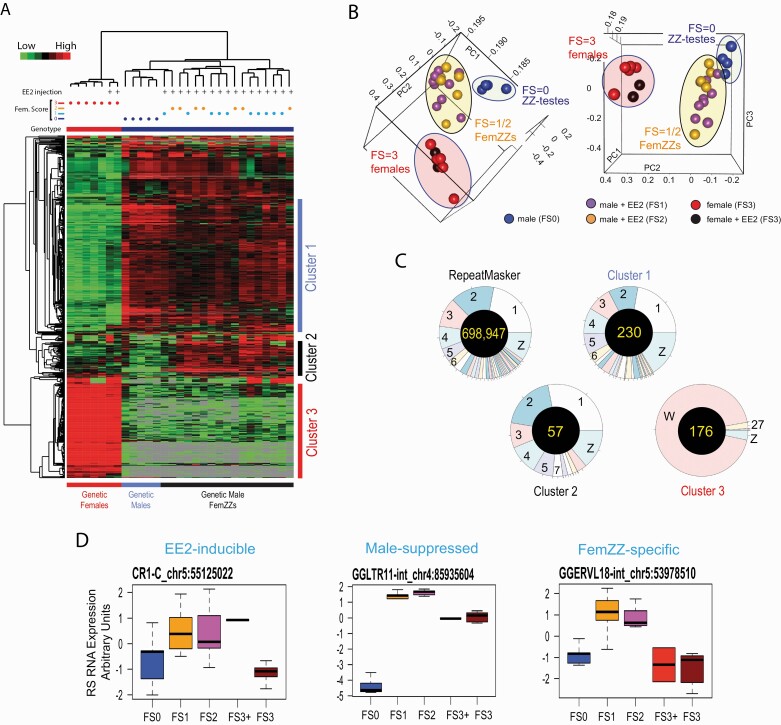
To obtain further insights into the genome-wide genomic alterations in the day-19 FemZZs, we extended our transcriptomic analysis to RSs. To this end, we adapted the recently published ERVmap pipeline (57), which was originally developed for quantitative detection of RNA expression from human endogenous retroviruses using RNA-seq data, to evaluate RNA expression from RSs in chicken genome. The adapted pipeline was applied to the 29 RNA-seq raw read data (fastq) to generate normalized counts for approximately 700 000 chicken RSs (Suppl. Table 7 (81)).
Unsupervised hierarchical clustering very clearly separated FS = 3 ZW-ovaries, with or without EE2 exposure, from ZZ-testes or FemZZs (Fig. 6A, heatmap). Normal ZZ-testes (FS = 0) were also separated from FemZZs, but weak (FS = 1) and strong (FS = 2) FemZZs were not separated. On the heatmap, clusters of RSs whose strong expression in normal ZZ-testes was unchanged in FemZZs (Fig. 6A-Cluster 1, blue bar; 231 RS), RSs specifically expressed in FemZZs (Fig. 6A-Cluster 2, black bar; 58 RSs), and RSs exclusively expressed in ZW-ovaries (Fig. 6A-Cluster 3; 176 RSs) were identified (Fig. 6A and Suppl. Table 8 (82)). Principal component analysis supported the clear separation of normal ZZ-testes (FS = 0), FemZZs (FS = 1 or 2), and ZW-ovaries (FS = 3), although weak (FS = 1) and strong (FS = 2) FemZZs were not separated (Fig. 6B).
Figure 6.
RNA expression from RSs in day-19 chicken embryonic gonads. A: Heatmap of unsupervised hierarchical clustering of RNA expression from RSs determined by RNA-seq. Vertical columns represent individual embryos and horizontal rows show RSs registered in the galGal6 RepeatMasker file. At the top, genetic sex (genotype; blue = ZZ males, red = ZW females), feminization scores, and EE2 injection are indicated for each gonad. At the right, 3 clusters of the RSs showing representative expression profiles are indicated: Cluster 1, suppressed in genetic females; Cluster 2, suppressed in genetic males or females but expressed in FemZZs; Cluster 3, specifically expressed in genetic females. B: Principal component analysis. Ovals indicate ZW-ovaries (red), ZZ-testes (green), and FemZZs (yellow). C: Chromosomal distributions of expressed RSs. Pie charts show distributions of all RepeatMasker-registered chicken RSs, and the RSs belonging to Clusters 1, 2, and 3 shown in panel (A). Numbers of total RSs for each chart are shown at the center. D: Examples of RNA expression profiles from RSs. Boxplots show median, upper, and lower quartiles, and the highest and lowest values of normalized RNA expression of the indicated RSs in gonads with varying feminization scores (FS0–FS3); FS3+ are genetic female gonads with exposure to EE2. Abbreviations: CpG, 5’-C-phosphate-G-3’; EE2, 17α-ethynylestradiol; MBD-seq, methyl-CpG binding domain protein-enriched genome sequencing; RNA, ribonucleic acid; RNA-seq, ribonucleic acid sequencing; RSs, repetitive sequences.
Chromosomal distributions of RSs belonging to Cluster 1 or 2 were comparable to those of the 698 947 RepeatMasker-registered RSs in the chicken reference genome sequence (Fig. 6C). Interestingly, whereas expression profiles of the Cluster 1 RSs were similar to those of the SKIV2L2/ACVR2B DEGs (Fig. 2D and 2E), their chromosomal location was not significantly biased to chromosome Z. In contrast, 94% of Cluster 3 RSs (165/176) were located in the female-specific sex chromosome W (Suppl. Table 8 (82)). Because 6 RSs in Cluster 3 appeared to be incorrectly mapped to the Z chromosome due to the presence of very similar genes in the W and Z chromosomes, nearly all (171/176) Cluster 3 RSs seemed to locate in chromosome W. Cluster 3 (176 RSs) represented 2.3% of all RepeatMasker-registered RSs located in chromosome W. The exceptionally strong expression of Cluster 3 RSs (Fig. 6A) may suggest epigenetically “permissive” status of chromosome W for RS expression.
