Abstract
Importance:
The American Joint Committee on Cancer, 8th edition (AJCC-8) contains a new staging system for human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OPSCC). Our study aim was to evaluate the effectiveness of the AJCC-8 relative to the AJCC 7th edition (AJCC-7).
Materials and Methods:
A retrospective chart review was performed on a multi- institutional, prospectively collected dataset from two tertiary referral centers. All patients had HPV+ OPSCC treated primarily with surgery. The prognostic value of AJCC-7 and AJCC-8 were compared for 5-year overall survival (OS) and disease-specific survival (DFS).
Results:
AJCC-8 pathological staging effectively risk stratified patients, creating a Cox model with a better fit (lower Akaike’s Information Criterion, p<0.0001) when compared to AJCC-7 pathological stages for both OS and DFS. The AJCC-8 pathologic staging did not produce a better fit than the AJCC-8 clinical staging (p=0.15) for OS, however, AJCC-8 pathologic was more effective than AJCC-8 clinical for DFS (p<0.0001). 76% of patients did not change their stage between clinical and pathologic AJCC-8 staging; 14% were upstaged by 1, <1% were upstaged by 2, 7% were downstaged by 1, and 3% downstaged by 2.
Conclusions and Relevance:
The new AJCC-8 staging system represents a significant improvement over AJCC-7 for risk stratification into groups that predict overall survival and disease-specific survival of surgically treated HPV+ OPSCC patients. The AJCC- 8 pathologic staging system was not significantly better than the AJCC-8 clinical staging system for overall survival, however, the pathologic staging system was better than the clinical for disease free survival.
Keywords: Human Papilloma Virus, HPV, Oropharyngeal Squamous Cell Carcinoma, Oropharyngeal Cancer, American Joint Committee on Cancer, AJCC, Cancer Staging
Introduction
Over the last two decades, human papillomavirus related (HPV+) oropharyngeal squamous cell carcinoma (OPSCC) has been recognized as a fundamentally different disease than tobacco and alcohol related (HPV-) OPSSC.1–3 While both diseases are squamous cell carcinomas that arise in the oropharynx, HPV+ disease tends to affect a younger cohort of patients with a different set of risk factors,1–3 and has been shown to have different molecular biology than HPV negative disease4. Importantly, patients with HPV+ disease have a substantially improved prognosis.1–3,5 Because of the improved survival in the HPV+ cohort, a new staging system separate from the HPV- OPSCC staging system was required to accurately risk stratify these patients.5,6
The American Joint Committee on Cancer (AJCC) recently released a new staging system in the 8th edition of their Cancer Staging Manual (AJCC-8) based primarily on work from the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S) and Haughey et. al.7–9 Compared to the AJCC-7, patients with HPV+ OPSCC are now substantially down-staged, primarily reflecting their excellent prognosis despite regional nodal disease.9,10 Additionally, AJCC-8 has different definitions for clinical and pathologic nodal stage and variations in the definitions of clinical and pathologic overall stage for HPV+ OPSCC. These differences reflect the fact that the ICON-S and Haughey study populations were treated differently, the former being primarily chemoradiated and the later having undergone surgery plus adjuvant therapy as indicated.7,8
Given that the AJCC-8 was recently released, it still requires validation. The goals of our study are to apply the AJCC-8 to a multi-institutional cohort of surgically treated patients with HPV-related OPSCC and validate the staging prognostic stratification. We also examine the clinical and pathologic staging of these patients to determine how pathologic data effects patients’ staging.
Methods:
University of Pittsburgh Medical Center (UPMC) and Oregon Health & Science University (OHSU) Institutional Review Board (IRB) approval was obtained. A retrospective review of a prospectively collected multi-institutional dataset was performed. Included patients had HPV+ OPSCC as determined by p16 immunohistochemistry and were treated primarily with surgery. All surgical approaches were included including traditional transoral surgery, transoral laser microsurgery and transoral robotic surgery (TORS).
