Abstract
Through a genome-wide analysis of bone mineral density (BMD) and muscle mass, identification of a signaling pattern on 17p11.2 recognized the presence of sterol regulatory element-binding factor 1 (SREBF1), a gene responsible for the regulation of lipid homeostasis. In conjunction with lipid-based metabolic functions, SREBF1 also codes for the protein, SREBP-1, a transcription factor known for its role in adipocyte differentiation. We conducted a quantitative correlational study. We established a zebrafish (ZF) SREBF1 knockout (KO) model and used a targeted customized lipidomics approach to analyze the extent of SREBF1 capabilities. For lipidomics profiling, we isolated the dorsal muscles of wild type (WT) and KO fishes, and we performed liquid chromatography-tandem mass spectrometry screening assays of these samples.
In our analysis, we profiled 48 lipid mediators (LMs) derived from various essential polyunsaturated fatty acids to determine potential targets regulated by SREBF1, and we found that the levels of 11,12 epoxyeicosatrienoic acid (11,12-EET) were negatively associated with the number of SREBF1 alleles (P = 0.006 for a linear model). We also compared gene expression between KO and WT ZF by genome-wide RNA-sequencing. Significantly enriched pathways included fatty acid elongation, linoleic acid metabolism, arachidonic acid metabolism, adipocytokine signaling, and DNA replication. We discovered trends indicating that BMD in adult fish was significantly lower in the KO than in the WT population (P < 0.03). These studies reinforce the importance of lipidomics investigation by detailing how the KO of SREBF1 affects both BMD and lipid-signaling mediators, thus confirming the importance of SREBF1 for musculoskeletal homeostasis.
Keywords: muscle and bone, lipid mediators, bone mineral density, gene expression, zebrafish
Genetic pleiotropy occurs when mutations in 1 gene affect at least 2 or multiple, seemingly unrelated, organs and phenotypes. These genetic alterations and their subsequent impacts on phenotypic characteristics may provide key information in correlating musculoskeletal (MSK) diseases with unique deoxyribonucleic acid (DNA) variants. This is supported by growing scientific evidence of the multiple mechanisms involved in the intricate co-regulation of bones and muscles via endocrine and paracrine feedback loops (1–5). These molecular signaling tracks include the growth hormone/insulin-like growth factor-1 (IGF-1) axis, sex steroid pathway, inflammation/tumor necrosis factor-α (TNF-α) pathway (reviewed in (1)), Wnt/β-catenin pathway (6), prostaglandin E2 (PGE2) pathway (7, 8), and the beta-aminoisobutyric acid signaling pathway (9). Even so, the list of biological pathways responsible for bone and muscle-related traits is continuously built upon in the scientific community and is far from complete. In fact, many organs and tissues communicate via yet unknown molecular pathways. For example, adipose tissue shares progenitors with bones (10, 11) and also secretes fatty acids (FAs), which may accumulate in the muscle. Not surprisingly, since muscles, adipose, and bone tissues are 3 key components of the body’s overall composition, there is increased interest to expand the understanding of the regulation of pleiotropic genetic effects on bone and muscle in health, disease, and during aging. Recently, we performed a bivariate genome-wide association study (GWAS) meta-analysis for paired bone-muscle traits (muscle [lean] mass and bone mineral density [BMD]) in children (12). The results of this bivariate GWAS identified several genomic loci influencing both traits (association P < 5 × 10−8). One signal was mapped to the locus at 17p11.2, the short-arm portion of chromosome 17. Ensuing bioinformatics analyses indicated that sterol regulatory element-binding factor 1 (SREBF1) is the most likely gene driving the associations of this chromosomal locus with both lean mass and BMD. It was also generally found that alleles from the single nucleotide polymorphisms (SNPs) at 17p11.2 were associated with higher BMD and lower lean mass.
SREBF1 codes for the protein, SREBP-1, a transcription factor expressed ubiquitously, although research suggests the expression is stronger in lipogenic tissues due to its key function in inducing liver lipogenesis. In humans, variants of the SREBF1 gene were found to associate with type 2 diabetes, glycemia, and insulin resistance (13). During human mesenchymal stem cell (hMSC) differentiation, the expression of SREBF1 was shown to peak at the onset of osteoblast mineralization. However, in mouse calvaria-derived cells, a model for osteoblastogenesis, SREBF1 was consistently expressed without displaying major changes in the transition from the preosteoblastic to mature osteoblastic stages (12). A further analysis shows that in skeletal muscle, the protein SREBP-1 indirectly downregulates the expression of MYOD1, MYOG, and MEF2C, and, interestingly, an overexpression of SREBP-1 produces an inhibition of myoblast-to-myotube differentiation and subsequently leads to loss of muscle-specific proteins in differentiated myotubes (14, 15). To note, SREBP-1 has also been shown to interact with lamin A, an intermediate filament protein implicated in the suppression of adipogenesis (16) and in functional disease processes such as muscular dystrophy. In agreement with the genetic associations (12), an overexpression of SREBF1 has been linked to decreases in muscle protein catabolism and an inverse rise in osteoblast mineralization.
In expansion of the aforementioned concept, SREBF1 is a known adipocyte differentiation factor, and since it directly regulates the transcription of over 200 genes involved in the de novo synthesis of FAs, triglycerides, and cholesterol (17–19), it raises its potential role in lipid homeostasis. The reciprocal relationship between the differentiation of adipocytes and osteoblasts from MSCs in the bone marrow (3) has been well documented (20). Through a molecular analysis of these inverse functions, we were able to identify key alterations in the homeostatic stability between adipogenesis and osteoblastogenesis and link this disequilibrium to the phenotype of reduced bone mineral quality in zebrafish (ZF) lacking SREBF1. This concept is illustrated via the identification of small molecule T63, a transcriptional activator that promotes osteoblastic differentiation and bone formation (21), thereby suppressing osteoporotic mechanisms within the body and maintaining physiologic BMD scores.
Lipids are essential cellular and extracellular molecules with important roles in nutrition and human health and disease. A very clear example is lipotoxicity, a multifactorial condition where increased circulating levels of lipids and metabolic alterations in utilization of FAs are often associated with functional impairments such as the onset of insulin resistance in skeletal muscle and cardiac dysfunction in obese and diabetic individuals. Various lipids, globally referred to as lipid mediators (LMs), are also important intra- and intercellular signaling molecules and include ligands to G-protein-coupled receptors and transcription factors, allosteric modulators, and direct covalent modifiers of proteins. Furthermore, the heterogeneity of acyl chains within general classes of lipids can result in distinct cellular signaling properties of critical pathophysiological relevance.
Of particular interest to the present study is a class of lipid-signaling molecules known as epoxyeicosatrienoic acids (EETs), metabolites formed from the synthesis of arachidonic acid (AA) through cytochrome P450/epoxygenase. These autocrine effectors associated with insulin signaling and inflammation have been linked to the positive exertion of cardioprotective mechanisms (22). Epoxyeicosatrienoic acids were also involved in osteoclast formation and activities through modulation of multiple pathways to suppress receptor activator of NF-κB ligand (RANKL) signaling (23). In the last decade it has been reported that NF-κB signaling mediates RANKL-induced osteoclastogenesis, and inhibition of NF-κB was an effective approach to inhibit osteoclast formation and bone resorptive activity (24). Knowledge from lipidomics profiling regarding EETs allows for the further identification and analysis of EET signaling on inflammatory pathogenesis and bone formation/resorption in MSK diseases. Taking everything into account, this emerging evidence points to an important role of lipid metabolism and lipid signaling as a crucial influence in MSK pathophysiology. When combined with the specific roles of lipids in inflammation and tissue regeneration, it is of paramount importance to decipher pathways coordinating these systemic responses.
To date, several knockout (KO) and/or transgenic mouse models have been established to study the function of SREBP pathway-related genes (25). The phenotypes of these published germline and tissue-specific KO and transgenic mice for the SREBP pathway were limited to reduced FA synthesis, enhanced insulin secretion, and altered inflammatory response in different organs such as the liver (26), intestine (27), pancreas (28), and immune system (29). Currently, to our knowledge, there is no data showing the direct effect of SREBP on bone and muscle, but conditional deletion of membrane-bound transcription factor proteinase, site 1 (Mbtps1) in bone, led to an increase in the expression of SREBF1, resulted in an age-related slow twitch muscle (soleus) phenotype with increased contractile force and size, as well as a 25% increase in the stiffness of bone. The reciprocal regulation between Mbtps1 and SREBF1 is complex, and further research is needed for understanding the function of SREBP in bone and muscle (30), but seems to suggest a positive correlation, in that, a higher amount of the gene associated with higher MSK function.