Taking a similar approach used to identify DEGs, we were able to classify differentially expressed RSs into 7 groups (Suppl. Table 8 (82)). While large numbers of RSs followed the expression profiles represented by the 3 clusters shown in Fig. 6A, we were able to identify 2 RSs displaying the “EE2-inducible” profile—namely, strongly expressed in the EE2-exposed ZW-ovaries and FemZZs but not in normal ZZ-testes or unexposed ZW-ovaries (Fig. 6D and Suppl. Fig. 3 (83)). Similarly, there were 12 RSs whose expression was specifically and strongly suppressed only in normal ZZ-males (Fig. 6D and Suppl. Fig. 3 (83)). From the RSs that belonged to Cluster 2, we selected 8 representative RSs displaying clear FemZZ-specific expression (Fig. 6D and Suppl. Fig. 3 (83)).
Taken together, our RS expression profiling showed that RSs strongly expressed in normal ZZ-testes largely maintained their expression in FemZZs regardless of the morphological degrees of feminization, whereas their expression was suppressed in normal ZW-ovaries. In contrast to the protein coding SKIV2L2/ACVR2 group DEGs displaying similar profiles of expression, these RSs were not enriched in chromosome Z. The existence of a significant number of FemZZ-specific RSs (Cluster 2, Fig. 6A and 6D) contributed to clear separation of FemZZs from FS = 0 normal ZZ-testes by unsupervised hierarchical clustering (Fig. 6A) or PCA (Fig. 6B).
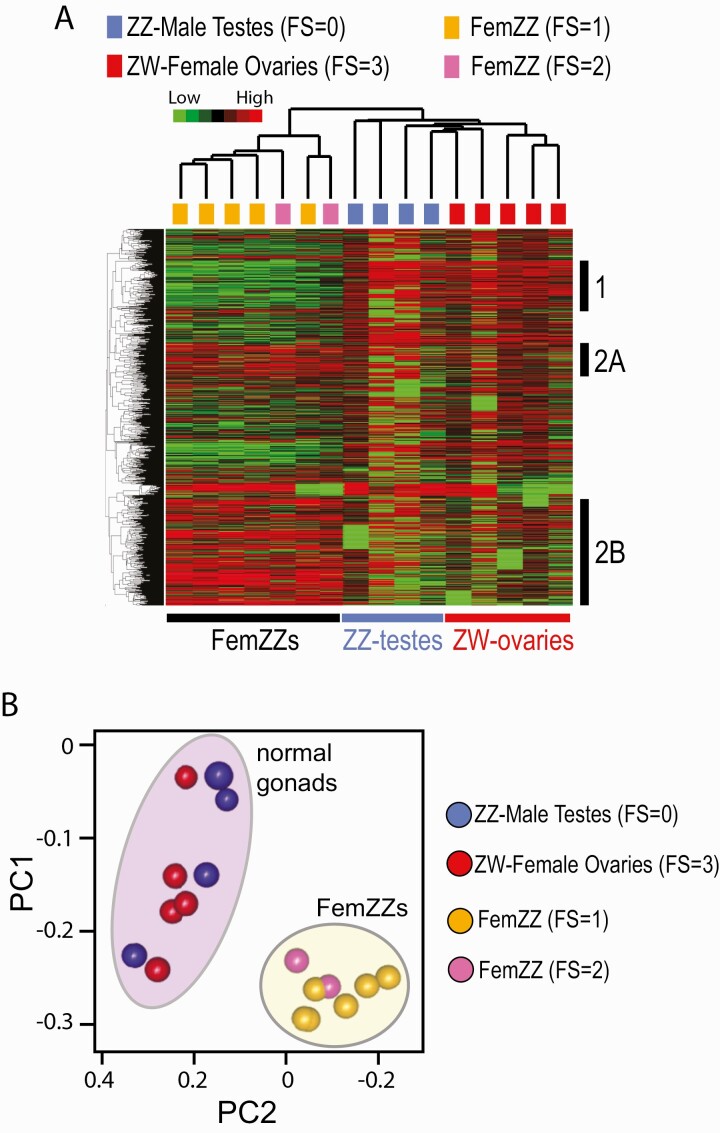
Genome-wide CpG methylation analysis distinguishes FemZZs from normally developed gonads
In our preceding study, we observed incomplete male-to-female epigenetic reprogramming involving DNA methylation and histone modifications at the CYP19A1/aromatase promoter, whereas CYP19A1 mRNA expression in those FemZZs was indistinguishable from that in ZW-ovaries (27). In the present study, our analysis of feminization-associated changes in DNA methylome was extended to genome-wide by taking the MBD-seq approach, as we previously described (50, 60, 61). Unsupervised hierarchical clustering clearly separated FS = 1/2 FemZZs from normal FS = 0 ZZ-testes or FS = 3 ZW-ovaries (Fig. 7A). Normal ZZ-testes and ZW-ovaries were separated but only modestly. In Fig. 7A, clusters of CpG sites more strongly methylated in normal gonads than FemZZs (Cluster 1), and CpG sites showing the opposite methylation profiles (Clusters 2A and 2B) were identified. Principal component analysis revealed a clear separation of FemZZs from normal gonads, but normal testes and ovaries were not separated (Fig. 7B). These results further supported the notion that FemZZs did not consist of simple mixtures of normally developed testicular and ovarian cells.