The tumor (T), lymph node (N) and distant metastatic (M) classifications vary from AJCC-7 to the AJCC-8. Additionally, AJCC-8 has different clinical and pathologic staging definitions. Each patient was staged both clinically and pathologically according to both the AJCC-7 and AJCC-8 criteria. Clinical T-stage was determined by pre-operative physical examination and imaging while pathologic T-stage was recorded on the surgical pathology report. Clinical N-stage was determined by preoperative computed tomography (CT) imaging while pathologic N-stage was determined by the surgical pathology report. Comparisons between the staging systems could then be performed.
Statistical Methods:
Exploratory data analysis quantified the demographic and medical characteristics and verified the assumptions of the corresponding statistical tests. Unadjusted Cox proportional hazard ratios were computed to determine the overall effect of the demographics on survival. Primary outcomes were overall survival (OS) and disease-free survival (DFS) at five years. OS was defined as the time from diagnosis to the date of last follow up or death from any cause. DFS was defined as the time from diagnosis to the date of last follow up or first detectable disease recurrence in any location. Censoring occurred when a patient was lost to follow up. The Kaplan-Meier survival curves were compared across stages within a given AJCC guideline with log-rank tests. Given the paucity of patients with Stage IV disease by AJCC-8 criteria, Stage IVa and IVb were grouped together. Multivariate Cox proportional hazard regression models were used to evaluate associations between clinical variables and survival outcomes. Akaike’s Information Criteria (AIC) was used to compare models representing AJCC guidelines. AIC is a value that quantifies the fit of the model to the data and penalizes models with more covariates. When comparing models, the one with the lower the AIC represents a better fit to the data. While giving no information about the absolute quality of a model, the AIC can help comparatively rank one model vs another. Confidence intervals for the AIC values were obtained using 1,000 bootstrap samples. This involves resampling the data with replacement creating a new dataset of the same size while retaining the staging results and outcomes together for each participant. The models are then run with this dataset and the AIC values recorded. This is repeated 1,000 times to create estimates of the alternative distributions for the appropriate comparisons. The confidence intervals are computed as the 2.5 and 97.5 percentiles of the estimated alternative distributions. The p-value for the AIC values was obtained using permutation tests. This involves taking the staging results and shuffling them so that they are no longer related to the outcomes. The models were then run with the dataset and the AIC values recorded. Again, this is was repeated 1,000 times to create estimates of the null distribution for the appropriate comparisions. The p-values were computed as the number null distribution simulations that were more extreme than the statistic obtained when using the full un-modified dataset. The c-statistic for each model was computed with a macro from Mayo Clinic11. All test were 2-tailed and our alpha-value was 0.05. Data was analyzed using SAS version 9.4.
Results
Patients (n=309) who were included in the study had dates of surgery from March 1983 to December 2015. Median follow up was 33 months (range 12–340). The mean age at diagnosis was 57 years (range of 30–80). Thirty-four percent of the primary tumors were located in the base of tongue (BOT) while 64% were found in the tonsil and 2% in other oropharyngeal sites. The remainder of the patient characteristic data can be seen in Table 1 with associated Cox univariate associations with OS. Of note, smoking status was associated with OS (Never smoker HR=1, Former smoker HR=2.3 p=0.07, Current smoker HR=2.9 p=0.004; overall p=0.015). Additionally, primary site was associated with OS (BOT HR=1, Tonsil HR=0.92 p=0.8, NOS HR=3.9 p=0.03, Soft Palate HR=7.3 p=0.06; overall p=0.03) Lastly, the decade of presentation was not associated with OS (p=0.22).