Zebrafish possess a rapid external development, amenability to mutagenesis, a relatively small genome, and short generation time. Their muscles are reminiscent of the mammal’s and the osseous matter of adult ZF show similar properties to their human counterpart, suggesting that it may be used as a successful model to study general human bone and MSK diseases (31). Another major advantage of ZF includes that of large clutches, which allow for the pooling of multiple siblings rather than a single individual animal. Each of these traits make the ZF a valuable genetic model to test the hypothesis that a human GWAS finding has a biological functional meaning, and determines whether this template can be further utilized for a better understanding of the age-related MSK impacts that lipid signaling have within the body. In this study we established a ZF SREBF1 KO model, which was selected due to its pleiotropic MSK nature and key lipid metabolism function. We used this new model to profile and quantify bone density and skeletal muscles for LMs in adult fish. The results are striking: SREBF1 KO ZF displaying phenotypic bone changes (ie, age- and sex-adjusted BMD was significantly lower in SREBF1-/- fish than in their WT siblings) associated with specific lipid modifications. These studies advance insights of lipid signaling in the MSK system, establish a new ZF model suitable for validation of GWAS, and demonstrate the key roles of EETs in optimal MSK health.
Materials and Methods
Chemicals and reagents
Sixteen isotope-labeled LM internal standards (IS) including arachidonic acid-d8 (AA-d8), 6-keto prostaglandin F1α-d4 (6-keto-PGF1α-d4), prostaglandin F2α-d4 (PGF2α-d4), prostaglandin E2-d4 (PGE2-d4), prostaglandin D2-d4 (PGD2-d4), thromboxane B2-d4 (TXB2-d4), leukotriene B4-d4 (LTB4-d4), leukotriene C4-d5 (LTC4-d5), 5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (5-HETE-d8), 15S-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (15-HETE-d8), 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic-5,6,8,9,11,12,14,15-d8 acid (12-HETE-d8), platelet-activating factor C-16-d4 (PAF C-16-d4), tetranor-prostaglandin E metabolite-d6 (tetranor-PGEM-d6), oleoyl ethanolamide-d4 (OEA-d4), docosahexaenoic acid-d5 (DHA-d5), and eicosapentaenoic acid-d5 (EPA-d5) were purchased from Cayman Chemical Co. (Ann Arbor, Michigan). Formic acid (reagent grade, ≥95%) was obtained from Sigma–Aldrich (St. Louis, Missouri). HPLC-MS–grade acetonitrile, water, methanol, and ethanol were purchased from J.T. Baker (Phillipsburg, New Jersey).
Zebrafish housing and maintenance
Zebrafish (Danio rerio) of AB strain were maintained at 28°C under 14-hour light, 10-hour dark cycles at the ZF research facility (Bar Ilan University [BIU] Azrieli campus). All animal research was approved by BIU IACUC protocol No. 023-b7241-24.
Genetic modification of the SREBF1 gene
CRISPR-Cas9 clustered regularly interspaced short palindromic repeats (CRISPR) was used to KO SREBF1 in ZF (32). The plasmids pCS2-nCas9n (47929) and pT7-gRNA (46759) were purchased from Addgene (Cambridge, Massachusetts). Two DNA oligomers (5`- TAGGGGTCATGGAGAGACAAAC-3` and 5`-AAACGTTTGTCTCTCCATGACC-3`) were annealed and ligated in to the pT7-gRNA plasmid after it was digested with the restriction enzymes BsmBI, BglII, and SalI. Next, pT7-gRNA plasmid was linearized using BamHI and used for in vitro transcription of the pT7-gRNA by T7 RNA polymerase (New England Biolabs, Ipswich, Massachusetts). This was followed by purification of the samples using MicroSpin G-50 Columns (GE Healthcare, Marlborough, Massachusetts). In the creation of Cas9 mRNA, the template DNA (pCS2-nCas9n) was linearized by NotI and purified using a QIAprep column (Qiagen, Hilden, Germany). Capped Cas9 mRNA was synthesized using mMESSAGE mMACHINE SP6 kit (Invitrogen, Waltham, Massachusetts) and purified using MicroSpin G-50 columns. A mix of SREBF1 gRNA and Cas9 mRNA was injected directly into 1-cell-stage embryos using a pneumatic Pico Pump (WPI, Worcester, Massachusetts).
Micro-CT
Euthanasia of ZF (5–8 months old) was carried out by 0.4% tricaine methanesulfonate (MS222), followed by submersion in ice water (0°C–4°C). Formaldehyde-fixed ZF samples were gently wrapped in Kimwipes (Kimtech Science, Roswell, Georgia), soaked with double distilled water, placed in an 8-mm diameter sample tube and scanned in a SkyScan 1172 micro-CT scanner (Bruker, Kontich, Belgium), according to the manufacturer’s instructions. The ZF skeleton was imaged at 4.8-μm isotropic voxel size. The applied x-ray voltage was 50 kV and the current was 100 μA; a power source of 10 W with 0.25-mm aluminum filtration was also used. Scans were taken over 360° with a 0.40° rotation step. A region of interest (ROI) was traced from the cephalic region to the fin. The region of interest was thresholded using a manually determined global threshold. Plug-ins like bitwise operations and despeckle were used to calculate whole ZF BMD in CTAn software. Calibration phantoms of known density (0.25 and 0.75 g/cm3 hydroxyapatite) were scanned at the same time as the ZF samples for the quantification of the density of ZF bones. Three-dimensional tomography images of the entire ZF body and vertebrae were generated using CTvox software (Bruker).
Lipidomics profiling of zebrafish
Collection of dorsal muscle.
We used dorsal muscles of adult (4–6 month) ZF (KO, heterozygotes, and WT, SREBF1+/+) for lipidomics profiling. Upon euthanasia, the weight and length of each fish was measured prior to the necessitated removal of the head and tail. Next, fish were dissected, the skin was removed, and the internal organs were dissected. During the dissection process, the sex of the fish was identified by observation of the gonads under binocular microscopy. Similar numbers of males and females were included in this study (17 and 19, respectively). Lastly, the muscle samples were weighed and flash frozen in liquid nitrogen.
Sample preparation.
Briefly, the dorsal muscle of each adult (4–6 month) ZF (50–100 mg) was minced into small pieces, and then transferred into 1.0 mL of ice-cold 80% methanol in water (v/v), with one 5-mm stainless steel bead (Qiagen, Germantown, Maryland) to perform homogenization using the TissueLyser II homogenizer (Qiagen, Germantown, Maryland) at a frequency of 30 seconds-1, in 8- × 30-second bursts, waiting 20 seconds in between to avoid high temperatures. Each obtained homogenate was added with 5 µL of internal IS mixture stock solution (5 µg/mL for AA-d8, 2 µg/mL for DHA-d5 and EPA-d5, and 0.5 µg/mL for all the rest IS), then agitated on ice and in the dark for 1 hour. Subsequently, the homogenates were centrifuged at 15 000 xg, 4°C for 10 minutes to remove any precipitated proteins. Although very limited, these muscle homogenates contained some connective tissue as well.
Solid phase extraction.
The clear supernatant obtained from dorsal muscle homogenates was cleaned-up and concentrated by solid phase extraction (SPE) before its injection into the liquid chromatography–mass spectrometry (LC-MS). Before loading samples to the preconditioned cartridge (Strata-X 33 µm polymeric reversed phase SPE cartridge, 10 mg/1 mL, Phenomenex, Torrance, California), 4 mL of ice-cold 0.1% formic acid was added into the supernatant in order to fully protonate the LM species. Once the sample had been totally loaded, the cartridges were washed with 0.1% formic acid (1 mL) and followed shortly after by another wash with 15% (v/v) ethanol in water (1 mL) to remove excess salts. The LMs from the SPE sorbent bed were eluted by methanol; the solvents were then removed using an Eppendorf 5301 concentrator centrifugal evaporator (Eppendorf, Hamburg, Germany) and the dried extracts were stored at -80°C immediately. Before initiating the liquid chromatography–tandem mass spectrometry (LC-MS/MS) experiments, 50 µL of methanol was added to reconstitute the dried extracts, and 10 µL of the mixed solution was directly injected using the autosampler. The relative peak area of each LM in lipidomic profiling analysis was normalized based on the peak area of the related IS and the weight of each muscle sample.
