Abstract
From 1970 to 2010 the foreign-born population in the United States has rapidly increased from 9.6 to 40.0 million individuals. Historically, differences in cancer rates have been observed between US-born and foreign-born individuals. However, comprehensive and up-to-date data on US cancer rates by birth place is lacking. To compare cancer mortality rates among foreign and US-born individuals, population-based cancer mortality data were obtained from the CDC’s National Center for Health Statistics. Utilizing data recorded on death certificates, individuals were categorized as US-born or foreign-born. Annual population estimates were obtained from the American Community Survey. Age-adjusted mortality rates and rate ratios (RRs) for all cancer sites were calculated using SEER*Stat. A total of 5,670,535 deaths from malignant cancers were recorded in the US from 2005 to 2014 and 9% of deaths occurred among foreign-born individuals. Overall, foreign-born individuals had a 31% lower cancer mortality rate when compared to US-born individuals (Rate Ratio (RR): 0.69 (95% CI: 0.68–0.69)), and similar results were observed when stratifying by sex, race/ethnicity, age, and geographic region. However, foreign-born individuals did have significantly elevated cancer mortality rates for seven cancers sites, of which five were infection-related, including: nasopharynx (RR: 2.01), Kaposi Sarcoma (RR: 1.94), stomach (RR: 1.82), gallbladder (RR: 1.47), acute lymphocytic leukemia (RR: 1.27), liver and intrahepatic bile duct (RR: 1.24), and thyroid (RR: 1.22) cancers. Many of these deaths could be avoided through improved access to prevention, screening, and treatment services for immigrant populations in the US or in their country of origin.
Keywords: Cancer, Foreign-born, Immigrant, US-born, Stomach, Cervical, Liver, Malignancy
1. Introduction
Foreign-born individuals in the United States (US) represent a rapidly growing segment of the general population. From 1970 to 2010 the foreign-born population in the US has increased from 9.6 to 40.0 million individuals, and in 2010 foreign-born individuals composed 12.9% of the general US population (U.S. Census Bureau, 2010; Singh and Hiatt, 2006). In contrast to 1970 when most immigrants into the US were from Europe, in 2010 most of the foreign-born population had immigrated from Latin America (51%), Asia (30%), or Europe (11%) (U.S. Census Bureau, 2010).
When compared to their US-born counterparts, foreign-born individuals are more likely to be uninsured, below the poverty line, have limited English proficiency, and lower educational attainment (Singh and Hiatt, 2006; Dominguez et al., 2015). Despite these disparities, after controlling for sociodemographic variables, immigrants on average are healthier, live longer, and have lower rates of hypertension, smoking, obesity, asthma, high cholesterol, heart disease, and mental health disorders than US-born individuals (Singh and Hiatt, 2006; Dominguez et al., 2015; Cunningham et al., 2008; Singh and Miller, 2004; Blue and Fenelon, 2011; Singh and Siahpush, 2001). This phenomenon is commonly referred to as the “immigrant paradox,” and there are a number of theories on why immigrants have improved health outcomes, including migration selectivity, higher social support, and a lower prevalence of harmful risk factors (e.g. smoking and obesity) when compared to their native-born counterparts (Singh and Hiatt, 2006; Cunningham et al., 2008; Singh and Miller, 2004; Singh and Siahpush, 2001).
Cancer outcome research reveals the same immigrant health advantage. Overall, immigrants have lower cancer mortality rates when compared to their respective US-born race/ethnicity groups (Singh and Hiatt, 2006; Singh and Miller, 2004; Singh et al., 2013). Due primarily to factors that occur prior to an individual’s immigration to the US (such as vaccine availability or acquisition of specific infections), foreign-born individuals have an elevated risk of developing and dying from a number of infection related cancers, including stomach (Singh and Hiatt, 2006; Cunningham et al., 2008; Singh and Miller, 2004; Singh and Siahpush, 2001; Singh et al., 2013), liver (Singh and Hiatt, 2006; Dominguez et al., 2015; Singh et al., 2013), and cervical (Singh and Hiatt, 2006; Dominguez et al., 2015; Singh and Miller, 2004; Seeff and McKenna, 2003) cancer. While they have lower cancer mortality rates overall, foreign-born individuals have lower access to healthcare, and are significantly less likely to undergo breast (Singh and Hiatt, 2006; Cunningham et al., 2008; Singh and Miller, 2004; Singh and Siahpush, 2001; White et al., 2017), cervical (Singh and Hiatt, 2006; Dominguez et al., 2015; White et al., 2017; Goel et al., 2003; Endeshaw et al., 2018a), and colorectal cancer screening when compared to their US-born counterparts (Singh and Hiatt, 2006; Dominguez et al., 2015; Singh and Miller, 2004; Seeff and McKenna, 2003).
The rapid rise observed in the foreign-born population has not been accompanied by an increase in immigrant health research (Singh and Hiatt, 2006). As such, the purpose of the present study is to use the most recent cancer mortality data to: a) describe cancer mortality rates among the foreign-born population; b) determine if any specific subgroups have elevated rates; c) identify specific cancers for which foreign-born individuals may have higher rates; and d) describe mortality rate changes over time.