Table 1:
Description of study population and hazard ratios (HR) from Cox univariate analysis for overall survival (OS).
| n (%) | HR | p-value | |
|---|---|---|---|
| Age (years) | |||
| Mean (standard deviation) | 56.9 (8.6) | 1.018 (0.98–1.05) | 0.2951 |
| Gender | |||
| Male | 254 (82) | 1 | Ref |
| Female | 55 (17.8) | 0.507 (0.21–1.21) | 0.1242 |
| Smoking Status | |||
| Never | 128 (41.4) | 1 | Ref |
| Former | 103 (33.3) | 2.305 (0.94–5.64) | 0.0673 |
| Current | 78 (25.2) | 2.886 (1.40–5.95) | 0.0041 |
| Primary Site | |||
| BOT | 105 (34) | 1 | Ref |
| Tonsil | 197 (63.8) | 0.921 (0.33–6.62) | 0.8023 |
| NOS | 5 (1.6) | 3.872 (1.10–13.61) | 0.0347 |
| Soft Palate | 2 (0.6) | 7.318 (0.94–57.10) | 0.0576 |
| Margins | |||
| Negative | 255 (83) | 1 | Ref |
| Positive | 54(18) | 1.627 (0.82–3.25) | 0.1672 |
| Extranodal Extension | |||
| None | 125 (48.4) | 1 | Ref |
| <1 mm | 16 (6.2) | 1.467 (0.33–6.62) | 0.6178 |
| >1 mm | 117 (45.3) | 1.818 0.91–3.62) | 0.0886 |
| Angiolymphatic Invasion | |||
| ALI+ | 172 (56) | 1 | Ref |
| ALI- | 137 (44) | 1.86 (0.95–3.64) | 0.07 |
| Perineural Invasion | |||
| PNI+ | 262 (85) | 1 | Ref |
| PNI- | 47 (15) | 1.944 (0.92–4.11) | 0.0819 |
| Treatment Type | |||
| Surgery Only | 74 (23.9) | 1 | Ref |
| Surgery+Radiation (XRT) | 102 (33) | 1.205 (0.43–3.41) | 0.7245 |
| Surgery+Chemoradiation (CRT) | 133 (43) | 2.331 (0.88–6.15) | 0.087 |
| Decade of Surgery | 0.2169 | ||
| 1980 | 8 (2.6) | 2.6 (0.63, 0.16) | 0.5138 |