LC-MS/MS conditions
All components of the LC-MS/MS system were from Shimadzu Scientific Instruments, Inc. (Columbia, Maryland). The LC system was equipped with 4-pumps (Pump A/B: LC-30AD, Pump C/D: LC-20AD XR), a SIL-30AC autosampler (AS), and a CTO-30A column oven containing a 2-channel 6-port switching valve. The LC separation was conducted on a RESTEK C8 column (Ultra C8, 150 × 2.1 mm, 3µm, RESTEK, Bellfonte, Pennsylvania), along with a Halo guard column (Optimize Technologies, Oregon City, Oregon). The MS/MS analysis was performed with a Shimadzu LCMS-8050 triple quadrupole mass spectrometer. The instrument was operated and optimized under both positive and negative electrospray and multiple reaction monitoring modes (+/- ESI MRM). The flow rate settings and gradient program for the LC system as well as MS/MS conditions were recommended by a software method package for 158 lipid mediators (Shimadzu Scientific, Tokyo, Japan), with optimizations (33). In a brief summation, the optimized conditions were as follows: interface voltage, 4.0 kV; interface temperature, 275°C; desolvation line temperature, 275°C; heating block temperature, 400°C; drying gas (N2), 10 L/minute; nebulizing gas (N2), 3 L/minute; heating gas (Air), 10 L/minute; and collision-induced dissociation gas (Ar), 230 kPa. The acquisition was divided into multiple segments. The m/z transitions (parents to product ions) and their tuning voltages were selected based on the best MRM responses from instrumental method optimization software. The mobile phase composition consists of (1) 0.1% formic acid in water and (2) acetonitrile. All analyses and data processing were completed on Shimadzu LabSolutions V5.65 software (Shimadzu Scientific).
RNA-sequencing and data analysis
In the analysis of the molecular mechanisms associated with SREBF1, we compared gene expression between KO (SREBF1-/-) and WT (SREBF1+/+) ZF using genome-wide RNA-sequencing. For RNA extraction, we collected 50 larvae (7 days postfertilization, dpf) into a 1.5-ml tube, added 250 μl TRI reagent (T9424; Sigma-Aldrich, St. Louis, Missouri) and homogenized the sample by pipetting up and down. Next, 750 μl of TRI reagent was added, followed by 200 μl of chloroform; the samples were then mixed gently and centrifuged at 12 000 xg for 15 minutes at 4°C. The top RNA-containing aqueous layer was transferred into a new tube and 0.5 ml isopropanol was added. The obtained samples were incubated for 10 minutes at room temperature, centrifuged at 12 000 xg for 10 minutes at 4°C, then the supernatant was removed, and the newly formed pellet was washed by adding 1 ml of 75% ethanol. Next, the samples were centrifuged at 7500 xg for 5 minutes at 4°C, the ethanol solution was removed, and the samples were re-suspended in 90 μl of RNAse-free water and followed by a quick transference to the 55°C incubator for 10 minutes. Lastly, DNase I (04716728001; Sigma-Aldrich) was added and the samples were incubated for another 15 minutes at 37°C. The samples were cleaned by Qiagen RNeasy (74704, Qiagen).
For RNA-sequencing we pooled RNA extracted from 50 larvae of each genotype; for each pool of RNA we had 3 repeats (technical triplicates). Polymerase chain reaction (PCR)-based cDNA library construction and next-generation sequencing were carried out at the Genome Technology Center at Bar Ilan University/Azrieli Faculty of Medicine. Quality control of initial RNA material was assessed using the RNA Pico kit on the Agilent 2100 Bioanalyzer, and only samples with an RNA integrity number (RIN) above 7 were included in this study. Starting with 100 ng of total RNA material, mRNA libraries were generated using the NEBNext library reagent (Cat #E7420; New England Biolabs, Ipswich, Massachusetts) in accordance with NEB’s protocols. The Illumina Hi-Seq 2500 platform (Illumina, La Jolla, California) was used to generate 61 base pair-long single-end reads. Libraries were applied to an Illumina flow cell (4 samples per lane) at a concentration of 10 pM, and we obtained an average of 41 million reads per sample (range 35–50 million).
Trimmed reads were generated by Illumina’s cloud tool Basespace. Pre- and postalignment quality control, genome alignment, and differential gene expression were analyzed with Partek Flow software (Partek, St. Louis, Missouri). A ZF genome (built GRCz10) database was utilized for alignment using Star2.5.2b, with the guidance of Gencode v24. Aligned reads were quantified to Gencode v24 using an expectation—maximization algorithm. Normalized counts were analyzed for differential expression with the gene-specific analysis method (we normalized the gene counts using the reads per kilobase per million reads, RPKM, and, additionally, total counts).
Bioinformatics analysis and annotation
In comparison of the gene expression between KO (SREBF1-/-) and WT (SREBF1+/+), we performed the Student’s t-test for further analysis and quantification of the similarities between each sample. Genes were considered to be differentially expressed if the false discovery rate (FDR) was less than 0.05, and there was at least a 2-fold difference in expression. In exploration of the functional features of the identified genes, we used the online tools (18) such as gene ontology term, KEGG Kyoto Encyclopedia of Genes and Genomes (KEGG) canonical pathways analysis, Ingenuity Pathway Analysis (IPA; www.ingenuity.com), and Webgestalt (34), all of which quantify functional enrichment analysis.
Selected genes (eg, hubs of the top-associated pathways) were verified by quantitative real-time polymerase chain reaction (RT-qPCR) on the samples identical to those used for RNA-Seq.
Gene expression by RT-qPCR
RNA was extracted per the process described in the previous section (RNA sequencing). cDNA was synthesized using PrimeScript First Strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan), according to the manufacturer’s instructions. An RT-qPCR assay was performed using ViiA 7 D Real-Time PCR Instrument (Life Technologies Corporation, Gaithersburg, Maryland). In this analysis, a template with 6 μl of cDNA (40 ng per well) was mixed with 0.15 μl of each primer (10 μM) and 7.5 μl of Fast SYBR Green Master Mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts) to generate a final volume of 15 μl. The standard cycling conditions were 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. All reactions were performed as technical triplicates, and an analysis of melting curves was performed in each reaction. Nontemplate controls (double distilled water) were incorporated into each PCR run. The relative expression values of the genes were normalized using expression levels of the housekeeping gene rpl32. The subsequent results of individual RT-qPCR were analyzed using the Delta Delta C(T) equation, as previously described (35). Graphs were created using GraphPad Prism V8.0 (GraphPad Prism Software, San Diego, California). Comparisons between the KO and WT were performed via unpaired Student’s t-test and the statistical significance was set at P < 0.05.
Statistical analysis.
All data were subjected to statistical analysis using SPSS Software (version 23).
The BMD in adult fish was analyzed using the linear model as follows: Y = α+β 1X + β 2S + β 3Z, where Y = BMD, X = genotype, Z = age, and S = sex. Distribution of each LM’s relative peak area was tested in a combined sample as well as by sex. Both Pearson and Spearman correlations were calculated between the LMs. For the genotype-association analyses, LM levels were controlled for both the sex and weight of an animal (unless specified otherwise). The goodness of curve fit was tested by comparing regression statistics (coefficients of determination [R2] and significance of quadratic term) from the linear and quadratic models. Other tests included analysis of variance (ANOVA) adjusted for sex of the fish and Kruskal-Wallis (stated in the respective figure legends), and if a deviation from normality was observed, a nonparametric Mann-Whitney U test was applied. For lipidomics profiling results, 1-way ANOVA with Tukey post hoc test (α = 0.05) was applied for multiple comparison analysis. Grubb’s test (α = 0.05) was applied to remove the significant outliers from each dataset. P-values were considered significant at 0.01 (to consider a large number of the LMs).
Results
Knockout of SREBF1 gene by introducing S23fs mutation
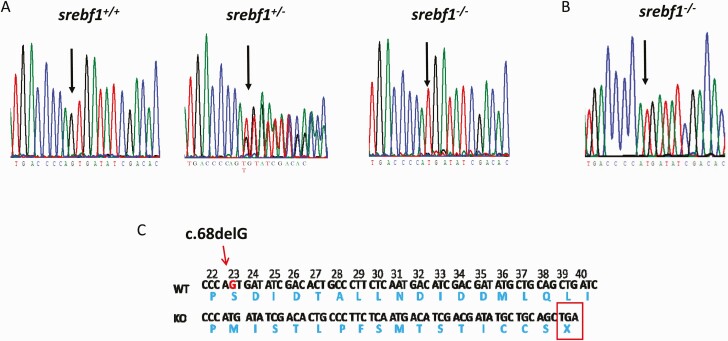
We obtained a null mutation of SREBF1 in ZF through CRISPR/Cas9-mediated gene editing. Guide RNA (gRNA) targeting exon 1 of ZF SREBF1 was co-injected with Cas9 mRNA into ZF embryos; the changes in sequence were detected by T7 endonuclease digestion. Several founders with indels were screened for germline transmission and the fish with 1bp deletion were propagated for the study. The 1 bp deletion is predicted to produce a termination codon of 39 amino acids after the initiation of a start codon (Fig. 1), subsequently leading to a gene KO (protein ablation).
