Abstract
This special report was developed to communicate policy and procedures for free-standing acute inpatient rehabilitation hospitals (AIRHs) to protect patients and healthcare personnel and to prevent further spread of severe acute respiratory syndrome coronavirus 2. The recommended policies were developed in conjunction with the New Mexico Department of Health and hospital leadership. As we attain additional knowledge and experience during this pandemic, suggestions of best practice will continue to evolve for AIRHs. The authors encourage readers to work with local regulatory officials to ensure regulatory compliance as well as respect of the availability of local resources.
Keywords: : acute rehabilitation, coronavirus disease, COVID-19, healthcare policy, severe acute respiratory syndrome coronavirus 2
Lay abstract
This special report communicates potential policy and procedure modifications for free-standing acute inpatient rehabilitation hospitals in response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease (COVID-19) pandemic. The goal of the communication is to help protect patients and healthcare personnel and to prevent further spread of SARS-CoV-2. The recommendations were developed in conjunction with the New Mexico Department of Health and hospital leadership. A comprehensive response policy to SARS-CoV-2/COVID-19 should include facility changes, enhanced personal protective equipment policy, enhanced admission policy and enhanced surveillance testing policy. In the setting of rapidly changing state and federal guidelines, hospital staff should work with state officials to ensure that potential policy and procedure modifications are in line with state and federal recommendations.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the infectious source for coronavirus disease (COVID-19) was first reported on 31 December 2019 [1]. As of 15 November 2020, there were 10,690,665 total positive cases of COVID-19 and 243,580 COVID-19 related deaths in the United States [2]. In New Mexico, there have been 63,171 total cases of individuals testing positive for COVID-19 out of 1,360,452 tests administered (4.6% positive) and 1208 COVID-related deaths [3]. There have also been 5523 total hospitalizations, with 480 being hospitalized as of 15 November 152020 [4]. In Bernalillo County, there have been 16,194 cases of individuals testing positive for COVID-19 (out of 439,038; 3.7% positive), with 256 deaths.
The rapid spread and sheer number of cases have exacted a global and national toll on the healthcare system as healthcare professionals balance the need for continued patient care with the need to control the spread of the virus [5]. This has been the case for all levels of patient care, including acute inpatient rehabilitation facilities, with societies such as the American Academy of Physical Medicine and Rehabilitation (AAPM&R) providing recommendations and resources to support care in the time of COVID-19 [6]. However, the authors agree with the AAPM&Rs statement that “Congress has been primarily focused on keeping COVID patients alive but hasn't moved on to considering what happens to patients after they survive. Many of these patients are still struggling with potentially long-term difficulties” [7]. More importantly, there has not been a consistent framework provided for free-standing acute inpatient rehabilitation facilities admitting patients with previous diagnosis of COVID-19, nor is there universally accepted guidelines for limiting the exposure of COVID-19 to the high-risk acute inpatient rehabilitation populations in these facilities.
Our free-standing acute inpatient rehabilitation facility is in a unique position because it is one of only four such facilities in the state of New Mexico capable of providing complex acute inpatient rehabilitation services. Our rehabilitation hospital is the only hospital in New Mexico accredited by the Commission on Accreditation of Rehabilitation Facilities for 3 years in six programs: Comprehensive Integrated Inpatient Rehabilitation Program, Inpatient Brain Injury Program, Inpatient and Outpatient Spinal Cord System of Care, Inpatient Stroke Specialty Program, Outpatient Medical Rehabilitation Program, and Outpatient Medical Rehabilitation Program (Children and Adolescents). The hospital has 265 employees with 43 contractors. There were 62 licensed acute inpatient rehabilitation beds and 1470 inpatient admissions in 2019. The hospital provides a full continuum of inpatient and outpatient rehabilitation care, including physical therapy, occupational therapy, speech and language pathology, rehabilitation nursing and case management services. The inpatient program is designed for patients with a variety of rehabilitation needs, offering general inpatient medical rehabilitation and three specialized programs: brain injury rehabilitation, stroke rehabilitation and spinal cord injury rehabilitation. Details of the program are supplied as Supplementary material to this article and can be found online [8].
On 6 July 2020, senior hospital leadership was informed of a patient who discharged on 3 July 2020 then tested positive for COVID-19 two days after being discharged. In response to this notification, all patients and staff were tested that same week as part of a swift and organized response. Testing resulted in identification of three patients and four staff members with positive COVID-19 tests. Given the acute inpatient rehabilitation population at our facility (e.g., older adults often with underlying chronic medical conditions) is at high risk of being affected by respiratory pathogens such as COVID-19, a comprehensive assessment was made with resulting action plans (Table 1).