Figure 7.
MBD-seq CpG methylation profiles of day-19 chicken embryonic gonads. A: Heatmap of unsupervised hierarchical clustering of gonadal CpG methylation. Vertical columns represent individual embryos and horizontal rows show CpG sites differentially methylated across the feminization scores, which are indicated on the top with color codes. At the right, 3 clusters of CpG sites with representative methylation profiles are indicated: Cluster 1, hypomethylated in FemZZs; Clusters 2A and 2B, hypermethylated in FemZZs. B: Principal component analysis. Ovals indicate normal gonads (purple) or FemZZs (yellow).
We next attempted to identify genes whose promoter region (3 kbp up- and downstream of TSS) was differentially methylated between normal ZZ-testes, normal ZW-ovaries, and FemZZs. We identified 6 groups of such TSS differentially methylated genes (TDMGs) (Suppl. Table 9 (84)). Our attempts to link DEGs to changes in TSS methylation revealed that 8 CTNNB1 group DEGs (AMH, COL18A1, COL20A1, NES, NTM, SALL4, SRC_CHICK, and TYRO3) were commonly found in the lists of genes whose TSS is hypermethylated along with feminization (Suppl. Table 9 (84); tabs FS0 < FS3, FS0 < FS1/2, and FS3 > FS1/2). The feminization-associating TSS hypermethylation of these genes may contribute to the suppression of their mRNA expression upon feminization (Figs. 2C and 2D, 3D–3J). Interestingly, similar feminization-correlating TSS hypermethylation was also observed with (1) 3 representative DEGs specifically expressed in FemZZs (Fig. 2D and 24, and Suppl. Fig. 2)—namely, DAZL, DMRTB1, and TCF7; (2) CTSS, whose expression is specifically suppressed in FemZZs (Fig. 4E), and (3) 2 feminization-inducible genes, FOSL2 and ZP3 (Figs. 2D and 3B, and Suppl. Fig. 2). The reason for these associations is unknown. The number of DEGs linked to changes in TSS methylation is relatively small, possibly due to cellular heterogeneity in the whole gonadal tissues. Note that CYP19A1 was not identified as a TDMG in the present study due to the MBD-seq approach. Only 3 CpG sites in the CYP19A1 promoter showed feminization-dependent demethylation, whereas methylation of many other CpG sites in the promoter and TSS region remained unchanged (27). Because MBD-seq does not detect a small number of differentially methylated CpG sites surrounded by other CpG sites whose methylation state is unchanged, CYP19A1 was not identified as a TDMG by this deep sequencing method, whereas the feminization-dependent demethylation of 3 CpG sites in the CYP19A1 promoter was readily detectable by bisulfite pyrosequencing (Fig. 1). The apparent sensitivity limit of MBD-seq may have limited the number of the detected TDMGs in the current study.
Discussion
Identification of feminization-sensitive and resistant genes among DEGs showing genetic sex-specific expression in normal chicken embryonic gonads
We previously reported generation of FemZZs showing varying degrees of feminization by direct injection of EE2-containing oil emulsion into the yolk of chicken eggs before completion of gonadal sex differentiation (27). Our present study reproduced the quantitative demethylation of 3 CpG sites in the promoter of the CYP19A1/aromatase gene along with increased degrees of morphological feminization and expression of the aromatase mRNA transcripts (Fig. 1). Taking advantage of this convenient and robust homeothermic animal model of environmentally induced gonadal sex reversal, we were able to classify DEGs expressed in day-19 chicken embryonic gonads into 11 groups based on their quantitative responsiveness to feminization (Table 1, Figs. 2–4, Suppl. Fig. 2 (76), and Suppl. Table 5 (75)). The significant heterogeneity in expression profiles among these DEG groups (Fig. 2) provides evidence that some of the sex-specific DEGs are relatively resistant to the environmentally induced feminization of ZZ-gonads while other DEGs are prone to be affected by minimal degrees of feminization. The latter, feminization-sensitive groups of DEGs may play predominant roles in morphological and transcriptomic gonadal feminization. The feminization-resistant DEGs, on the other hand, might be responsible for the posthatching reversal of FemZZs to masculinized phenotype (37) and so could act as transcriptomic memory of the genetic sex.
Our previous study assessed the degree of EE2-induced feminization of day-19 chicken embryonic gonads by gross morphology (FS) and methylation of CpG sites at the promoter of the CYP19A1 gene (27). In the present study, we demonstrated the strong link between the FS (a coarse and subjective marker of feminization) and CYP19A1 mRNA expression (an objectively quantitative marker) (Fig. 2C). Then, we have shown that various known markers of gonadal sex differentiation have widely diversified degrees of sensitivities to EE2-induced feminization (Fig. 2D and Suppl. Fig. 2). Thus, our study has vastly extended the arsenal of sex differentiation markers, behaviors of which are summarized in Table 1. Future studies, including histological assessments of feminization, can take advantage of the new knowledge on differential sensitivities of individual and/or groups of markers shown in Table 1.