2. Methods
2.1. Selection criteria, mortality data, and population estimates
Population-based cancer mortality data were obtained from the National Center for Health Statistics (NCHS) for all deaths occurring in the 50 states and the District of Columbia. NCHS collects information on sex, race, ethnicity, birth place, age at death, state of residence, and cause of death from death certificates filed in each state. Birth place is assigned by NCHS as one of the 50 states, the District of Columbia, Puerto Rico, Virgin Islands, Guam, Northern Mariana Islands, American Samoa, Canada, Cuba, Mexico, or the “remainder of the world”. Individuals whose death certificates stated they were born in one of the 50 states, the District of Columbia, or the US territories were categorized as US-born. Remaining cases with a recorded place of birth outside of the US and its territories were categorized as foreign-born (Singh et al., 2013). Mortality data for malignant cancers were selected using the Tenth Revision of the International Classification of Diseases (ICD-10) codes C00-C97. A total of 5,725,710 deaths from malignant cancer were recorded between 2005 and 2014. Cases with no recorded place of birth (0.96%) were excluded from all analyses, for a final sample size of 5,670,535 deaths.
Annual population estimates were obtained from the US Census Bureau’s American Community Survey (ACS). ACS is a continuous national survey of randomly selected households and collects detailed information on the US population including information on birth place (American Community Survey, 2016). ACS also provides public-use microdata sample (PUMS) files that can be used to estimate the US population using social, housing and demographic characteristics. We obtained the annual ACS PUMS files from 2005 to 2014 and extracted the US-born and foreign-born population (based on place of birth) for age, race/ethnicity, sex, and Census region subgroups using provided person weights (American Community Survey, 2016).
2.2. Statistical analysis
Mortality data from NCHS and population estimates from ACS for 2005–2014 were formatted using the Surveillance Epidemiology End Results (SEER) Program SEER*Prep (Surveillance Research Program National Cancer Institute, 2016) software to create a SEER*Stat database (Surveillance Research Program National Cancer Institute, 2017). We calculated age-adjusted mortality rates of malignant cancers by birth place, age-adjusting to the 2000 US standard population. Race/ethnicity was categorized into four mutually exclusive categories including: non-Hispanic white, non-Hispanic black, non-Hispanic Asian/Pacific Islander (API), and Hispanic (henceforth referred to as whites, blacks, APIs and Hispanics). Due to the lack of foreign-born American Indian/Alaskan Natives, this group was excluded from all analyses. SEER*Stat was used to calculate rate ratios by birth place (US-born vs. foreign-born), sex, age, race/ethnicity, and cancer site. When utilizing rate ratios to identify cancers for which foreign-born individuals may have higher rates, the Bonferroni correction was performed to account for multiple comparisons in which p = 0.05 is divided by the number of comparisons (i.e. the number of cancer types evaluated). The average annual percent change (AAPC) of cancer death rates from 2005 to 2014 by birth place, sex, race/ethnicity, and cancer site was calculated using Joinpoint. The Tiwari et al., modification was used to calculate AAPC confidence intervals (Tiwari et al., 2006).