Table 1. . SARS-CoV-2/COVID-19 action plan and desired outcomes.
| Action plan | Desired outcome |
|---|---|
| All hospital filters to be changed to MERV-13 | Prevent or reduce the airborne transfer of infectious organisms and viruses through airborne transmission |
| Enhanced PPE Policy | Enhanced PPE (i.e., KN95, face shields) delivered and deployed |
| SARS-CoV-2/COVID-19 Enhanced Admission Policy | Prevent COVID-19–positive patient admission |
| SARS-CoV-2/COVID-19 Enhanced Surveillance Testing Policy | Identify staff and patient COVID-19 exposure and asymptomatic active infections |
| SARS-CoV-2/COVID-19 Updated Facility Policy | Maintain average census |
| Response to Positive SARS-CoV-2/COVID-19 Test | Limit likelihood of an undiagnosed COVID-19 exposure for staff and patients |
MERV-13: Minimum efficiency reporting value-13; PPE: Personal protective equipment; SARS-CoV-2/COVID-19: Severe acute respiratory syndrome coronavirus 2/coronavirus disease.
Our hospital has an established infection prevention and control program. It became clear, however, that new measures were needed in the setting of this unique pandemic. The medical director, in conjunction with state officials from the Department of Health and senior hospital leadership, developed new procedures and policies as detailed below.
Goals & specific aims of proposed SARS-CoV-2/COVID-19 enhanced policy to limit airborne transfer, enhanced admission, enhanced surveillance & updated facility policy
The goal of the SARS-CoV-2/COVID-19 specific policies and procedures is to protect patients and healthcare personnel and to prevent further spread of the virus. These policies and procedures may be of benefit to other free-standing acute inpatient rehabilitation facilities.
The specific aims of the program are as follows:
Identify SARS-CoV-2 exposures
Contain SARS-CoV-2 exposures
Safely maintain average daily census
The efficient and timely identification of SARS-CoV-2 exposures mitigates risk of an outbreak across our hospital system and protects the vulnerable patient population. Containment limits exposures to a single unit, allowing for continued patient flow in other units, ultimately allowing for uninterrupted admission and the ability to maintain the average census. This enables ongoing, high-level rehabilitation services while also allowing admission from overwhelmed acute care hospitals.
SARS-CoV-2/COVID-19 enhanced policy to limit airborne transfer
To help prevent or reduce airborne transfer of infectious organisms and viruses through airborne transmission, all hospital filters were changed to minimum efficiency reporting value-13 (MERV-13). The MERV-13 filters are reported to filter 90% of particles in the 3–10 μm range, 85% of particles in the 1–3 μm range and 50% of particles in the range 0.3–1 μm range (exact ranges differ by brand, and the reader is encouraged to work with local officials to determine the most appropriate choice).