Characteristics of the feminization-sensitive DEGs
The CTNNB1 group DEGs represent the feminization-sensitive genes that are prone to be suppressed with minimal degrees of feminization (Fig. 2D). Gene ontology analysis links this group of DEGs to “cell adhesion” and “male genitalia development” GO terms (Suppl. Table 4 (74)). Seven genes encoding collagens belong to this group (Fig. 3H, Suppl. Fig. 2), and some of them are located in the vicinities of the mesenchymal stem cell/epithelial-mesenchyme transition (MSC/EMT) gene TWIST2 (Fig. 3H and 3I) (85). Several DEGs involved in collagen synthesis and regulation were closely localized in chromosomes 7 and 20 (Fig. 4F); it is interesting to speculate that long-spanning epigenetic changes such as increased chromosomal accessibility might simultaneously regulate these DEGs. Another MSC/EMT gene LIFR (85) also belongs to the CTNNB1 group (Suppl. Fig. 2), implying possible roles of MSC/EMT in testicular development. Also in this group is LUM encoding lumican, a regulator of collagen fibril organization ((86), Suppl. Fig. 2). Feminization-suppressible expression of these DEGs suggests possible testis-specific roles of collagen fibrils and/or MSC/EMT, which may be relevant to the robust morphological changes of FemZZs. The CTNNB1 group also includes genes relevant to Wnt signaling (Fig. 3D), BMP signaling (Fig. 3E), pluripotency (Fig. 3F), and myosin light chain expression and regulation (Fig. 3G); these pathways may also play roles in testicular gonadal differentiation.
Characteristics of the feminization-resistant DEGs
Expression of the ACVR2B/SKV2L2 group DEGs was not significantly diminished, even partly, upon the EE2-induced feminization of ZZ-gonads, defining a transcriptomic hallmark of genetic male sex unaffected by phenotypic feminization. The persistent expression of the ACVR2B/SKV2L2 group DEGs in normal and feminized ZZ-gonads at day 19 (Figs. 2D and 2E, 3K–3P) implies their possible role as transcriptomic memory of the genetic sex. If this expression pattern is maintained beyond hatching, these DEGs may contribute to the posthatching re-masculinization of once feminized ZZ-gonads, which was reported to occur within 1 year of age (37).
The SKIV2L2 group DEGs include the master masculinization gene DMRT1 and a critical regulator of germline cell development KIT (Fig. 3K). This group also includes genes relevant to DNA repair (Fig. 3L), maintenance of chromosomal structure (Fig. 3M), cytoskeleton (Fig. 3N), and mitochondrial ribosome formation (Fig. 3O). The relevance of these pathways to possible transcriptomic memory of genetic sex in FemZZs needs to be investigated in future studies.
The feminization-resistant DEG are characterized by their very biased chromosomal localization—namely, the vast majority (21/25, 84%) of the ACVR2B/SKV2L2 group DEGs are localized in the sex chromosome Z (Fig. 2F, Suppl. Table 5 (75)). This is in stark contrast to the absence of Z-chromosomal enrichment among the feminization-sensitive, CTNNB1 group DEGs (Fig. 2F, Suppl. Table 5 (75)). Unlike mammalian sex chromosome X, avian chromosome Z is not subjected to global dosage compensation in ZZ-males (9, 14–21). Thus, in the chicken, Z-linked genes are expressed more strongly in ZZ-male cells than ZW-female cells by 1.4- to 1.8-fold across various tissues, including gonads (9, 87–89). Chromosome Z has been strongly “masculinized” over evolution—namely, this sex chromosome is enriched for male-biased DEGs as well as genes related to sex differentiation and reproduction (9, 15, 20, 89–94). Our observation that as many as 21% of all Z-linked genes followed the SKIV2L2/ACVR2B profile of differential expression, whereas no Z-linked genes were inducible upon feminization, agree with the highly masculinized aspect of this sex chromosome. Thus, our current study suggests a somewhat “stubborn” aspect of gene expression from chromosome Z, which might contribute to the CASI.
In normal chicken embryonic gonads, DMRT1 is strongly expressed in the proliferating germ cells in both differentiating ZW-ovaries and ZZ-testes and then suppressed soon after meiosis starts (11, 95). DMRT is also expressed in the somatic cells involved in seminiferous tubules formation (96). Guioli et al recently feminized ZZ-gonads through exposure to estradiol starting on days 7 to 7.5 or later and observed that the medulla of day-17 gonads consisted of somatic cells expressing female markers and cells expressing male markers (29). The medulla of the day-17 feminized gonads were overlaid by a cortex containing germ cells that did not properly enter meiosis (29). Consistent with their observations, our FemZZs (ie, EE2-feminized ZZ-gonads) contained SOX9-positive medullary cells on day 19, while they were overlaid by a clearly formed cortex (Fig. 5). The FS = 1 FemZZs (more weakly feminized) still maintained large numbers of SOX9-positive cells that apparently formed tubule-like structures throughout the medullary area, whereas FS = 2 FemZZs (more strongly feminized) showed smaller numbers of SOX9 restricted in the central regions of the medulla. In the medulla of both FS = 1 and FS = 2 FemZZs, DMRT1-positive cells were co-localized with the SOX9-positive cells, and some of them were identical to the SOX9-positive cells, but some of them were not. The SOX9+/DMRT1+ cells likely belonged to the Sertoli cell lineage of gonadal somatic cells, while the SOX9-/DMRT1+ medullary cells seemed to be germline cells. The cortex of both FemZZs contained large numbers of DMRT1-positive cells, showing a stark contrast to the ZW-ovarian cortex that was completely devoid of DMRT1-positive cells. Taken together, agreeing with the model proposed by Guioli et al that the medulla of estrogen-feminized ZZ-gonads are resistant to full feminization and that germline cells were blocked or delayed to enter meiosis (29), our data (Fig. 5) suggest that cells persistently expressing DMRT1 in FemZZs consist of the medullary, feminization-resistant somatic cells as well as the germline cells distributed in both the medullar and the cortex. Based on the numbers of DMRT1-positive cells in the cortex and the medulla, we predict that the cortex germline cells may contribute to the RNA-seq–detected total mRNA expression from the DMRT1 gene (Figs. 2E and 3K) in a greater degree than the medullary somatic/germline cells. The exact identity of the cortex DMRT1-positive cells (eg, germline cell lineage showing normal or blocked/delayed entry to meiosis) is not determined in our current study due to technical limitations and remained to be determined in future studies.