3. Results
From 2005 to 2014, 5,670,535 deaths from malignant cancers occurred in the United States, of which 528,694 (9.3%) occurred among foreign-born individuals. Overall, foreign-born individuals had a lower cancer mortality rate when compared to US-born individuals (RR: 0.69 (95% CI: 0.68, 0.69)). When stratified by sex, this pattern remained significant with foreign-born men and women having a 32% and 30% lower cancer mortality rate when compared to their respective counterparts. When stratifying by sex, age, race/ethnicity, and geographic regions, foreign-born individuals had a significantly lower cancer mortality rates for all stratification groups (Table 1).
Table 1.
Age-adjusted cancer mortality rates in the United States by birth place, sex, age, race/ethnicity, and US Census Region: 2005–2014.a
Source: National Center for Health Statistics (NCHS) & American Community Survey.
| US-born | Foreign-born | Rate ratioc (95% CI) | |||
|---|---|---|---|---|---|
| No. of cases | Average annual rate (95% CI) | No. of cases | Average annual rate (95% CI) | ||
| Overallb | 5,141,841 | 178.87 (178.72, 179.03) | 528,694 | 122.76 (122.43, 123.10) | 0.69* (0.68, 0.69) |
| Sex | |||||
| Men | 2,700,236 | 217.42 (217.15, 217.68) | 261,419 | 148.04 (147.44, 148.63) | 0.68* (0.68, 0.68) |
| Women | 2,441,605 | 151.73 (151.54, 151.93) | 267,275 | 106.88 (106.47, 107.29) | 0.70* (0.70, 0.71) |
| Age (years) – male | |||||
| < 35 | 30,105 | 4.76 (4.71, 4.82) | 4480 | 4.50 (4.31, 4.69) | 0.94* (0.90, 0.99) |
| 35–49 | 117,152 | 43.58 (43.33, 43.83) | 17,158 | 26.20 (25.81, 26.60) | 0.60* (0.59, 0.61) |
| 50–64 | 684, 193 | 278.33 (277.67, 278.99) | 64,028 | 160.99 (159.75, 162.25) | 0.58* (0.57, 0.58) |
| 65–79 | 1,117,658 | 994.21 (992.35, 996.07) | 101,756 | 649.78 (645.74, 653.83) | 0.65* (0.65, 0.66) |
| 80+ | 751,128 | 2117.56 (2112.77, 2122.37) | 73,997 | 1651.83 (1639.94, 1663.79) | 0.78* (0.77, 0.79) |
| Age (years) – female | |||||
| < 35 | 26,468 | 4.36 (4.30, 4.41) | 3992 | 4.00 (3.84, 4.17) | 0.92* (0.88, 0.96) |
| 35–49 | 138,378 | 50.73 (50.46, 51.00) | 22,676 | 34.95 (34.49, 35.40) | 0.69* (0.68, 0.70) |
| 50–64 | 567,445 | 218.36 (217.79, 218.94) | 62,201 | 141.68 (140.57, 142.80) | 0.65* (0.64, 0.65) |
| 65–79 | 921,146 | 683.94 (682.54, 685.34) | 94,943 | 446.38 (443.53, 449.24) | 0.65* (0.65, 0.66) |
| 80+ | 788,168 | 1238.89 (1236.15, 1241.65) | 83,463 | 1017.85 (1010.95, 1024.79) | 0.82* (0.82, 0.83) |
| Race/ethnicity – male | |||||
| Non-Hispanic White | 2,274,490 | 214.79 (214.51, 215.08) | 112,397 | 177.29 (176.24, 178.35) | 0.83* (0.82, 0.83) |
| Non-Hispanic Black | 310,684 | 285.95 (284.88, 287.03) | 15,788 | 149.33 (146.68, 152.02) | 0.52* (0.51, 0.53) |
| Non-Hispanic APId | 13,930 | 157.77 (155.07, 160.49) | 54,785 | 128.80 (127.64, 129.97) | 0.82* (0.80, 0.83) |
| Hispanic | 83,172 | 163.36 (162.17, 164.56) | 77,785 | 133.62 (132.59, 134.66) | 0.82* (0.81, 0.83) |
| Race/ethnicity – female | |||||
| Non-Hispanic White | 2,049,881 | 151.65 (151.44, 151.86) | 120,229 | 131.58 (130.80, 132.37) | 0.87* (0.86, 0.87) |
| Non-Hispanic Black | 293,425 | 178.69 (178.04, 179.35) | 17,465 | 106.33 (104.68, 108.01) | 0.60* (0.59, 0.60) |
| Non-Hispanic APId | 11,927 | 113.71 (111.58, 115.86) | 53,813 | 90.82 (90.02, 91.62) | 0.80* (0.78, 0.82) |