Enhance personal protective equipment (PPE) (i.e., KN95 and face shields) were delivered and deployed to hospital staff. This enhanced PPE protocol was made mandatory; the use of cloth, homemade masks was initially discouraged and later banned.
SARS-CoV-2/COVID-19 enhanced admission policy
Revised patient admission policy
All potential patients must be free of symptoms typically associated with SARS-CoV-2 for 7 days before admission, unless attributed to other etiologies (e.g., negative for COVID-19 and positive for influenza)
All potential patients not previously identified as COVID-19 positive are required to have at least one negative test within 72 h before admission
Patients previously identified as COVID-19 positive require two negative tests >24 h apart before admission
SARS-CoV-2/COVID-19 enhanced surveillance testing policy
Patient and staff testing
All newly admitted patients are tested upon admission regardless of the last COVID-19 test
All admitted patients tested weekly
Weekly rotating surveillance testing of 25% hospital staff
SARS-CoV-2/COVID-19 updated facility policy
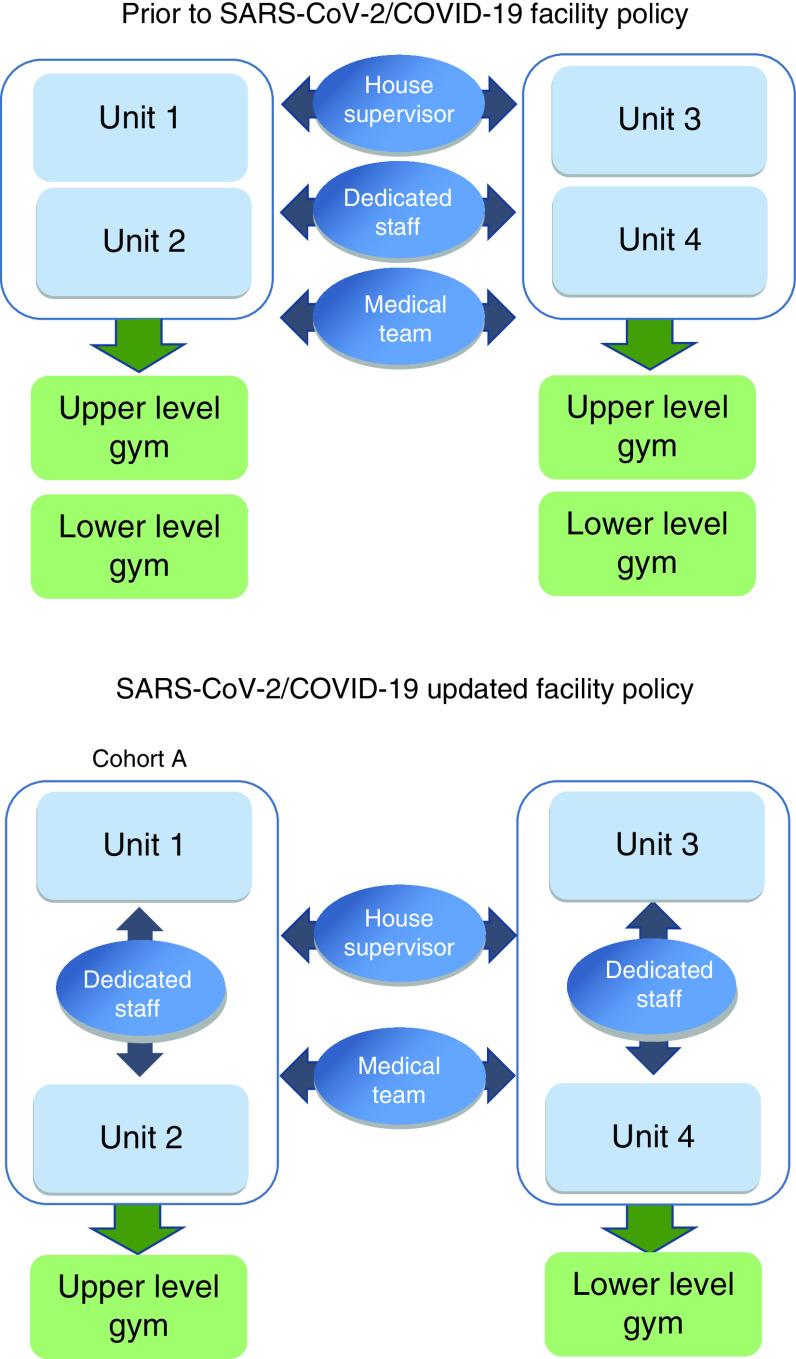
Cohorts established with designated units, rehabilitation gym space, nursing, therapy and ancillary staff (Figure 1)
Each cohort consisting of 31 patient rooms (double occupancy) with dedicated nursing, therapy (assigned to unit) and ancillary staff (assigned to cohort)
Nursing, therapy and ancillary staff cannot visit/aid units not in designated cohort
Social distancing procedures [9] are enforced throughout facility including nursing stations, break rooms, work rooms and gyms
Figure 1. . SARS-CoV-2/COVID-19 updated facility policy.
Dedicated staff: nursing, therapy and ancillary staff. Medical team: medical doctors, nurse practitioners and physician assistants.
SARS-CoV-2/COVID-19: Severe acute respiratory syndrome coronavirus 2/coronavirus disease.
Response to positive SARS-CoV-2/COVID-19 test
Patients identified as positive through the admission testing or weekly surveillance testing process will be immediately transferred to acute care hospital for medical triage and treatment
Affected unit would be quarantined for at least 2 weeks:
— Comingling between adjacent units in cohort will cease immediately and continue to be prohibited until such time as the quarantine is lifted
— No new patients admitted to affected unit and no patients will be transferred out of affected unit other than to discharge (acute, home, or other appropriate facility)
Designated staff responsible for cohort in which patient was assigned will undergo immediate testing and subsequent weekly testing until no new positive results are detected
Discussion
The goal of the developed policy and procedures is to protect patients and healthcare personnel and to prevent further spread of SARS-CoV-2. The policies were developed in conjunction with an infectious disease epidemiologist at the New Mexico Department of Health. The authors feel that these processes and procedures may be of benefit to other free-standing acute inpatient rehabilitation facilities.