Characteristics of the FemZZ-specific DEGs
Our transcriptomic analysis identified DEGs specifically inducible in FemZZs (Figs. 2D and 2E, 4A and 4B; Suppl. Table 5 (75)). Although the reason of such an expression pattern is unknown, the presence of germline marker genes DAZL and DDX4/VASA (29, 97, 98) in this group of DEGs may imply a possibility that germline cells are overproliferated in FemZZs. This speculation is consistent with the recent histological study by Guioli et al, which showed meiotically compromised germline cells in the cortex of estradiol-feminized ZZ-testes (29). The clear separation of FemZZ DNA methylomes from those of normal gonads (Figs. 7A and 7B) may reflect possible over-representation of certain types of cell populations, although it is unknown as to whether such cells are within the context of normal sexual differentiation or transcriptionally aberrant, nonphysiological cells. Future studies involving histological analyses, including identification of cell populations expressing known sex-specific somatic and germline markers such as SOX9, FOXL2, DMRT1, and/or single cell RNA sequencing would be helpful to test the above speculations. We also identified DEGs specifically suppressible in FemZZs (Figs. 2D and 2E, 4C–4F; Suppl. Table 5 (75)), which included genes involved in mitochondria formation and suppression of mitochondria-dependent apoptosis (Fig. 4C and 4D), although functional roles of such genes in the FemZZ-suppressible expression are to be examined in future studies.
Differential expression of RSs in chicken embryonic gonads with varying degrees of feminization
Out of the approximately 700 000 RSs in the chicken genome (Suppl. Table 7 (81)), we identified nearly 500 differentially expressed RSs by unsupervised hierarchical clustering (Fig. 6A). On the heatmap, a cluster of RSs whose expression is specifically suppressed in ZW-ovaries (Cluster 1, 231 RSs), specifically induced in FemZZs (Cluster 2, 58 RSs) and specifically induced in ZW-ovaries (Cluster 3, 176 RSs) were identified (Fig. 6A). Expression of Cluster 1 RSs was largely maintained in both normal ZZ-testes and FemZZs, with no significant evidence of feminization-associated suppression, resembling the SKIV2L2/ACVR2B groups of protein-coding DEGs (Fig. 2D). However, in contrast to the SKIV2L2/ACVR2B group DEGs, Cluster 1 RSs were not enriched to sex chromosome Z (Figs. 2F and 6C, Suppl. Table 8 (82); note that each of Clusters 1 and 2 contained 1 unmapped RS). Because expression of RSs is often suppressed through epigenetic mechanisms such as heterochromatin formation (99), the large number of autosomal RSs strongly expressed in ZZ-gonads regardless of feminization suggests that a significant portion of autosomes in FemZZs may maintain the masculine types of epigenetic regulation of chromatin accessibility. The FemZZ-specific RS Cluster 2 distinguishes FemZZs from normal gonads (Fig. 6A and 6B). Although the reason of this expression pattern is presently unknown, these RSs may be relevant to germline cell development, as discussed earlier for protein-coding genes. Expression of Cluster 3 RSs in ZW-ovaries was extremely strong and, to our surprise, 94% to 97% (165–171 out of 176) of Cluster 3 RSs were linked to the female-specific sex chromosome W (Fig. 6C, Suppl. Table 8 (82)). The chicken W chromosome is a degraded version of chromosome Z, and it largely consists of heterochromatins and RSs with only ~25 protein coding genes therein (9, 30, 100). Our results indicate that a significant number of the RSs in chromosome W was expressed with exceptional strength that is rarely seen in autosomal or Z-linked RSs (Fig. 6A). This observation suggest that W-linked RSs may be localized outside the dominating heterochromatin regions.
In summary, our present study showed that a group of Z-linked genes, including the masculinization master gene DMRT1, specifically retain their testis-like strong mRNA expression in estrogen-feminized ZZ-male chicken embryonic gonads immediately before hatching. Testis-specific expression of autosomal RSs is also largely resistant to gonadal feminization. Transcriptomic and DNA methylome data are consistent with the notion that germline cells in FemZZs may be protected from apoptosis and maintain their bipotency of sexual differentiation. These characteristics of FemZZs may contribute to the (epi)genomic memory of genetic sex of temporarily feminized ZZ-gonads as well as CASI.
Acknowledgments
We appreciate Maria Tokuyama and Akiko Iwasaki for their help in modifying the ERVmap pipeline for quantitative detection of RNA transcripts expressed from chicken RSs. We also thank all members of the Shioda lab for their technical assistance.
Financial Support: This work was supported by the RICBAC Foundation and the US National Institutes of Health (grants R21ES024861, R01ES023316, and R01ES020454) to T.S.