| 1990 | 27 (8.7) | 8.7 (1.79, 0.74) | 0.1956 |
| 2000 | 29 (9.4) | 9.4 (1.69, 0.69) | 0.2512 |
| 2010 | 245 (79.3) | 1.0 | Ref |
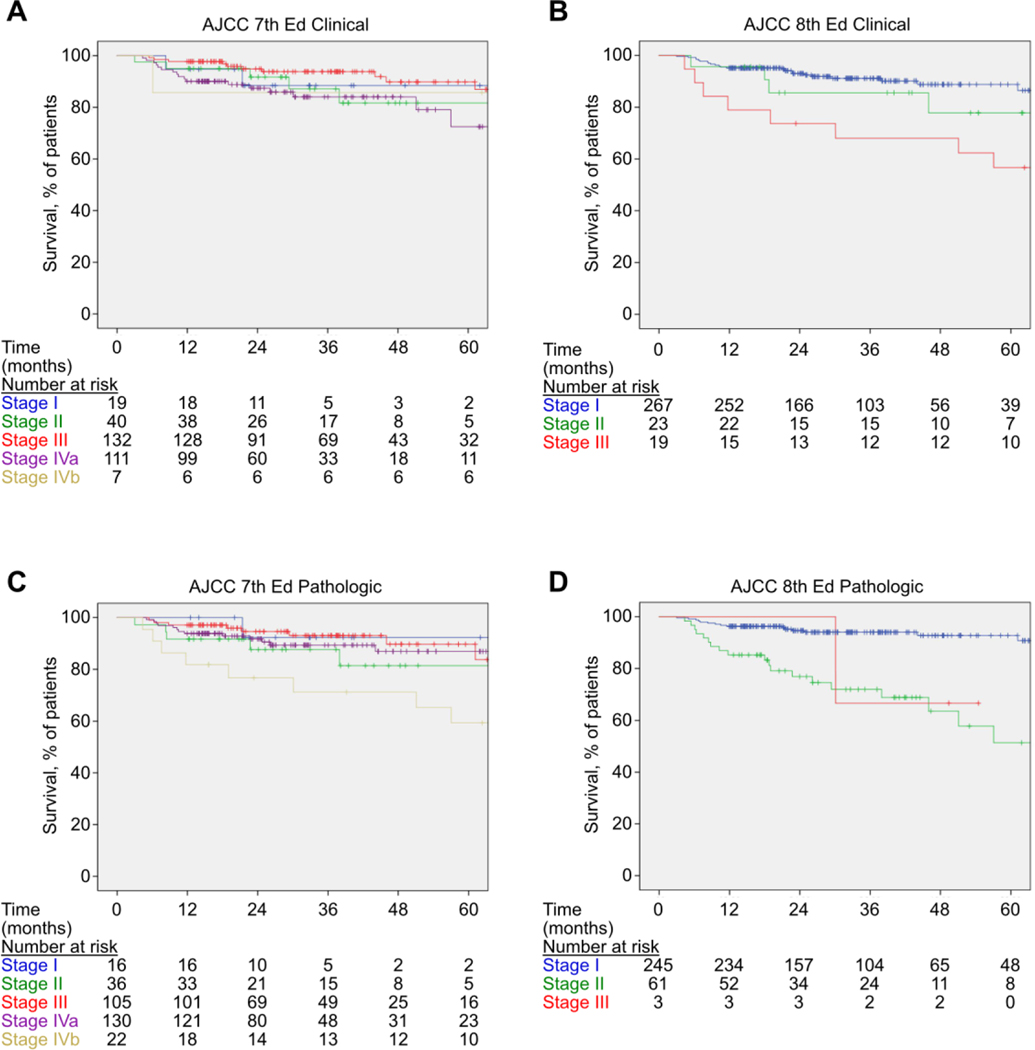
The patients’ clinical and pathologic staging as categorized by AJCC-7 and AJCC-8 can be seen in Table 2. Table 3 shows the 5-year overall survival for the different staging systems. The 5-year Kaplan-Meier overall survival curves can be seen in Figure 1, and disease-free survival is shown in Figure 2. After five years, the AJCC-7 pathologic stages did not have different survival probabilities (78.2% to 88.8%, p=0.87), nor did the AJCC-7 clinical stages (73.8% to 88.9%, p=0.09). However, the differences in five-year survival using the AJCC-8 pathologic stages did significantly differ (0.0% to 99.2%, p<0.0001), as did the five-year survival using the AJCC-8 clinical stages (51.3% to 89.7%, p=0.0001). After 1,000 bootstrap samples, the AIC value for the Cox model using AJCC-7 pathologic stages was 18.8 (95% CI: 2.5, 40.2) points higher than the Cox model using the AJCC-8 pathologic stages (p<0.0001), indicating that using the AJCC-8 pathologic stages produced a significantly better model fit for overall survival. Additionally, the AIC value for the Cox model using AJCC-8 pathologic stages does not have a statistically different model fit than the Cox model using the AJCC-8 clinical stages (difference=4.3, 95% CI: −−12.7, 22.7, p=0.152) for overall survival.