| Hispanic | 70,392 | 106.60 (105.79, 107.41) | 75,153 | 93.13 (92.44, 93.83) | 0.87* (0.86, 0.88) |
| Regions of the United States | |||||
| Northeast | 964,618 | 179.52 (179.16, 179.89) | 132,568 | 124.33 (123.65, 125.02) | 0.69* (0.69, 0.70) |
| Midwest | 1,227,103 | 183.33 (183.01, 183.65) | 58,821 | 130.56 (129.49, 131.64) | 0.71* (0.71, 0.72) |
| South | 1,974,091 | 182.05 (181.79, 182.31) | 150,023 | 120.34 (119.71, 120.96) | 0.66* (0.66, 0.66) |
| West | 926,029 | 166.18 (165.84, 166.53) | 187,282 | 121.19 (120.63, 121.76) | 0.73* (0.73, 0.73) |
Rates are age-adjusted to 2000 standard U.S. population and shown per 100,000.
Columns may not sum to total because of missing values.
US-born were used as a reference group to generate rate ratios.
Asian and Pacific Islander (API).
p < 0.05.
Overall, cancer mortality rates significantly decreased among US-born (AAPC: −1.68; 95% CI: −2.26, −1.10) and foreign-born individuals (AAPC: −1.62; 95% CI: −2.25, −0.99) from 2005 to 2014. While cancer mortality rates remained constant over the study period for US-born API and foreign-born Hispanics, cancer mortality rates significantly decreased among all other race/ethnicity stratifications (Fig. 1). Despite significant reductions in cancer mortality from 2005 to 2014 across most race/ethnicity stratifications, the associated rate ratios comparing US and foreign-born by race/ethnicity remained largely unchanged (data not shown).
Fig. 1.
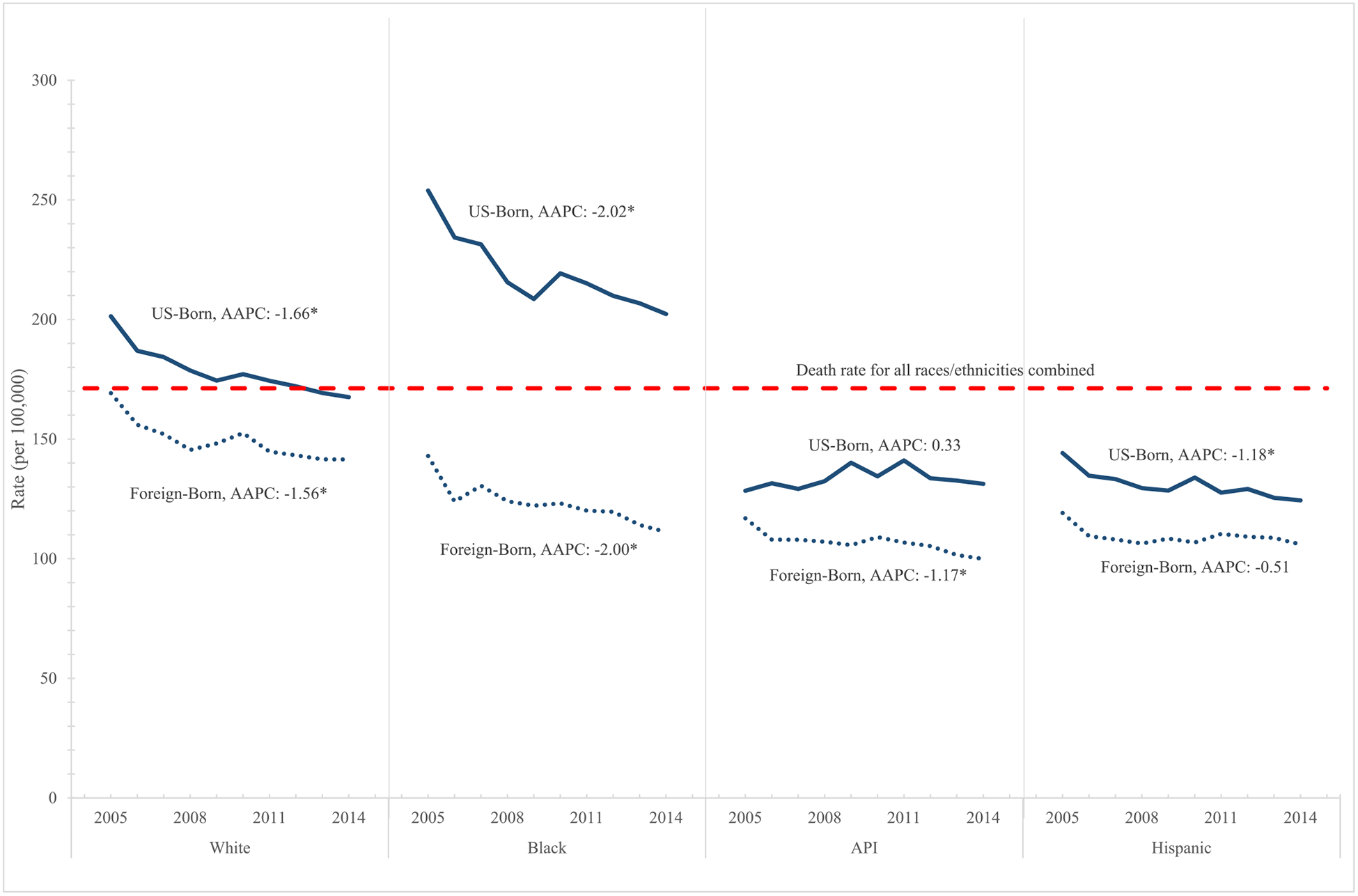
Trends in age-adjusted cancer mortality rates by race/ethnicity: United States 2005–2014.
*AAPC significantly different from 0 at the p = 0.05 level.
When comparing cancer mortality rates among US and foreign-born individuals for the top 12 causes of cancer mortality among men and women in the United States, foreign-born individuals had significantly lower cancer mortality rates for most cancer sites, with the exception of liver and intrahepatic bile duct among men and women and stomach among men (Table 2) (Ryerson et al., 2016). Of the sixty-five cancer sites evaluated, foreign-born individuals had significantly elevated mortality rates for seven selected cancer sites: nasopharynx (RR: 2.01), Kaposi sarcoma (RR: 1.94), stomach (RR: 1.82), gallbladder (RR: 1.47), acute lymphocytic leukemia (RR: 1.27), liver and intrahepatic bile duct (RR: 1.24), and thyroid (RR: 1.22). Liver and intrahepatic bile duct and stomach cancer mortality rates were not only elevated among the foreign-born population, but accounted for a large number of cancer-related deaths (31,097 and 24,583 respectively). Among the foreign-born population, there were racial/ethnic differences in the mortality rates of specific cancer sites when compared to US-born individuals (Table 3).
Table 2.
Age-adjusted cancer mortality rates in the United States 2005–2014 for the most common cancers, by sex and birth place.a
Source: National Center for Health Statistics (NCHS) & American Community Survey.
| Cancer site | Overall | US-born | Foreign-born | Rate ratiob | |||
|---|---|---|---|---|---|---|---|
| No. of cases | Average annual rate (95% CI) | No. of cases | Average annual rate (95% CI) | No. of cases | Average annual rate (95% CI) | Rate ratio (95% CI) | |
| Top 12 cancer sites among men | |||||||
| Lung and bronchus | 865,639 | 60.04 (59.91, 60.17) | 806,077 | 63.88 (63.74, 64.03) | 59,562 | 33.91 (33.63, 34.19) | 0.53* (0.53, 0.54) |