Figure 1.
srebf1 S23fs mutation (c.68delG) established in zebrafish by CRISPR-Cas9. A: Genomic DNA sequence of adult zebrafish, wild type (WT), heterozygote (Het), and homozygote (knockout [KO]) for c.68delG, arrow indicating deletion point. B: RNA extraction from adult srebf1-/- show c.68delG in cDNA sequence. C: Schematic representation of srebf1 S23fs; deletion of Guanine (G) (indicated in red) generated a stop codon at locus 115 (codon 39).
Lipidomics profiling of ZF
As described above, adult ZF dorsal muscles were utilized for lipidomics profiling. In total, 48 LMs, derived from various essential polyunsaturated FAs, were identified from these ZF samples in order to determine potential targets regulated by SREBF1. The values of LMs were normalized to the weight of the animal and of the 48 LMs, 7 showed differences by sex (higher in males): 12-HEPE, 12-HETE, 15-HETrE, PGD3, PGE2, PGE3, and PGF2α (Table 1 and Table S1 (36)).
Table 1.
Relative peak areas of lipid mediators in the muscle of adult zebrafish: descriptive statistics by sex
| Lipid Mediator | Mean | Female | Mean | Male | Median | ANOVA | ||
|---|---|---|---|---|---|---|---|---|
| SD | Median | SD | F Statistics | P value | ||||
| PGF2α | 0.0009 | 0.0005 | 0.0007 | 0.0005 | 0.0002 | 0.0005 | 12.664 | 0.001 |
| PGE2 | 0.0008 | 0.0004 | 0.0007 | 0.0005 | 0.0003 | 0.0004 | 6.787 | 0.014 |
| 12-HETE | 0.0043 | 0.0019 | 0.0041 | 0.0027 | 0.0015 | 0.0023 | 7.187 | 0.011 |
| PGE3 | 0.0030 | 0.0018 | 0.0023 | 0.0016 | 0.0010 | 0.0013 | 8.421 | 0.006 |
| PGD3 | 0.0030 | 0.0018 | 0.0026 | 0.0016 | 0.0010 | 0.0013 | 8.918 | 0.005 |
| 12-HEPE | 0.0117 | 0.0045 | 0.0119 | 0.0065 | 0.0033 | 0.0063 | 15.961 | 0.000 |
| DHA | 0.6760 | 0.1491 | 0.7008 | 0.5815 | 0.1364 | 0.5429 | 3.941 | 0.055 |
| 15-HETrE | 0.0003 | 0.0002 | 0.0003 | 0.0001 | 0.0001 | 0.0001 | 16.849 | 0.000 |
Relative peak area of each LM in lipidomic profiling analysis was normalized based on the peak area of internal standards (IS) and the weight of the sample.
Abbreviations: SD, standard deviation; PGF2alpga, Prostaglandin F2 Alpha; PGE2, Prostaglandin E2; 12-HETE=12-Hydroxyeicosatetraenoic acid; PGE3, Prostaglandin E3; PGD3, Prostaglandin D3; 12-HEPE=12-12-HETE, 12- Hydroxyeiosapentaenoic acid; DHA, Docosahexaenoic acid; 15-HETrE, 15-Hydroxyeicosatrienoic acid.
Association between the number of SREBF1 alleles and lipid mediator values
A curve fit was performed through the comparison of regression statistics from the linear and quadratic models to determine the association between SREBF1 genotypes (SREBF1+/+, SREBF1+/-, SREBF1-/-) and LMs.
Through comparison of the regression statistics associated with each model type, it was found that the coefficient of determination (R2) was generally higher in the quadratic model. It is worth noting that in the linear regression model, only one LM phenotype, 11,12-EET, was significantly associated with the number of SREBF1 alleles (P = 0.006, Table 2). Also, the R2 for the linear model displayed similar statistical qualities to that of the quadratic model (0.446 and 0.446, respectively), thus the addition of the quadratic term did not result in more variance in 11,12-EET explained. Figure 2 shows a strong linearity of the relationship between the number of alleles and 11,12-EET levels, with zero alleles (SREBF1-/-) corresponding to the highest 11,12-EET, while having 2 alleles (SREBF1+/+) corresponds to the lowest levels of 11,12-EET. In the quadratic regression model, the number of quadratic terms linked to the SREBF1 alleles that were associated with 16 LM phenotypes were nominally significant (P < 0.05; Table 2, Table S2 (36)). Both linear and quadratic terms were nominally significant (P < 0.05) upon analysis of the associated levels of 10 LMs: 12-HETE, 9-HODE, 9-KODE, 13-KODE, 12-HEPE, 17-HDoHE, 10-HDoHE, 14-HDoHE, DHA, and 15-HETrE. Figure 3 shows the difference in relative peak areas of these LMs with the number of SREBF1 alleles.
Table 2.
Adult zebrafish muscle Srebf1 allelea association with Lipid Mediator concentration
| Lipid Mediator | Linear Model | Quadratic Model | ||||
|---|---|---|---|---|---|---|
| 6-keto-PGF1a | R | R 2 | R | R 2 | ||
| 0.234 | 0.055 | 0.441 | 0.194 | |||
| B | P-value | B | P-value | |||
| Allele | 0 | 0.170 | Allele | 3.132E-05 | 0.071 | |
| – | – | – | Allele2 | -1.748E-05 | 0.023 | |
| 5S,6S-LXA4 | R | R 2 | R | R 2 | ||
| 0.173 | 0.030 | 0.390 | 0.152 | |||
| B | P-value | B | P-value | |||
| Allele | -0.001 | 0.313 | Allele | 0.003 | 0.084 | |
| – | – | – | Allele2 | -0.002 | 0.037 | |
| 12-HETE | R | R2 | R | R2 | ||
| 0.061 | 0.004 | 0.558 | 0.312 | |||
| B | P-value | B | P-value | |||
| Allele | 0.000 | 0.724 | Allele | 0.005 | 0.001 | |
| – | – | – | Allele2 | -0.002 | 0.001 | |
| 11,12-EET | R | R2 | R | R2 | ||
| 0.446 | 0.199 | 0.449 | 0.202 | |||
| B | P-value | B | P-value | |||
| Allele | -1.339E-05 | 0.006 | Allele | -7.850E-06 | 0.635 | |
| – | – | – | Allele2 | -2.517E-06 | 0.727 | |
| 13-HODE | R | R2 | R | R2 | ||
| 0.134 | 0.018 | 0.369 | 0.136 | |||
| B | P-value | B | P-value | |||
| Allele | 0.000 | 0.435 | Allele | 0.004 | 0.081 | |
| – | – | – | Allele2 | -0.002 | 0.041 | |
| 9-HODE | R | R2 | R | R2 | ||
| 0.205 | 0.042 | 0.457 | 0.209 | |||
| B | P-value | B | P-value | |||
| Allele | 0.000 | 0.231 | Allele | 0.003 | 0.039 | |
| – | – | – | Allele2 | -0.001 | 0.013 | |
| 13-KODE | R | R2 | R | R2 | ||
| 0.261 | 0.068 | 0.500 | 0.250 | |||
| B | P-value | B | P-value | |||
| Allele | 0.000 | 0.123 | Allele | 0.001 | 0.034 | |
| – | – | – | Allele2 | 0.000 | 0.008 | |
| 9-KODE | R | R2 | R | R2 | ||
| 0.163 | 0.026 | 0.446 | 0.199 | |||
| B | P-value | B | P-value | |||
| Allele | -2.579E-05 | 0.344 | Allele | 0.000 | 0.031 | |
| – | – | – | Allele2 | 0.000 | 0.012 | |
| 12-HEPE | R | R2 | R | R2 | ||
| 0.039 | 0.001 | 0.614 | 0.377 | |||
| B | P-value | B | P-value | |||
| Allele | 0.000 | 0.823 | Allele | 0.013 | 0.000 | |
| – | – | – | Allele2 | -0.006 | 0.000 | |
| 17-HDoHE | R | R2 | R | R2 | ||
| 0.027 | 0.001 | 0.342 | 0.117 | |||
| B | P-value | B | P-value | |||
| Allele | 1.713E-05 | 0.877 | Allele | 0.001 | 0.049 | |
| – | – | – | Allele2 | 0.000 | 0.045 | |
| 10-HDoHE | R | R2 | R | R2 | ||
| 0.009 | 0.000 | 0.382 | 0.146 | |||
| B | P-value | B | P-value | |||
| Allele | -1.073E-05 | 0.958 | Allele | 0.002 | 0.030 | |
| – | – | – | Allele2 | -0.001 | 0.023 | |
| 14-HDoHE | R | R2 | R | R2 | ||
| 0.023 | 0.001 | 0.424 | 0.180 | |||
| B | P-value | B | P-value | |||
| Allele | 1.324E-05 | 0.896 | Allele | 0.001 | 0.013 | |
| – | – | – | Allele2 | 0.000 | 0.011 | |
| DHA | R | R2 | R | R2 | ||
| 0.037 | 0.001 | 0.398 | 0.158 | |||
| B | P-value | B | P-value | |||
| Allele | -0.007 | 0.832 | Allele | 0.263 | 0.027 | |
| – | – | – | Allele2 | -0.123 | 0.018 | |
| 15-HETrE | R | R2 | R | R2 | ||
| 0.021 | 0.000 | 0.428 | 0.183 | |||
| B | P-value | B | P-value | |||
| Allele | 5.080E-06 | 0.902 | Allele | 0.000 | 0.013 | |
| – | – | – | Allele2 | 0.000 | 0.010 |
a Allele: number of SREBF1 WT alleles (0, 1, or 2); allele2: quadratic term of “allele.”