Author Contributions: T.S. and K.J.I. conceived the study and designed the experiments. K.S., J.O., B.C., Misato K., Mutsumi K., and T.S. performed the experiments. K.S., B.C., Mutsumi K., and T.S. analyzed the data and wrote the manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All high-throughput sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under the accession code GSE160494. Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Adolfi MC, Carreira AC, Jesus LW, et al. Molecular cloning and expression analysis of dmrt1 and sox9 during gonad development and male reproductive cycle in the lambari fish, Astyanax altiparanae. Reprod Biol Endocrinol. 2015;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dang Z, Kienzler A. Changes in fish sex ratio as a basis for regulating endocrine disruptors. Environ Int. 2019;130:104928. [DOI] [PubMed] [Google Scholar]
- 3. Sakae Y, Oikawa A, Sugiura Y, et al. Starvation causes female-to-male sex reversal through lipid metabolism in the teleost fish, medaka (Olyzias latipes). Biol Open. 2020;9(4):bio050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Q, Ye D, Wang H, Wang Y, Sun Y. Zebrafish cyp11c1 knockout reveals the roles of 11-ketotestosterone and cortisol in sexual development and reproduction. Endocrinology. 2020;161(6):bqaa048. [DOI] [PubMed] [Google Scholar]
- 5. Wu K, Song W, Zhang Z, Ge W. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish - a novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis. Development. 2020;147(4):dev182758. [DOI] [PubMed] [Google Scholar]
- 6. Nagabhushana A, Mishra RK. Finding clues to the riddle of sex determination in zebrafish. J Biosci. 2016;41(1):145–155. [DOI] [PubMed] [Google Scholar]
- 7. Rich AL, Phipps LM, Tiwari S, Rudraraju H, Dokpesi PO. The increasing prevalence in intersex variation from toxicological dysregulation in fetal reproductive tissue differentiation and development by endocrine-disrupting chemicals. Environ Health Insights. 2016;10:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber C, Zhou Y, Lee JG, et al. Temperature-dependent sex determination is mediated by pSTAT3 repression of Kdm6b. Science. 2020;368(6488):303–306. [DOI] [PubMed] [Google Scholar]
- 9. Hirst CE, Major AT, Smith CA. Sex determination and gonadal sex differentiation in the chicken model. Int J Dev Biol. 2018;62(1-2-3):153–166. [DOI] [PubMed] [Google Scholar]
- 10. Kuroiwa A. Sex-determining mechanism in avians. Adv Exp Med Biol. 2017;1001:19–31. [DOI] [PubMed] [Google Scholar]
- 11. Guioli S, Nandi S, Zhao D, Burgess-Shannon J, Lovell-Badge R, Clinton M. Gonadal asymmetry and sex determination in birds. Sex Dev. 2014;8(5):227–242. [DOI] [PubMed] [Google Scholar]
- 12. Smith CA, Sinclair AH. Sex determination: insights from the chicken. Bioessays. 2004;26(2):120–132. [DOI] [PubMed] [Google Scholar]
- 13. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195(4):231–272. [DOI] [PubMed] [Google Scholar]
- 14. Arnold AP, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu Rev Genomics Hum Genet. 2008;9:109–127. [DOI] [PubMed] [Google Scholar]
- 15. Ellegren H. Emergence of male-biased genes on the chicken Z-chromosome: sex-chromosome contrasts between male and female heterogametic systems. Genome Res. 2011;21(12):2082–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellegren H, Hultin-Rosenberg L, Brunström B, Dencker L, Kultima K, Scholz B. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh Y, Melamed E, Yang X, et al. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuroda Y, Arai N, Arita M, et al. Absence of Z-chromosome inactivation for five genes in male chickens. Chromosome Res. 2001;9(6):457–468. [DOI] [PubMed] [Google Scholar]
- 19. Melamed E, Arnold AP. Regional differences in dosage compensation on the chicken Z chromosome. Genome Biol. 2007;8(9):R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naurin S, Hasselquist D, Bensch S, Hansson B. Sex-biased gene expression on the avian Z chromosome: highly expressed genes show higher male-biased expression. Plos One. 2012;7(10):e46854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q, Mank JE, Li J, Yang N, Qu L. Allele-specific expression analysis does not support sex chromosome inactivation on the chicken Z chromosome. Genome Biol Evol. 2017;9(3):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirst CE, Major AT, Ayers KL, et al. Sex reversal and comparative data undermine the W chromosome and support Z-linked DMRT1 as the regulator of gonadal sex differentiation in birds. Endocrinology. 2017;158(9):2970–2987. [DOI] [PubMed] [Google Scholar]
- 23. She ZY, Yang WX. Sry and SoxE genes: how they participate in mammalian sex determination and gonadal development? Semin Cell Dev Biol. 2017;63:13–22. [DOI] [PubMed] [Google Scholar]
- 24. Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346(6281):245–250. [DOI] [PubMed] [Google Scholar]
- 25. Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351(6322):117–121. [DOI] [PubMed] [Google Scholar]
- 26. Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–244. [DOI] [PubMed] [Google Scholar]
- 27. Ellis HL, Shioda K, Rosenthal NF, Coser KR, Shioda T. Masculine epigenetic sex marks of the CYP19A1/aromatase promoter in genetically male chicken embryonic gonads are resistant to estrogen-induced phenotypic sex conversion. Biol Reprod. 2012;87(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halldin K, Berg C, Brandt I, Brunström B. Sexual behavior in Japanese quail as a test end point for endocrine disruption: effects of in ovo exposure to ethinylestradiol and diethylstilbestrol. Environ Health Perspect. 1999;107(11):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guioli S, Zhao D, Nandi S, Clinton M, Lovell-Badge R. Oestrogen in the chick embryo can induce chromosomally male ZZ left gonad epithelial cells to form an ovarian cortex that can support oogenesis. Development. 2020;147(4):dev181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Major AT, Smith CA. Sex reversal in birds. Sex Dev. 2016;10(5–6):288–300. [DOI] [PubMed] [Google Scholar]