| Prostate | 279,970 | 21.74 (21.66, 21.82) | 254,407 | 22.40 (22.31, 22.49) | 25,563 | 16.85 (16.64, 17.06) | 0.75* (0.74, 0.76) |
| Colon and rectum | 266,503 | 18.74 (18.67, 18.82) | 240,268 | 19.39 (19.31, 19.47) | 26,235 | 14.59 (14.41, 14.78) | 0.75* (0.74, 0.76) |
| Pancreas | 181,499 | 12.50 (12.44, 12.56) | 163,609 | 12.90 (12.83, 12.96) | 17,890 | 9.95 (9.79, 10.10) | 0.77* (0.76, 0.78) |
| Leukemia | 128,641 | 9.34 (9.29, 9.39) | 116,566 | 9.68 (9.63, 9.74) | 12,075 | 6.96 (6.83, 7.10) | 0.72* (0.70, 0.73) |
| Liver and intrahepatic bile duct | 132,978 | 8.66 (8.61, 8.71) | 113,666 | 8.49 (8.44, 8.54) | 19,312 | 9.92 (9.78, 10.07) | 1.17* (1.15, 1.19) |
| Non-Hodgkin lymphoma | 110,353 | 7.95 (7.90, 8.00) | 99,337 | 8.20 (8.15, 8.25) | 11,016 | 6.22 (6.10, 6.35) | 0.76* (0.74, 0.77) |
| Urinary bladder | 101,622 | 7.65 (7.60, 7.69) | 93,367 | 7.98 (7.93, 8.03) | 8255 | 5.30 (5.19, 5.42) | 0.66* (0.65, 0.68) |
| Esophagus | 111,207 | 7.46 (7.42, 7.51) | 104,539 | 8.05 (8.00, 8.10) | 6668 | 3.60 (3.51, 3.69) | 0.45* (0.44, 0.46) |
| Kidney and renal pelvis | 83,287 | 5.72 (5.68, 5.76) | 76,254 | 6.00 (5.96, 6.05) | 7033 | 3.83 (3.74, 3.93) | 0.64* (0.62, 0.65) |
| Brain and other nervous system | 78,987 | 5.24 (5.20, 5.27) | 71,277 | 5.48 (5.44, 5.52) | 7710 | 3.84 (3.75, 3.93) | 0.70* (0.68, 0.72) |
| Stomach | 66,218 | 4.64 (4.60, 4.68) | 52,527 | 4.23 (4.20, 4.27) | 13,691 | 7.39 (7.26, 7.52) | 1.75* (1.71, 1.78) |
| Top 12 cancer sites among women | |||||||
| Lung and bronchus | 695,744 | 37.64 (37.55, 37.73) | 650,829 | 40.76 (40.66, 40.87) | 44,915 | 18.22 (18.06, 18.40) | 0.45* (0.44, 0.45) |
| Breast | 405,140 | 21.93 (21.86, 22.00) | 363,520 | 22.91 (22.84, 22.99) | 41,620 | 16.02 (15.86, 16.17) | 0.70* (0.69, 0.71) |
| Colon and rectum | 251,251 | 13.19 (13.13, 13.24) | 224,969 | 13.63 (13.57, 13.68) | 26,282 | 10.49 (10.36, 10.62) | 0.77* (0.76, 0.78) |
| Pancreas | 179,126 | 9.49 (9.45, 9.54) | 159,150 | 9.73 (9.68, 9.77) | 19,976 | 8.13 (8.01, 8.24) | 0.84* (0.82, 0.85) |
| Ovary | 143,599 | 7.74 (7.70, 7.78) | 128,022 | 8.00 (7.96, 8.05) | 15,577 | 6.13 (6.03, 6.23) | 0.77* (0.75, 0.78) |
| Leukemia | 96,631 | 5.19 (5.16, 5.23) | 86,250 | 5.33 (5.29, 5.36) | 10,381 | 4.31 (4.22, 4.40) | 0.81* (0.79, 0.83) |
| Non-Hodgkin lymphoma | 92,289 | 4.88 (4.85, 4.91) | 82,190 | 4.99 (4.96, 5.03) | 10,099 | 4.11 (4.02, 4.19) | 0.82* (0.81, 0.84) |
| Corpus and uterus, NOS | 81,607 | 4.36 (4.33, 4.39) | 72,297 | 4.47 (4.44, 4.50) | 9310 | 3.66 (3.58, 3.73) | 0.82* (0.80, 0.84) |
| Liver and intrahepatic bile duct | 65,579 | 3.51 (3.48, 3.54) | 53,794 | 3.32 (3.29, 3.35) | 11,785 | 4.80 (4.71, 4.89) | 1.44* (1.42, 1.48) |
| Brain and other nervous system | 62,299 | 3.48 (3.45, 3.51) | 55,833 | 3.63 (3.60, 3.66) | 6466 | 2.66 (2.59, 2.73) | 0.73* (0.71, 0.75) |
| Myeloma | 51,199 | 2.72 (2.69, 2.74) | 45,853 | 2.80 (2.77, 2.83) | 5346 | 2.17 (2.11, 2.23) | 0.77* (0.75, 0.80) |
| Kidney and renal pelvis | 47,286 | 2.51 (2.49, 2.54) | 42,910 | 2.63 (2.61, 2.66) | 4376 | 1.77 (1.72, 1.83) | 0.67* (0.65, 0.70) |
p < 0.05.
Rates are age-adjusted to 2000 standard U.S. population and shown per 100,000.
US-born were used as a reference group to generate rate ratios.
Table 3.
Age-adjusted cancer mortality rates that are elevated among foreign-born individuals, by race/ethnicity: United States 2005–2014.a
Source: National Center for Health Statistics (NCHS) & American Community Survey.