Figure 2.
Relative levels of 11,12-EET in dorsal muscles from adult zebrafish of different srebf1 genotype. Wild type (WT, srebf1+/+); heterozygote (Het, srebf1+/-), and homozygote (knockout [KO], srebf1-/-). Mean ± SD, n = 15 for WT, n = 15 for Het, and n = 6 for KO. One-way analysis of variance (ANOVA) with Tukey post hoc test (α = 0.05) was applied for statistical analysis.
Figure 3.
Relative Lipid Mediators levels in dorsal muscles from adult zebrafish of different srebf1 genotypes. Wild type (WT, srebf1+/+), heterozygote (Het, srebf1+/-), and homozygote (knockout [KO], srebf1-/-). Mean ± standard deviation (SD), n = 15 for WT, n = 15 for Het, and n = 6 for KO. One-way ANOVA with Tukey post hoc test (α = 0.05) was applied for statistical analysis.
Bone Mineral Density Analysis
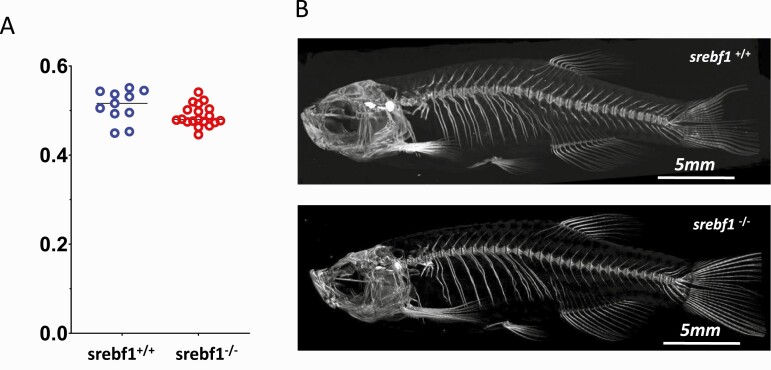
Whole the body of adult ZF was scanned in a SkyScan 1172 micro-CT scanner. Age- and sex-adjusted BMD was significantly lower (P < 0.03 in males) in SREBF1-/- fish than in their WT siblings (Fig. 4). Overall, the skeleton, as well as bones of the spine and head regions show no morphological differences between KO and WT.
Figure 4.
Lower BMD found in srebf1-/- adult zebrafish compared with srebf1+/+. A: Quantification of BMD in adult zebrafish (P < 0.03). B: MicroCT images of representative srebf1+/+ and srebf1-/- adult male fish.
RNA sequencing and bioinformatics
A total of 1142 of the ZF genes were found differentially expressed (the FDR was <0.05 and there was at least a 2-fold difference in expression), namely 630 up-expressed and 512 down-expressed. Table 3 lists the enriched categories (pathways), the number of Entrez genes in the corresponding gene list, in the categories and also in the FDR. Significantly enriched pathways included fatty acid elongation, linoleic acid metabolism, AA metabolism, adipocytokine signaling, and DNA replication. Figures S1 and S2 provide a graphical representation of gene networks (ZF-specific) based on the Ingenuity software.
Table 3.
Enriched pathways based on the differentially expressed genes in RNA-seq of SREBF1+/+ and SREBF1-/- larvae
| Gene Set (KEGG) | Description | Size (# Genes) | Leading Edge Number | P-value | FDR |
|---|---|---|---|---|---|
| dre00062 | Fatty acid elongation – Danio rerio | 30 | 6 | 0 | 0.01077 |
| dre03030 | DNA replication – Danio rerio | 35 | 25 | 0 | 0.02049 |
| dre00591 | Linoleic acid metabolism – Danio rerio | 20 | 7 | 0.00388 | 0.04446 |
| dre00590 | Arachidonic acid metabolism – Danio rerio | 46 | 11 | 0.00879 | 0.05007 |
| dre04920 | Adipocytokine signaling pathway – Danio rerioa | 78 | 30 | 0 | 0.05064 |
a down-regulated.
Validation of RNA-seq results by RT-qPCR
Due the high propensity to receive false-positives in RNA sequencing, we sought to confirm gene expression differences through a RT-qPCR assay using the same RNA samples that were used in the RNA-seq experiment. We selected top genes from the RNA-sequencing results: hmgcra, mtmr12, ndstl1, cyp2p7, cyp2p8, cyp2p9, and aaca2 in addition to others. We analyzed the expression of these top genes by RT-qPCR, using the ViiA7 Real-time PCR System. RT-qPCR analysis showed upregulation of cyp2p7, cyp2p9 (P < 0.001), cyp2p8 and ndstl1 (P < 0.05), and hmgcra (P > 0.05) expression in SREBF1-/- ZF when compared with SREBF1+/+ (see (36)). It is important to note that these changes were in concordance with those observed via the RNA-seq. The gene mtmr12 showed lower (nonsignificant, P > 0.05) expression in SREBF1-/- compared with SREBF1+/+, which is directionally opposite to the results obtained in RNA-seq.
Conclusions and Discussion
A major goal of our study was to prove the feasibility of using the ZF model as an effective system model for biological validation of GWAS studies. Particularly, we were interested in bivariate studies that are concomitantly relevant for bone and muscle traits. A top priority gene identified in our studies was SREBF1, a genetic factor responsible for the regulation of lipid homeostasis and known for its keystone role in adipocyte differentiation. Obviously, this candidate gene is extremely intriguing in the context of bone-muscle crosstalk given the importance of lipid metabolism and homeostasis for these organ systems.
All in all, this study provides evidence that not only does KO of SREBF1 affect lipid-signaling mediators in the ZF model, but it also has a direct impact on BMD. This might be attributed to the pathway of 11,12-EET. Interestingly, the LM, 11,12-epoxyeicosatrienoic acid (11,12-EET), had a direct and linear correlation with the quantity of SREBF1 alleles analyzed. While lacking alleles (SREBF1-/-) corresponds with the highest 11,12-EET levels in the dorsal muscles of adult ZF, having 2 alleles (SREBF1+/+) corresponds to the lowest 11,12-EET. It is noteworthy to mention that the levels of CYP epoxy-metabolites, including 11,12-EET, significantly increased with exercise levels in humans (27). These investigators proposed that CYP epoxy-metabolites could contribute to the cardiovascular response associated with maximal exercise (37). Epoxyeicosatrienoic acids were widely reported for suppressing NF-κB signaling, which plays an important role in osteoclastogenesis and bone formation (23, 38). An osteoclast is a giant multinucleated cell that is differentiated from the hematopoietic stem cell by the cooperative action of macrophage-colony stimulating factor and RANKL (39). Osteoclast formation and bone resorptive activity were significantly reduced by the inhibition of NF-κB (24). Additionally, Yang et al has reported that SREBP-1 was positively correlated with NF-κB activation in SREBP-1-overexpressed renal carcinoma cell line ccRCC (40), suggesting a declined NF-κB signaling when SREBP-1 expression reduced. These findings help to explain the marked bone loss observed in our new ZF model. Furthermore, there is an abundance of reports demonstrating the key positive role EETs play in hematopoietic stem and progenitor cell engraftment, angiogenesis, and resolution of inflammation, an essential process for tissue development, maintenance, and healing (41–45).
To our knowledge, these data sets are the 1st to provide some initial evidence and a potential association between SREBF1 genotype 11,12-EET levels, and BMD in an animal model.