- 31. Jessl L, Lenz R, Massing FG, Scheider J, Oehlmann J. Effects of estrogens and antiestrogens on gonadal sex differentiation and embryonic development in the domestic fowl (Gallus gallus domesticus). Peerj. 2018;6:e5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith CA, Katz M, Sinclair AH. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol Reprod. 2003;68(2):560–570. [DOI] [PubMed] [Google Scholar]
- 33. Elbrecht A, Smith RG. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255(5043):467–470. [DOI] [PubMed] [Google Scholar]
- 34. Vaillant S, Magre S, Dorizzi M, Pieau C, Richard-Mercier N. Expression of AMH, SF1, and SOX9 in gonads of genetic female chickens during sex reversal induced by an aromatase inhibitor. Dev Dyn. 2001;222(2):228–237. [DOI] [PubMed] [Google Scholar]
- 35. Hudson QJ, Smith CA, Sinclair AH. Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary. Dev Dyn. 2005;233(3):1052–1055. [DOI] [PubMed] [Google Scholar]
- 36. Lambeth LS, Cummins DM, Doran TJ, Sinclair AH, Smith CA. Overexpression of aromatase alone is sufficient for ovarian development in genetically male chicken embryos. PLoS One. 2013;8(6):e68362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23(Suppl):S87–S92. [DOI] [PubMed] [Google Scholar]
- 38. Lambeth LS, Morris KR, Wise TG, et al. Transgenic chickens overexpressing aromatase have high estrogen levels but maintain a predominantly male phenotype. Endocrinology. 2016;157(1):83–90. [DOI] [PubMed] [Google Scholar]
- 39. Burke WH, Henry MH. Gonadal development and growth of chickens and turkeys hatched from eggs injected with an aromatase inhibitor. Poult Sci. 1999;78(7):1019–1033. [DOI] [PubMed] [Google Scholar]
- 40. Vaillant S, Dorizzi M, Pieau C, Richard-Mercier N. Sex reversal and aromatase in chicken. J Exp Zool. 2001;290(7):727–740. [DOI] [PubMed] [Google Scholar]
- 41. Morris KR, Hirst CE, Major AT, et al. Gonadal and endocrine analysis of a gynandromorphic chicken. Endocrinology. 2018;159(10):3492–3502. [DOI] [PubMed] [Google Scholar]
- 42. Zhao D, McBride D, Nandi S, et al. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464(7286):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clinton M, Zhao D, Nandi S, McBride D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012;20(1):177–190. [DOI] [PubMed] [Google Scholar]
- 44. Rosenthal NF, Ellis H, Shioda K, Mahoney C, Coser KR, Shioda T. High-throughput applicable genomic sex typing of chicken by TaqMan real-time quantitative polymerase chain reaction. Poult Sci. 2010;89(7):1451–1456. [DOI] [PubMed] [Google Scholar]
- 45. Owa C, Poulin M, Yan L, Shioda T. Technical adequacy of bisulfite sequencing and pyrosequencing for detection of mitochondrial DNA methylation: sources and avoidance of false-positive detection. PLoS One. 2018;13(2):e0192722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 1 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401030. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 48. Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47(8):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nikolayeva O, Robinson MD. edgeR for differential RNA-seq and ChIP-seq analysis: an application to stem cell biology. Methods Mol Biol. 2014;1150:45–79. [DOI] [PubMed] [Google Scholar]
- 50. Chamorro-Garcia R, Diaz-Castillo C, Shoucri BM, et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat Commun. 2017;8(1):2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 2 – Transcriptomaicand epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401141. Accessed October 20, 2020. [DOI]
- 52. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Figure 1 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401180. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 53. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saldanha AJ. Java Treeview – extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. [DOI] [PubMed] [Google Scholar]
- 55. Mitsunaga S, Odajima J, Yawata S, et al. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc Natl Acad Sci U S A. 2017;114(46):E9913–E9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dennis G Jr, Sherman BT, Hosack DA, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 57. Tokuyama M, Kong Y, Song E, Jayewickreme T, Kang I, Iwasaki A. ERVmap analysis reveals genome-wide transcription of human endogenous retroviruses. Proc Natl Acad Sci U S A. 2018;115(50):12565–12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huda A, Jordan IK. Analysis of transposable element sequences using CENSOR and RepeatMasker. Methods Mol Biol. 2009;537:323–336. [DOI] [PubMed] [Google Scholar]
- 59. Tempel S. Using and understanding RepeatMasker. Methods Mol Biol. 2012;859:29–51. [DOI] [PubMed] [Google Scholar]
- 60. Diaz-Castillo C, Chamorro-Garcia R, Shioda T, Blumberg B. Transgenerational self-reconstruction of disrupted chromatin organization after exposure to an environmental stressor in mice. Sci Rep. 2019;9(1):13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taylor JA, Shioda K, Mitsunaga S, et al. Prenatal exposure to bisphenol A disrupts naturally occurring bimodal DNA methylation at proximal promoter of fggy, an obesity-relevant gene encoding a carbohydrate kinase, in gonadal white adipose tissues of CD-1 mice. Endocrinology. 2018;159(2):779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lienhard M, Grimm C, Morkel M, Herwig R, Chavez L. MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics. 2014;30(2):284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ramirez F, Ryan DP, Gruning B, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44(W1):W160–W165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. RRID:AB_2877113.