| Elevated cancers among foreign-born individuals, by race/ethnicity | US-born | Foreign-born | Rate ratiob (99.92% CI) | ||
|---|---|---|---|---|---|
| No. of cases | Average annual rate (99.92% CI) | No. of cases | Average annual rate (99.92% CI) | ||
| Overall | |||||
| Nasopharynx | 4839 | 0.17 (0.16, 0.18) | 1635 | 0.34 (0.31, 0.37) | 2.01* (1.82, 2.21) |
| Kaposi sarcoma | 360 | 0.01 (0.01, 0.02) | 107 | 0.03 (0.02, 0.04) | 1.94* (1.31, 2.90) |
| Stomach | 87,135 | 3.05 (3.01, 3.08) | 24,583 | 5.56 (5.44, 5.68) | 1.82* (1.78, 1.87) |
| Gallbladder | 16,785 | 0.58 (0.57, 0.60) | 3690 | 0.86 (0.81, 0.91) | 1.47* (1.38, 1.56) |
| Acute lymphocytic leukemia | 11,874 | 0.43 (0.42, 0.45) | 2321 | 0.55 (0.51, 0.60) | 1.27* (1.16, 1.39) |
| Liver and intrahepatic bile duct | 167,460 | 5.69 (5.64, 5.73) | 31,097 | 7.04 (6.91, 7.18) | 1.24* (1.21, 1.27) |
| Thyroid | 14,025 | 0.49 (0.47, 0.50) | 2554 | 0.60 (0.56, 0.64) | 1.22* (1.14, 1.32) |
| Non-Hispanic White | |||||
| Kaposi sarcoma | 255 | 0.01 (0.01, 0.01) | 44 | 0.02 (0.01, 0.05) | 2.33* (1.27, 5.26) |
| Stomach | 61,252 | 2.53 (2.49, 2.56) | 8022 | 5.18 (4.99, 5.39) | 2.05* (1.97, 2.14) |
| Gallbladder | 13,151 | 0.54 (0.52, 0.55) | 1110 | 0.70 (0.63 0.78) | 1.30* (1.17, 1.45) |
| Non-Hispanic Black | |||||
| Gallbladder | 2336 | 0.87 (0.81, 0.93) | 305 | 1.13 (0.91, 1.39) | 1.30* (1.04, 1.62) |
| Non-Hispanic API | |||||
| Liver and intrahepatic bile duct | 1300 | 6.76 (6.13, 7.44) | 11,581 | 10.84 (10.49, 11.20) | 1.60* (1.45, 1.78) |
| Nasopharynx | 121 | 0.62 (0.44, 0.83) | 1101 | 0.91 (0.82, 1.01) | 1.47* (1.07, 2.10) |
| Gallbladder | 99 | 0.53 (0.36, 0.73) | 782 | 0.78 (0.69, 0.88) | 1.48* (1.04, 2.20) |
| Hispanic | |||||
| Gallbladder | 965 | 0.87 (0.78, 0.97) | 1485 | 1.06 (0.97, 1.16) | 1.22* (1.06, 1.41) |
| Melanoma of the skin | 826 | 0.67 (0.59, 0.76) | 1290 | 0.85 (0.77, 0.94) | 1.26* (1.08, 1.49) |
| Acute lymphocytic leukemia | 1642 | 0.65 (0.59, 0.72) | 1399 | 0.77 (0.69, 0.86) | 1.19* (1.02, 1.38) |
The Bonferroni adjustment was performed to account for multiple comparisons: p < 0.0008.
Rates are age-adjusted to 2000 standard U.S. population and shown per 100,000.
US-born were used as a reference group to generate rate ratios.
Foreign-born individuals did not have elevated cancer mortality rates for cancers that have screening and early detection technologies for which the United States Preventive Service Task Force has a recommendation (Recommendations for Primary Care Practice, 2018): breast, cervical, colorectal, or lung and bronchus cancers, both overall, and when stratified by race/ethnicity (data not shown). Overall, similar declines in cancer mortality trends were observed when comparing these cancers between US and foreign-born individuals. While cervical cancer mortality significantly decreased among foreign-born individuals overall, no significant decrease was observed among US-born individuals (data not shown).
4. Discussion
In the present study, foreign-born individuals in the US had lower rates of cancer mortality when compared to the US-born population. When stratifying by age, race/ethnicity, sex, and geographic location, no subgroup of the foreign-born population had higher death rates. Despite overall lower rates of cancer mortality, foreign-born individuals did have significantly elevated cancer mortality rates for seven cancers sites including: nasopharynx (RR: 2.01), Kaposi Sarcoma (RR: 1.94), stomach (RR: 1.82), gallbladder (RR: 1.47), acute lymphocytic leukemia (RR: 1.27), liver and intrahepatic bile duct (RR: 1.24), and thyroid (RR: 1.22) cancers. Despite the high cancer mortality rate observed among US-born blacks when compared to other races/ethnicities in the US-born population, the highest cancer death rates were observed among foreign-born whites. When analyzing trends over time, cancer death rates among foreign and US-born individuals significantly decreased at near identical rates during the study period.
The findings of this study are consistent with other studies that have observed lower cancer mortality rates among foreign-born populations overall, but higher cancer death rates for specific infection-related cancers (stomach and liver and intrahepatic bile duct) (Singh and Hiatt, 2006; Dominguez et al., 2015; Cunningham et al., 2008; Singh and Miller, 2004; Singh and Siahpush, 2001; Singh et al., 2013). These findings also align with previous trends on a widening disparity in cancer mortality when comparing US-and foreign-born individuals, with the foreign-born population experiencing 12% lower cancer death rate in 1979–1981, 21% lower in 1989–1991, 25% lower in 1999–2001, and 31% lower in the present study (Singh and Hiatt, 2006).
Despite an overall elevated rate of liver cancer mortality, when stratified by race/ethnicity foreign-born APIs were the only racial/ethnic group to experience elevated rates of liver and intrahepatic bile duct cancer mortality when compared to their US-born counterparts (Endeshaw et al., 2018b). The higher mortality rates of liver and intrahepatic bile duct cancer observed among APIs can likely be explained by elevated rates of hepatitis B acquired prior to immigrating to the United States (Pollack et al., 2014; Kowdley et al., 2012; El-Serag et al., 2007). As global hepatitis B childhood vaccination programs continue to improve, it is likely that the mortality rates observed among foreign-born APIs will begin to decline (Ryerson et al., 2016).