These suggestions for updated facility policies in response to the SARS-CoV-2/COVID-19 pandemic are in line with the experience of other facilities [10–15]. As we attain additional knowledge and experience during this pandemic, suggestions of best practice will continue to evolve for acute inpatient rehabilitation hospitals. However, as we develop policies and procedures, prior practice guidelines, such as those learned from severe acute respiratory syndrome should be considered [15].
The development of our hospital's policies and procedures follows current recommendations and prior experience with respiratory pandemics. In the setting of rapidly changing state and federal guidelines we also worked with New Mexico state officials to ensure our recommendations were in line with state and federal recommendations. The authors encourage readers to work with local regulatory officials to ensure any polices or procedures developed are within regulatory compliance, respects the availability of local resources and addresses specific local conditions.
Future perspective
There will be a need to develop detailed federal and national guidelines for acute inpatient rehabilitation hospitals in response to SARS-CoV-2/COVID-19 pandemic and future viral pandemics. These guidelines will ensure rehabilitation hospital policies and procedures are within regulatory compliance, respect the availability of national and local resources and appropriately address the evolving pandemic. In the absence of such guidelines, rehabilitation hospitals will have to work with state officials to develop guidelines in response to the SARS-CoV-2/COVID-19 pandemic.
Executive summary.
Policy and procedures should be developed for acute inpatient rehabilitation hospitals to protect patients and healthcare personnel and to prevent further spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease (COVID-19).
In the setting of rapidly changing state and federal guidelines hospital staff should work with state officials to ensure recommendations are in line with state and federal recommendations.
A comprehensive response policy to SARS-CoV-2/COVID-19 should include facility changes, enhanced personal protective equipment policy, enhanced admission policy and enhanced surveillance testing policy.
Footnotes
Author contributions
R Ehsanian, JH Sloan and J Workman: formal analysis, methodology; project administration; visualization; writing – original draft; writing – review and editing. D Jones: resources; project administration; writing – review and editing. WE Rivers: formal analysis; writing – review and editing. AK Manole: writing – review and editing.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
For studies reporting the original results of a clinical trial or the secondary analysis of clinical trial data, authors should include a data sharing statement, as described on the ICMJE website: http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html. Authors are asked to specify whether their manuscript reports either the original results of a clinical trial, or the secondary analysis of clinical trial data that have been shared with them, and in either case include a suitable data sharing statement. Further information, and example wording, can be found in the Author Guidelines on our website.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.WorldHealth Organization. Novel Coronavirus – China (2020). www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 2.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) in the U.S., Centers for Disease Control and Prevention, Nov. 15, 2020. (2020). www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 3.New Mexico Department of Health. Coronavirus Updates – coronavirus updates in New Mexico. (2020). https://cv.nmhealth.org/
- 4.New Mexico Department of Health. COVID-19 public dashboard. (2020). https://cv.nmhealth.org/dashboard
- 5.Rosenbaum L. The untold toll – the pandemic's effects on patients without COVID-19. N. Engl. J. Med. 382(24), 2368–2371 (2020). [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Physical Medicine and Rehabilitation. COVID-19. (2020). www.aapmr.org/news-publications/covid-19
- 7.American Academy of Physical Medicine and Rehabilitation. Regulation changes. (2020). www.aapmr.org/news-publications/covid-19/regulation-changes
- 8.LoveLace. LoveLace UNM Rehabilitation Hospital 2018 Report Card. (2020). https://lovelace.com/sites/default/files/file-uploads/LRH_UNM_report%20card_2018%20%281%29.pdf
- 9.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) – prevention & treatment. (31 July 2020). (2020). www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
- 10.McNeary L, Maltser S, Verduzco-Gutierrez M. Navigating coronavirus disease 2019 (Covid-19) in physiatry: a CAN report for inpatient rehabilitation facilities. PMR 12(5), 512–515 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Chang MC, Park D. How should rehabilitative departments of hospitals prepare for coronavirus disease 2019? Am. J. Phys. Med. Rehabil. 99(6), 475–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 323(20), 2007–2008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choon-Huat Koh G, Hoenig H. How should the rehabilitation community prepare for 2019-nCoV? Arch. Phys. Med. Rehabil. 101(6), 1068–1071 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negrini S, Ferriero G, Kiekens C, Boldrini P. Facing in real time the challenges of the COVID-19 epidemic for rehabilitation. Eur. J. Phys. Rehabil. Med. 56(3), 313–315 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Lim PA, Ng YS, Tay BK. Impact of a viral respiratory epidemic on the practice of medicine and rehabilitation: severe acute respiratory syndrome. Arch. Phys. Med. Rehabil. 85(8), 1365–1370 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]