- 66. RRID:AB_2194160.
- 67. RRID:AB_2087193.
- 68. RRID:AB_2571722.
- 69. RRID:AB_2725786.
- 70. Xu M, Chen L. An empirical likelihood ratio test robust to individual heterogeneity for differential expression analysis of RNA-seq. Brief Bioinform. 2018;19(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 3 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401147. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 72. Feng Y, Peng X, Li S, Gong Y. Isolation and characterization of sexual dimorphism genes expressed in chicken embryonic gonads. Acta Biochim Biophys Sin (Shanghai). 2009;41(4):285–294. [DOI] [PubMed] [Google Scholar]
- 73. Carré GA, Couty I, Hennequet-Antier C, Govoroun MS. Gene expression profiling reveals new potential players of gonad differentiation in the chicken embryo. PLoS One. 2011;6(9):e23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 4 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401153. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 75. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 5 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401156. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 76. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Figure 2 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401186. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 77. Ayers KL, Lambeth LS, Davidson NM, Sinclair AH, Oshlack A, Smith CA. Identification of candidate gonadal sex differentiation genes in the chicken embryo using RNA-seq. BMC Genomics. 2015;16:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 6m – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401162. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 79. Onderak AM, Anderson JT. Loss of the RNA helicase SKIV2L2 impairs mitotic progression and replication-dependent histone mRNA turnover in murine cell lines. Rna. 2017;23(6):910–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McRae HM, Garnham AL, Hu Y, et al. PHF6 regulates hematopoietic stem and progenitor cells and its loss synergizes with expression of TLX3 to cause leukemia. Blood. 2019;133(16):1729–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 7 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401165. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 82. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 8 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401171. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 83. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Figure 3 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401189. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 84. Shioda K, Odajima J, Cordazzo B, Isselbacher KJ, Shioda T. Data from: Supplemental Table 9 – Transcriptomic and epigenetic preservation of genetic sex identity in estrogen-feminized male chicken embryonic gonads. NIH Figureshare. Deposited May 30, 2020. ProMED-mail website. 10.35092/yhjc.12401177. Accessed October 20, 2020. [DOI] [PMC free article] [PubMed]
- 85. Gurung S, Williams S, Deane JA, Werkmeister JA, Gargett CE. The transcriptome of human endometrial mesenchymal stem cells under TGFβR inhibition reveals improved potential for cell-based therapies. Front Cell Dev Biol. 2018;6:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karamanou K, Perrot G, Maquart FX, Brézillon S. Lumican as a multivalent effector in wound healing. Adv Drug Deliv Rev. 2018;129:344–351. [DOI] [PubMed] [Google Scholar]
- 87. Arnold AP, Itoh Y. Factors causing sex differences in birds. Avian Biol Res. 2011;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McQueen HA, Clinton M. Avian sex chromosomes: dosage compensation matters. Chromosome Res. 2009;17(5): 687–697. [DOI] [PubMed] [Google Scholar]
- 89. Wright AE, Moghadam HK, Mank JE. Trade-off between selection for dosage compensation and masculinization on the avian Z chromosome. Genetics. 2012;192(4):1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kaiser VB, Ellegren H. Nonrandom distribution of genes with sex-biased expression in the chicken genome. Evolution. 2006;60(9):1945–1951. [PubMed] [Google Scholar]
- 91. Mank JE, Ellegren H. All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity (Edinb). 2009;102(3):312–320. [DOI] [PubMed] [Google Scholar]
- 92. Storchová R, Divina P. Nonrandom representation of sex-biased genes on chicken Z chromosome. J Mol Evol. 2006;63(5):676–681. [DOI] [PubMed] [Google Scholar]
- 93. Mank JE, Axelsson E, Ellegren H. Fast-X on the Z: rapid evolution of sex-linked genes in birds. Genome Res. 2007;17(5):618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mořkovský L, Storchová R, Plachý J, Ivánek R, Divina P, Hejnar J. The chicken Z chromosome is enriched for genes with preferential expression in ovarian somatic cells. J Mol Evol. 2010;70(2):129–136. [DOI] [PubMed] [Google Scholar]
- 95. Omotehara T, Smith CA, Mantani Y, et al. Spatiotemporal expression patterns of doublesex and mab-3 related transcription factor 1 in the chicken developing gonads and Mullerian ducts. Poult Sci. 2014;93(4):953–958. [DOI] [PubMed] [Google Scholar]
- 96. Lambeth LS, Raymond CS, Roeszler KN, et al. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev Biol. 2014;389(2):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim YM, Han JY. The early development of germ cells in chicken. Int J Dev Biol. 2018;62(1-2-3):145–152. [DOI] [PubMed] [Google Scholar]
- 98. Yang SY, Lee HJ, Lee HC, et al. The dynamic development of germ cells during chicken embryogenesis. Poult Sci. 2018;97(2):650–657. [DOI] [PubMed] [Google Scholar]
- 99. Allshire RC, Madhani HD. Ten principles of heterochromatin formation and function. Nat Rev Mol Cell Biol. 2018;19(4):229–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bellott DW, Skaletsky H, Cho TJ, et al. Avian W and mammalian Y chromosomes convergently retained dosage-sensitive regulators. Nat Genet. 2017;49(3):387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All high-throughput sequencing data that support the findings of this study have been deposited in the Gene Expression Omnibus (GEO) under the accession code GSE160494. Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.