Foreign-born individuals had higher rates of stomach cancer mortality when compared to US-born individuals (RR: 1.82; 95% CI: 1.80, 1.85), and this remained after stratifying by sex, geographic location, and age. Of note, however, the excess stomach cancer mortality observed among foreign-born individuals was primarily a function of relatively low stomach cancer mortality among US-born whites (2.53 per 100,000) when compared to foreign-born whites (5.18), blacks (US-born: 6.55; foreign-born: 6.05), APIs (US-born: 5.92; foreign-born: 6.31), and Hispanics (US-born: 5.35; foreign-born: 5.49) (Hallowell et al., 2018). As roughly 89% of stomach cancers can be attributed to H. pylori infection worldwide (Plummer et al., 2015), the elevated cancer death rates observed among US-born minority groups when compared to US-born whites is likely reflective of elevated H. pylori infection rates in these populations, which has previously been documented (Grad et al., 2011). Worldwide, over half of the world’s population is infected with H. pylori, with the prevalence of H. pylori varying widely by geographic region (Africa (79.1%), Latin America (63.4%), Asia (54.7%), Europe (34.3–62.8%), North America (37.1%)) (Hooi et al., 2017). Subsequently, individuals born in these areas have a high risk of H. pylori infection and the subsequent development of stomach cancer, despite moving to lower risk countries such as the United States after birth (El-Serag et al., 2018). To help address this issue, H. pylori screening is beginning to be recommended for first generation immigrants who migrate from high burden countries (El-Serag et al., 2018).
In addition to stomach and liver and intrahepatic bile duct cancers, foreign-born individuals in this study had higher cancer mortality rates for a number of other rare infection-related cancers when compared to their US-counterparts, including: nasopharynx (Epstein-Barr virus), Kaposi Sarcoma (human herpesvirus type 8), and gallbladder (liver fluke) cancers (Plummer et al., 2016). This closely aligns with global trends as only 4.0% of new cancer cases in North America can be attributed to infections, in contrast to 15.4% world-wide and up to 40% in some sub-Saharan regions (Plummer et al., 2016). Unfortunately, the limited information available in this data set with regards to place of birth did not allow us to identify specific subgroups of the foreign-born population that may have higher cancer death rates from these infection-related cancers. Other work, however, has found these infection-related cancers to be elevated based on geographic location, with elevated rates of Kaposi Sarcoma observed among individuals of Mediterranean or Eastern European descent (Iscovich et al., 2000), elevated gallbladder cancer rates among individuals from Chile, Korea, Japan, Czech Republic, Hungary, and Poland (Torre et al., 2017), and higher nasopharynx cancer rates among individuals from East and Southeast Asia (Wei and Sham, 2005). In contrast to previous work, foreign-born individuals did not have higher rates of cervical cancer mortality (RR: 0.95 (95% CI: 0.92, 0.97)) (Singh and Hiatt, 2006; Singh and Miller, 2004; Seeff and McKenna, 2003; Hallowell et al., 2019) overall or when stratified by race/ethnic group (data not shown). However, when stratified by age, older (65+) foreign-born women did have significantly higher mortality rates when compared to their respective US-born counterparts (age 65–79 years (rate ratio = 1.15, 95% CI = 1.09, 1.22); ≥80 years (rate ratio = 1.43, 95% CI = 1.32, 1.55)) (Hallowell et al., 2019).
Other cancers which foreign-born individuals experienced higher mortality, including acute lymphocytic leukemia and thyroid cancer, have relatively high survival rates (Inaba et al., 2013; Davies and Welch, 2010). As such, the mortality differentials observed for these cancers could reflect known disparities in access to healthcare and health insurance among the foreign-born population (Singh and Hiatt, 2006; Dominguez et al., 2015). With improved access to healthcare services among the foreign-born population, observed mortality differences may disappear.
Despite facing numerous sociodemographic disadvantages (Singh and Hiatt, 2006; Dominguez et al., 2015) the overall lower cancer death rate observed among the foreign-born population is expected. Smoking is one of the leading causes of cancer and cancer related mortality in the US, and overall foreign-born individuals are 50% less likely to report smoking cigarettes when compared to their US-born counterparts, even when controlling for socioeconomic and demographic variables (Singh and Hiatt, 2006; Dominguez et al., 2015; Bosdriesz et al., 2013). While other work has documented the higher life expectancy observed among the foreign-born population, most of the improved life expectancy for both men and women can be attributed to differences in smoking prevalence when compared to their US-born counterparts (Blue and Fenelon, 2011). The lower prevalence of obesity, alcohol consumption, and unhealthy diets observed among the foreign-born population are likely further reducing their cancer risk (Singh and Hiatt, 2006; Dominguez et al., 2015; Cunningham et al., 2008; Singh and Miller, 2004; Blue and Fenelon, 2011; Singh and Siahpush, 2001). Finally, the health advantage observed in the foreign-born population could be due to migration selectivity (i.e. individuals who migrate to the US are healthier and have better health outcomes than those who remain behind) or the salmon bias (i.e. the return of less healthy immigrants to their country of origin prior to death) (Singh and Hiatt, 2006; Cunningham et al., 2008; Singh and Miller, 2004; Singh and Siahpush, 2001).
The main strength of this study is that it provides the most up-to-date comprehensive examination of cancer death rates by birth place, sex, age, and race/ethnicity for the entire US population over the last decade. Unfortunately, similar analyses for cancer incidence cannot be performed due to the large proportion of individuals with missing birth place information in cancer registry data (Singh and Hiatt, 2006). As the data in this study were based on information obtained from death certificates, one major limitation of this study is the lack of information on immigration variables that play a key role in mortality, such as length of residency (Singh and Hiatt, 2006; Cunningham et al., 2008; Singh and Miller, 2004), English proficiency, and legal and citizenship status (Singh and Hiatt, 2006; Reyes and Miranda, 2015) or specific country of origin. Additionally, we did not have access to other variables of interest such as socioeconomic position, education level, health insurance status, and other risk factor data which would likely impact cancer death rates.