Many LMs, including EETs, which are metabolites formed via the catabolism of AA by cytochrome P450/epoxygenase, play an important role in insulin-mediated augmentation of microvascular blood flow in skeletal muscle (46). Epoxyeicosatrienoic acids are additionally associated with the promotion of healthy insulin signaling, inflammation, and bone formation, all of which can lead to physical decompensation and debility if found to be functionally inept. An interesting and intriguing example is their involvement in the metformin pathway, a mechanism where hepatic glucose production is inhibited and glucose uptake is promoted in order to maintain physiologic serum glucose levels in those diagnosed with type II diabetes (47). All of these highly complex pathways are maintained because EETs are able to efficiently attenuate inflammatory signaling, thus implying their therapeutic potential in the treatment of diseases, particularly MSK disorders characterized by acute and chronic inflammation (48).
Upon molecular-genetic analyses, it has been reported that the gene CYP2J2, which encodes an anti-inflammatory epoxygenase with significant expression in heart and muscle, plays a predominant role in 11,12-EET synthesis. CYP2J2 expression upregulates in response to increased proinflammatory signaling in human adult ventricular myocytes, as previously reported, and is directly associated with SREBF1 KO in our current study. Since FA metabolism programed by SREBF1 is important for resolving inflammation, we postulate that SREBF1 KO results in enhanced inflammatory signaling and bone loss, which is compensated by elevated CYP2J2 expression and increased 11,12-EET production to promote anti-inflammatory responses and restoration of FA metabolism (22, 29, 49). Additionally, 9 LMs derived from AA, linoleic acid (LA), EPA, DHA, and dihomo-γ-linolenic acid, namely 12-HETE, 9-HODE, 13-KODE, 12-HEPE, 17-HDoHE, 10-HdoHE, 14-HdoHE, DHA, and 15-HETrE, were significantly but nonlinearly associated with the number of SREBF1 alleles. Observation of the LM’s value distribution per number of alleles and a frank quadratic relationship between these measures, reflects a heterosis-like relationship between the gene mutation and these 9 LMs (50–52). The relationship between the fish genotype and BMD is relatively straightforward. KO fish had a significantly lower BMD than WT controls of the same age, which corresponds to the effect originally seen in (12), where overexpression of SREBF1 enhances osteoblast mineralization (thus increases BMD). This finding proposes the idea that therapies and processes focused on enhancing SREBF1 expression and downstream LMs can be hugely beneficial for those suffering from age-related osteoporosis or a genetic condition that produces generalized low bone density.
In mice, SREBP-1 plays an important role in controlling hepatic lipogenesis (26, 53, 54). Hepatic lipogenesis is nutritionally regulated, ie, downregulated during fasting and upregulated during the postprandial state, as an adaptation to the nutritional environment. Upon fasting, the expression of the SREBF1 is markedly decreased, resulting in reduced amounts of SREBF1 protein in liver, with corresponding decreases in the mRNAs for SREBF1-activated target genes such as FA synthase (Fasn), a rate-limiting enzyme for lipogenesis (55). The fasting-induced Krüppel-like factor 15 (KLF15) is upregulated in fasting mouse liver and is known to contribute to the regulation of hepatic gluconeogenesis (56, 57). Furthermore, KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. SREBF1 is the target gene regulated by KLF15 that involved in the regulation of lipogenesis and gluconeogenesis in response to nutritional conditions (58). In our current study, lipogenesis was also affected in SREBF1 KO ZF.
Our RNA-seq analyses, which compared juvenile WT and KO fish, revealed that out of 5 enriched pathways, 4 were directly associated with lipid signaling mediators. Due to the richness and broadness of these analyses, the focal points of this discussion will be regarding some of the key protein coding genes in each pathway. Through an analysis of the association noted between LMs and protein coding genes we can foster a deeper understanding of the upregulatory and synergistic cellular mechanisms that develop during pathogenesis, thus resulting in consequential alterations to metabolic capabilities. For example, in the mitochondria the fatty acid elongation pathway is maintained by the protein, acetyl CoA Acyltransferase 2 (ACAA2), via catalyzation of the last step in the mitochondrial β-oxidation pathway, an aerobic process breaking down FAs into acetyl-CoA, thus playing a central role in the supply of energy for the animal (59). ACAA2 appears to be responsible for cholesterol ester formation and secretion in lipoproteins (60). The reduction in expression of this specific enzyme would lead to changes in the production and levels of LMs derived from the AA/Cytochrome P450 complex, such as EETs. This is clinically significant because alterations in circulating serum lipid levels, particularly in the disequilibrium between HDLs and LDLs, can increase the risk of an individual developing a hyperlipidemic pathology such as coronary artery disease (61).
In our experiments, 4 major ZF genes that underwent alterations were noted to be very closely interrelated with the cytochrome P450-system (cyp2j20, cyp2p7, cyp2p8, and cyp2p9) and displayed an association with the linoleic acid metabolism pathway. In humans, all 4 genes are orthologous to CYP2J2 (cytochrome P450 family 2 subfamily j member 2), which encodes 1 of the cytochrome P450 enzymes implicated in the catalyzation of many reactions involving drug metabolism and the synthesis of cholesterol, steroids, and other lipids. CYP2J2 metabolizes AA to 4 regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EET) (62), which have the propensity to facilitate insulin-mediated augmentation in skeletal muscle perfusion and blood volume (46). Interestingly, CYP2J2 is additionally involved in the metabolism of polyunsaturated FAs (PUFA) in the cardiovascular system. In addition to the negative correlation between 11,12-EET levels and the number of SREBF1 alleles (Fig. 1), the upregulated cyp2p7, cyp2p8, and cyp2p9 expression was also observed in SREBF1 KO ZF when compared to WT. CYP2J2 overexpression alleviated age-related insulin resistance and metabolic dysfunction (62). We postulate that this upregulation might be a reason for the increased 11,12-EET levels due to KO of SREBF1. We also found a significant change in leukotriene C4 synthase (LTC4S), which is encoded by LTC4S, a member of the membrane-associated proteins in the eicosanoid and glutathione (MAPEG) metabolism family involved in the AA metabolism pathway. Leukotrienes are biologically active lipid mediators that play important roles in inflammation and are involved in pathological states with an inflammatory component, such as asthma, cardiovascular disease, or cancer (63, 64). LTC4S catalyzes the committed step in the biosynthesis of cysteinyl leukotriene, a potent biological compound derived from AA (65) through the conjugation of leukotriene A4 (LTA4) with glutathione. Specifically, LTA4 is a key precursor to the production of leukotriene B4 (LTB4) and leukotriene C4 (LTC4), which are essential in the production of leukotrienes. In response to biological and nonbiological stimuli and several inflammatory conditions, these lipid mediators quantifiably increase as a result (66–69). Notably, LTC4S deficiency is associated with a neurometabolic developmental disorder characterized by, among other symptoms, severe muscular hypotonia (70).
Another enriched pathway was that of adipocytokine signaling, during which the traf2b, whose ortholog in humans is TRAF2 (the TNF receptor associated factor 2), encodes a member of the TNF receptor associated factor (TRAF) protein family. TRAF proteins associate with and mediate the signal transduction from members of the TNF receptor superfamily (71), thereby facilitating inflammatory and apoptotic processes. TRAF2 is a key intracellular signaling mediator that acts downstream of not only TNFα but also various members of the TNFα superfamily. It is essential for mediating cell survival, normal adaptive immune responses, and lymphocyte homeostasis (72, 73). TRAF2 is involved in the activation of NF-κB signaling that contributes to autoimmunity (74, 75). A similar gene affected is the Acyl-CoA synthetase bubblegum family member 2 (acsbg2), which mediates the activation of long-chain FAs for both the synthesis of cellular lipids and their degradation via beta-oxidation (76, 77). It is also able to activate very long-chain FAs and, although the relevance of such activity in vivo is unclear, we can anticipate the degree of interconnectedness this gene has on cardiomuscular maintenance. We were additionally very intrigued by our finding of acyl-CoA synthetase long-chain family member 5 (acsl5), a gene that has been reported to act like other ACSL family members through its engagement in lipid biosynthesis and FAs degradation (78). Previous studies of mouse models show that ACSL5 is a target gene of SREBF1, expression of ACSL5 is increased in SREBF1 transgenic mice and decreased in SREBF1 KO mice (79–81). ACSL5 in human skeletal muscle functions to increase mitochondrial fatty acid oxidation (82). In a recent study of the common carp (Cyprinus carpio) (83), it was noted that the acsl5 gene showed higher expression and lower methylation levels in its promoter region in relation to high or low α-linolenic acid (ALA) content in the muscular tissue. While different in substrate specificity, subcellular position, and tissue distribution, all of acsl5’s isozymes convert free long-chain FAs into fatty acyl-CoA esters and thus play a key role in lipid biosynthesis and degradation of FAs. One other gene found in RNA-Seq that plays a pivotal role in the control of body mass index is the leptin receptor (lepr), which participates in the regulation of fat metabolism by coding for a specific hormone linked to the weight management (84). Leptin is a key factor important for the regulation of skeletal muscle mass and can act directly on its receptors to regulate cellular proliferation and differentiation of muscle (85). A recent study (83) found that lepr was associated with AA, DHA, and EPA in common carp (C. carpio) muscles, thereby showing its close interrelatedness with key FAs necessary for the maintenance of cellular health. Leptin is a physiologic regulator of skeletal muscle angiogenesis (86) and is known to increase glucose uptake and enhance insulin signaling in skeletal muscle (87), meaning that alterations in lepr gene expression can result in atrophic changes to the body’s voluntary muscle. Lepr signaling is a negative modulator of bone mechanosensitivity (88); therefore, leptin-deficient and lepr-deficient mice have an increased bone formation leading to high bone mass (89).
In summary, this first ever application of our unique combination of the ZF and CRISPR technique, along with a novel lipidomics profiling of LMs led to a new understanding of SREBF1 biology and potential new applications in the fatty metabolism and MSK domains. Of the key changes uncovered by our RNA-seq analyses, the direct relationship between lipid signaling mediators and enriched pathways, the under expression of SREBF1, and the quantification of LM involvement in homeostatic processes provides opportunities to further the understanding of molecular–genetic muscular pathogenesis. This data points towards a scenario where in KO muscles, higher levels of lipid peroxidation and inflammatory cytokines would lead to bone-muscle injury due to the activation of “inflammasome,” as illustrated by our pathway/network assembly. It is remarkable that 11,12-EET was the key LM associated with the number of alleles of SREBF1, since EETs are associated with insulin signaling, inflammation, and bone formation.
All things considered, the association between SREBF1 and 11,12-EET seem to have a direct effect on fish muscle and a secondary effect on the bone. This finding could provide laboratories with an opportunity to explore the interrelatedness between intrahepatic adipogenesis and skeletal muscle development. Most importantly, we found that the KO of SREBF1 may lead to changes in FA metabolism through cytochrome P450 pathways. The change in P450 metabolites could further alter the activities of NFkB and PPAR pathways, which have been shown to be important for signaling in adipose tissues. These findings support the use of ZF as a suitable model for the investigation of lipid signaling mechanisms in addition to the central role of SREBP-1 biology in MSK homeostasis and maturity.
All in all, the progression of lipidomics profiling continues to offer opportunities for the advancement of knowledge needed to analyze the impacts that lipid-altering gene expression, along with other lipid signaling roles, has on bone and muscle so that in the future, we can utilize this information in the preservation of human health and the mitigation of disease.
Acknowledgments
The authors thank Dr Einav Wircer for her help with the zebrafish mutant model phenotyping.
Financial Support: This work was supported by Israel Science Foundation (ISF) grant #1121/19 (D.K.), we are grateful for instrumentation support from Shimadzu Scientific Instruments, Inc., and the George W. and Hazel M. Jay and Evanston Research Endowments (M.B.). C.L.M., J.H., M.B., Z.W., were partially supported by NIH-National Institutes of Aging PO1 AG03935, R01AG056504, and R01 AG060341 (M.B.).
Author Contributions: M.B. and D.K. designed and supervised the study; C.S. created the fish model and performed the bone density experiments; R.D. performed the fish muscle measurements; Z.W. and C.M. performed the lipidomics experiments; C.S., S.N., and J.H. interpreted the data; and C.S., D.K., and M.B. wrote the manuscript. All authors read and approved the final version of the manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All supplementary material and figures are located in a digital research materials repository (36).
References
- 1. Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5): 1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karasik D, Zinder M. The genetic pleiotropy of musculoskeletal aging. Front Physiol. 2012;3:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep. 2014;12(2):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang J, Hsu YH, Mo C, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J Bone Miner Res. 2014;29(7):1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of inter-organ communication. Cell. 2016;164(6):1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J, Romero-Suarez S, Lara N, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus. 2017;1(2):86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jahn K, Lara-Castillo N, Brotto L, et al. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–209; discussion 209–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitase Y, Vallejo JA, Gutheil W, et al. β-aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22(6):1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urs S, Henderson T, Le P, Rosen CJ, Liaw L. Tissue-specific expression of sprouty1 in mice protects against high-fat diet-induced fat accumulation, bone loss and metabolic dysfunction. Br J Nutr. 2012;108(6):1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ackert-Bicknell CL, Demissie S, Marín de Evsikova C, et al. PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res. 2008;23(9): 1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medina-Gomez C, Kemp JP, Dimou NL, et al. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat Commun. 2017;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grarup N, Stender-Petersen KL, Andersson EA, et al. Association of variants in the sterol regulatory element-binding factor 1 (SREBF1) gene with type 2 diabetes, glycemia, and insulin resistance: a study of 15 734 Danish subjects. Diabetes. 2008;57(4):1136–1142. [DOI] [PubMed] [Google Scholar]
- 14. Dessalle K, Euthine V, Chanon S, et al. SREBP-1 transcription factors regulate skeletal muscle cell size by controlling protein synthesis through myogenic regulatory factors. PLoS One. 2012;7(11):e50878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lecomte V, Meugnier E, Euthine V, et al. A new role for sterol regulatory element binding protein 1 transcription factors in the regulation of muscle mass and muscle cell differentiation. Mol Cell Biol. 2010;30(5):1182–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11(7):769–777. [DOI] [PubMed] [Google Scholar]
- 17. Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. [DOI] [PubMed] [Google Scholar]
- 18. Foretz M, Pacot C, Dugail I, et al. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19(5):3760–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JB, Sarraf P, Wright M, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao XL, Chen JJ, Zhang GN, et al. Small molecule T63 suppresses osteoporosis by modulating osteoblast differentiation via BMP and WNT signaling pathways. Sci Rep. 2017;7(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012;2012:605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan H, Zhao L, Cao H, Chen A, Xiao J. Epoxyeicosanoids suppress osteoclastogenesis and prevent ovariectomy-induced bone loss. Faseb J. 2015;29(3):1092–1101. [DOI] [PubMed] [Google Scholar]
- 24. Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24(9):2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engelking LJ, Cantoria MJ, Xu Y, Liang G. Developmental and extrahepatic physiological functions of SREBP pathway genes in mice. Semin Cell Dev Biol. 2018;81:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimano H, Yahagi N, Amemiya-Kudo M, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274(50):35832–35839. [DOI] [PubMed] [Google Scholar]
- 27. McFarlane MR, Cantoria MJ, Linden AG, January BA, Liang G, Engelking LJ. Scap is required for sterol synthesis and crypt growth in intestinal mucosa. J Lipid Res. 2015;56(8):1560–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kato T, Shimano H, Yamamoto T, et al. Palmitate impairs and eicosapentaenoate restores insulin secretion through regulation of SREBP-1c in pancreatic islets. Diabetes. 2008;57(9):2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oishi Y, Spann NJ, Link VM, et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25(2):412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorski JP, Huffman NT, Vallejo J, et al. Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) gene in osteocytes stimulates soleus muscle regeneration and increased size and contractile force with age. J Biol Chem. 2016;291(9):4308–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mariotti M, Carnovali M, Banfi G. Danio rerio: the Janus of the bone from embryo to scale. Clin Cases Miner Bone Metab. 2015;12(2):188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A. 2013;110(34):13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Bian L, Mo C, Kukula M, Schug KA, Brotto M. Targeted quantification of lipid mediators in skeletal muscles using restricted access media-based trap-and-elute liquid chromatography-mass spectrometry. Anal Chim Acta. 2017;984:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45(W1):W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 36.Supplementary tables and figures. 2020. doi: 10.5061/dryad.s7h44j158. [DOI]
- 37. Gollasch B, Wu G, Dogan I, Rothe M, Gollasch M, Luft FC. Maximal exercise and erythrocyte epoxy fatty acids: a lipidomics study. Physiol Rep. 2019;7(22):e14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Son HS, Lee J, Lee HI, et al. Benzydamine inhibits osteoclast differentiation and bone resorption via down-regulation of interleukin-1 β expression. Acta Pharm Sin B. 2020;10(3):462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473(2):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang H, Zhang X, Liu F, Fan J, Wang B, Dong C. SREBP1-driven lipid desaturation supports clear cell renal cell carcinoma growth through regulation of NF-κB signaling. Biochem Biophys Res Commun. 2018;495(1):1383–1388. [DOI] [PubMed] [Google Scholar]