In summary, foreign-born individuals in the US have lower cancer mortality rates when compared to the US-born population, despite numerous sociodemographic disparities. However, foreign-born individuals have higher cancer mortality rates for select infection-related cancers, in particular liver and stomach. Efforts to address the major risk factors for these cancers (hepatitis B and H. pylori) are needed to relieve this health disparity, and reduce the burden of these cancers in the US.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- American Community Survey, 2016. In: U.S.C. Bureau (Ed.), Design and Methodology Report. [Google Scholar]
- Blue L, Fenelon A, 2011. Explaining low mortality among US immigrants relative to native-born Americans: the role of smoking. Int. J. Epidemiol 40 (3), 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosdriesz JR, et al. , 2013. Smoking prevalence among migrants in the US compared to the US-born and the population in countries of origin. PLoS One 8 (3), e58654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SA, Ruben JD, Narayan KV, 2008. Health of foreign-born people in the United States: a review. Health Place 14 (4), 623–635. [DOI] [PubMed] [Google Scholar]
- Davies L, Welch HG, 2010. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch. Otolaryngol. Head Neck Surg 136 (5), 440–444. [DOI] [PubMed] [Google Scholar]
- Dominguez K, et al. , 2015. Vital signs: leading causes of death, prevalence of diseases and risk factors, and use of health services among Hispanics in the United States-2009–2013. MMWR. Morb. Mortal. Wkly Rep 64 (17), 469–478. [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, et al. , 2007. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch. Intern. Med 167 (18), 1983–1989. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, et al. , 2018. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin. Gastroenterol. Hepatol 16 (7), 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeshaw M, Clarke T, Senkomago V, Saraiya M, 2018a. Cervical cancer screening among women by birthplace and percent of lifetime living in the United States. J. Lower Genital Tract Dis 22 (4), 280–287 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endeshaw M, et al. , 2018b. Trends in liver cancer mortality in the United States: dual burden among foreign-and US-born persons. Cancer 125 (5), 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel MS, et al. , 2003. Racial and ethnic disparities in cancer screening. J. Gen. Intern. Med 18 (12), 1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad YH, Lipsitch M, Aiello AE, 2011. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am. J. Epidemiol 175 (1), 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallowell BD, Endeshaw M, Razzaghi H, Senkomago V, McKenna MT, Saraiya M, 2018. Stomach Cancer Mortality Rates among US and Foreign-Born Persons: United States 2005–2015. [DOI] [PMC free article] [PubMed]
- Hallowell BD, et al. , 2019. Cervical Cancer Death Rates Amolng U.S. and Foreign-Born Individuals: U.S. 2005–2014. AJPM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooi JK, et al. , 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153 (2), 420–429. [DOI] [PubMed] [Google Scholar]
- Inaba H, Greaves M, Mullighan CG, 2013. Acute lymphoblastic leukaemia. Lancet 381 (9881), 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscovich J, et al. , 2000. Classic Kaposi sarcoma. Cancer 88 (3), 500–517. [PubMed] [Google Scholar]
- Kowdley KV, et al. , 2012. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 56 (2), 422–433. [DOI] [PubMed] [Google Scholar]
- Plummer M, et al. , 2015. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 136 (2), 487–490. [DOI] [PubMed] [Google Scholar]
- Plummer M, et al. , 2016. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health 4 (9), e609–e616. [DOI] [PubMed] [Google Scholar]
- Pollack HJ, et al. , 2014. Chronic Hepatitis B and Liver Cancer Risks Among Asian Immigrants in New York City: Results From a Large, Community-based Screening, Evaluation, and Treatment Program. AACR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommendations for Primary Care Practice. Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/recommendations.
- Reyes AM, Miranda PY, 2015. Trends in cancer screening by citizenship and health insurance, 2000–2010. J. Immigr. Minor. Health 17 (3), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson AB, et al. , 2016. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 122 (9), 1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeff LC, McKenna MT, 2003. Cervical cancer mortality among foreign-born women living in the United States, 1985 to 1996. Cancer Detect. Prev 27 (3), 203–208. [DOI] [PubMed] [Google Scholar]
- Singh GK, Hiatt RA, 2006. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003. Int. J. Epidemiol 35 (4), 903–919. [DOI] [PubMed] [Google Scholar]
- Singh GK, Miller BA, 2004. Health, life expectancy, and mortality patterns among immigrant populations in the United States. Can. J. Public Health 95 (3), 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Siahpush M, 2001. All-cause and cause-specific mortality of immigrants and native born in the United States. Am. J. Public Health 91 (3), 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh GK, Rodriguez-Lainz A, Kogan MD, 2013. Immigrant health inequalities in the United States: use of eight major national data systems. Sci. World J 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Research Program National Cancer Institute, 2016. SEER*Prep Software. National Cancer Institute, Bethesda, MD. [Google Scholar]
- Surveillance Research Program National Cancer Institute, 2017. SEER*Stat Software. National Cancer Institute, Bethesda, MD. [Google Scholar]
- Tiwari RC, Clegg LX, Zou Z, 2006. Efficient interval estimation for age-adjusted cancer rates. Stat. Methods Med. Res 15 (6), 547–569. [DOI] [PubMed] [Google Scholar]
- Torre LA, et al. , 2017. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin. Gastroenterol. Hepatol 16 (3), 427–437. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, U.S. Census Bureau (Eds.), 2010. U.S. Census Bureau, 2010 American Community Survey. [Google Scholar]
- Wei WI, Sham JS, 2005. Nasopharyngeal carcinoma. Lancet 365 (9476), 2041–2054. [DOI] [PubMed] [Google Scholar]
- White A, et al. , 2017. Cancer screening test use-United States, 2015. MMWR Morb. Mortal. Wkly Rep 66 (8), 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