- 41. Li P, Lahvic JL, Binder V, et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523(7561):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rand AA, Rajamani A, Kodani SD, et al. Epoxyeicosatrienoic acid (EET)-stimulated angiogenesis is mediated by epoxy hydroxyeicosatrienoic acids (EHETs) formed from COX-2. J Lipid Res. 2019;60(12):1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao H, Chen J, Chai J, et al. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab Invest. 2017;97(7):782–791. [DOI] [PubMed] [Google Scholar]
- 45. Zhao Q, Huang J, Wang D, Chen L, Sun D, Zhao C. Endothelium-specific CYP2J2 overexpression improves cardiac dysfunction by promoting angiogenesis via Jagged1/Notch1 signaling. J Mol Cell Cardiol. 2018;123:118–127. [DOI] [PubMed] [Google Scholar]
- 46. Shim CY, Kim S, Chadderdon S, et al. Epoxyeicosatrienoic acids mediate insulin-mediated augmentation in skeletal muscle perfusion and blood volume. Am J Physiol Endocrinol Metab. 2014;307(12):E1097–E1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22(11):820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evangelista EA, Lemaitre RN, Sotoodehnia N, Gharib SA, Totah RA. CYP2J2 expression in adult ventricular myocytes protects against reactive oxygen species toxicity. Drug Metab Dispos. 2018;46(4):380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shapira R, David L. Genes with a combination of over-dominant and epistatic effects underlie heterosis in growth of saccharomyces cerevisiae at high temperature. Front Genet. 2016;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blein-Nicolas M, Albertin W, da Silva T, et al. A systems approach to elucidate heterosis of protein abundances in yeast. Mol Cell Proteomics. 2015;14(8):2056–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaeppler S. Heterosis: many genes, many mechanisms— end the search for an undiscovered unifying theory. ISRN Botany. 2012;2012:12. [Google Scholar]
- 53. Yahagi N, Shimano H, Hasty AH, et al. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J Biol Chem. 1999;274(50):35840–35844. [DOI] [PubMed] [Google Scholar]
- 54. Yahagi N, Shimano H, Hasty AH, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277(22):19353–19357. [DOI] [PubMed] [Google Scholar]
- 55. Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95(11):5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Teshigawara K, Ogawa W, Mori T, et al. Role of Krüppel-like factor 15 in PEPCK gene expression in the liver. Biochem Biophys Res Commun. 2005;327(3):920–926. [DOI] [PubMed] [Google Scholar]
- 57. Gray S, Wang B, Orihuela Y, et al. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007;5(4):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeuchi Y, Yahagi N, Aita Y, et al. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. Cell Rep. 2016;16(9):2373–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bartlett K, Eaton S. Mitochondrial beta-oxidation. Eur J Biochem. 2004;271(3):462–469. [DOI] [PubMed] [Google Scholar]
- 60. Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12(2):121–127. [DOI] [PubMed] [Google Scholar]
- 61. Linton MRF, Yancey PG, Davies SS, et al. The role of lipids and lipoproteins in atherosclerosis. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. [Google Scholar]
- 62. Yang Y, Dong R, Chen Z, et al. Endothelium-specific CYP2J2 overexpression attenuates age-related insulin resistance. Aging cell. 2018;17(2):e12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peters-Golden M, Henderson WR Jr. Leukotrienes. N Engl J Med. 2007;357(18):1841–1854. [DOI] [PubMed] [Google Scholar]
- 64. Fürstenberger G, Krieg P, Müller-Decker K, Habenicht AJ. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis? Int J Cancer. 2006;119(10):2247–2254. [DOI] [PubMed] [Google Scholar]
- 65. Haeggström JZ, Wetterholm A. Enzymes and receptors in the leukotriene cascade. Cell Mol Life Sci. 2002;59(5):742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. 2010;85(6):336–349. [DOI] [PubMed] [Google Scholar]
- 67. Bankova LG, Lai J, Yoshimoto E, et al. Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A. 2016;113(22):6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142(1):112–119. [DOI] [PubMed] [Google Scholar]
- 69. Laidlaw TM, Kidder MS, Bhattacharyya N, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mayatepek E. Leukotriene C4 synthesis deficiency: a member of a probably underdiagnosed new group of neurometabolic diseases. Eur J Pediatr. 2000;159(11):811–818. [DOI] [PubMed] [Google Scholar]
- 71. Yang HJ, Youn H, Seong KM, Jin YW, Kim J, Youn B. Phosphorylation of ribosomal protein S3 and antiapoptotic TRAF2 protein mediates radioresistance in non-small cell lung cancer cells. J Biol Chem. 2013;288(5):2965–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yeh WC, Shahinian A, Speiser D, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7(5):715–725. [DOI] [PubMed] [Google Scholar]
- 73. Nguyen LT, Duncan GS, Mirtsos C, et al. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity. 1999;11(3):379–389. [DOI] [PubMed] [Google Scholar]
- 74. Vallabhapurapu S, Matsuzawa A, Zhang W, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9(12): 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin WJ, Su YW, Lu YC, et al. Crucial role for TNF receptor-associated factor 2 (TRAF2) in regulating NFκB2 signaling that contributes to autoimmunity. Proc Natl Acad Sci U S A. 2011;108(45):18354–18359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pei Z, Jia Z, Watkins PA. The second member of the human and murine bubblegum family is a testis- and brainstem-specific acyl-CoA synthetase. J Biol Chem. 2006;281(10):6632–6641. [DOI] [PubMed] [Google Scholar]
- 77. Fraisl P, Tanaka H, Forss-Petter S, Lassmann H, Nishimune Y, Berger J. A novel mammalian bubblegum-related acyl-CoA synthetase restricted to testes and possibly involved in spermatogenesis. Arch Biochem Biophys. 2006;451(1):23–33. [DOI] [PubMed] [Google Scholar]
- 78. Bowman TA, O’Keeffe KR, D’Aquila T, et al. Acyl CoA synthetase 5 (ACSL5) ablation in mice increases energy expenditure and insulin sensitivity and delays fat absorption. Mol Metab. 2016;5(3):210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Achouri Y, Hegarty BD, Allanic D, et al. Long chain fatty acyl-CoA synthetase 5 expression is induced by insulin and glucose: involvement of sterol regulatory element-binding protein-1c. Biochimie. 2005;87(12):1149–1155. [DOI] [PubMed] [Google Scholar]
- 80. Horton JD, Shah NA, Warrington JA, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100(21):12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stoeckman AK, Towle HC. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J Biol Chem. 2002;277(30):27029–27035. [DOI] [PubMed] [Google Scholar]
- 82. Kwak HB, Woodlief TL, Green TD, et al. Overexpression of long-chain Acyl-CoA synthetase 5 increases fatty acid oxidation and free radical formation while attenuating insulin signaling in primary human skeletal myotubes. Int J Environ Res Public Health. 2019;16(7):1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang H, Xu P, Jiang Y, et al. Genomic, transcriptomic, and epigenomic features differentiate genes that are relevant for muscular polyunsaturated fatty acids in the common carp. Front Genet. 2019;00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Park KS, Shin HD, Park BL, et al. Polymorphisms in the leptin receptor (LEPR)–putative association with obesity and T2DM. J Hum Genet. 2006;51(2):85–91. [DOI] [PubMed] [Google Scholar]
- 85. Arounleut P, Bowser M, Upadhyay S, et al. Absence of functional leptin receptor isoforms in the POUND (Lepr(db/lb)) mouse is associated with muscle atrophy and altered myoblast proliferation and differentiation. PLoS One. 2013;8(8):e72330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nwadozi E, Ng A, Strömberg A, et al. Leptin is a physiological regulator of skeletal muscle angiogenesis and is locally produced by PDGFRα and PDGFRβ expressing perivascular cells. Angiogenesis. 2019;22(1):103–115. [DOI] [PubMed] [Google Scholar]
- 87. Shiuchi T, Toda C, Okamoto S, et al. Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle β2-adrenergic receptor but not by AMPK. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kapur S, Amoui M, Kesavan C, et al. Leptin receptor (Lepr) is a negative modulator of bone mechanosensitivity and genetic variations in Lepr may contribute to the differential osteogenic response to mechanical stimulation in the C57BL/6J and C3H/HeJ pair of mouse strains. J Biol Chem. 2010;285(48):37607–37618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All supplementary material and figures are located in a digital research materials repository (36).