Table 2:
Patients as categorized by AJCC-7 vs AJCC-8 criteria
| AJCC-7 | ||
|---|---|---|
| Clinical | Pathologic | |
| NO | 60 | 43 |
| N1 | 92 | 63 |
| N2a | 43 | 36 |
| N2b | 89 | 141 |
| N2c | 13 | 14 |
| N3 | 12 | 12 |
| Stage I | 19 | 16 |
| Stage II | 40 | 36 |
| Stage III | 132 | 105 |
| Stage IVa | 111 | 130 |
| Stage IVb | 7 | 22 |
| AJCC-8 | ||||
|---|---|---|---|---|
| Clinical | Patdologic | |||
| N0 | 60 | 42 | ||
| N1 | 224 | 223 | ||
| N2 | 13 | 44 | ||
| N3 | 12 | - | ||
| Stage I | 267 | 245 | ||
| Stage II | 23 | 61 | ||
| Stage III | 19 | 3 | ||
Table 3:
5-Year Overall survival and Disease-Free Survival for patients as grouped by AJCC-7 vs AJCC-8
| 5-Year Overall Survival | ||||||
|---|---|---|---|---|---|---|
| AJCC-7 | AJCC-8 | |||||
| N | OS | N | OS | |||
| cStage I | 19 | 82.40% | cStage I | 267 | 89.70% | |
| cStage II | 40 | 84.60% | cStage II | 23 | 51.30% | |
| cStage III | 132 | 88.90% | cStage III | 19 | 71.70% | |
| cStage IV | 118 | 73.80% | Overall | 309 | 82.60% | |
| Overall | 309 | 82.60% | ||||
| pStage I | 245 | 92.20% | ||||
| pStage I | 16 | 79.60% | pStage II | 61 | 58.80% | |
| pStage II | 36 | 88.20% | pStage III | 3 | 0.00% | |
| pStage III | 105 | 88.80% | Overall | 309 | 82.60% | |
| pStage IV | 152 | 78.20% | ||||
| Overall | 309 | 82.60% | ||||
| 5-Year Disease-Free survival | ||||||
| AJCC-7 | AJCC-8 | |||||
| N | DFS | N | DFS | |||
| cStage I | 19 | 88.40% | cStage I | 267 | 86.70% | |
| cStage II | 40 | 75.90% | cStage II | 23 | 77.80% | |
| cStage III | 132 | 89.20% | cStage III | 19 | 56.70% | |
| cStage IV | 118 | 74.70% | Overall | 309 | 82.00% | |
| Overall | 309 | 82.00% | ||||
| pStage I | 245 | 91.40% | ||||
| pStage I | 16 | 92.30% | pStage II | 61 | 49.40% | |
| pStage II | 36 | 74.60% | pStage III | 3 | 66.70% | |
| pStage III | 105 | 89.00% | Overall | 309 | 82.00% | |
| pStage IV | 152 | 79.00% | ||||
| Overall | 309 | 82.00% | ||||
Figure 1.
Kaplan-Meier curves of overall survival in this surgical cohort based on AJCC 7th Edition clinical stage (A), AJCC 8th Edition clinical stage (B), AJCC 7th Edition pathologic stage (C), and AJCC 8th Edition pathologic stage (D).
Figure 2.
Kaplan-Meier curves of disease-specific survival in this surgical cohort based on AJCC 7th Edition clinical stage (A), AJCC 8th Edition clinical stage (B), AJCC 7th Edition pathologic stage (C), and AJCC 8th Edition pathologic stage (D).
Table 3 shows the 5-year disease-free survival for the different staging systems. The 5-year Kaplan-Meier disease-free survival is shown in Figure 2. After five years, the AJCC-7 pathologic stages did not have different survival probabilities (74.6% to 92.3% p=0.47), nor did the AJCC-7 clinical stages (74.7% to 89.2% p=0.17). However, the differences in five-year survival using the AJCC-8 pathologic stages did significantly differ (66.6% to 91.3%, p<0.0001), as did the five-year survival using the AJCC-8 clinical stages (56.7% to 86.7%, p=0.014). After 1,000 bootstrap samples, the AIC value for the Cox model using AJCC-7 pathological categories was not significantly higher than Cox model using AJCC-7 clinical (2.2, p=0.38) supporting that the pathological and clinical stages do not differ. In addition, the AIC value for the Cox model using AJCC-7 pathologic stages was 22.3 (95% CI: 2.8–45.5) points higher than the Cox model using the AJCC-8 pathologic stages (p<0.0001), indicating that using the AJCC-8 pathologic stages produced a significantly better model fit for disease-free survival. Additionally, the AIC value for the Cox model using AJCC-8 pathologic stages does have a statistically different model fit than the Cox model using the AJCC-8 clinical stages (difference=17.6, 95% CI: 1.7 to 38.2, p=<0.0001) for disease-free survival.
When examining AJCC-8, pathologic staging did not change the clinical staging for 235 patients (76%) while 43 patients (14%) were upstaged by 1 stage, 1 patient (0.32%) was upstaged by 2 stages, 21 patients (7%) were downstaged by 1 and 9 patients (3%) were downstaged by 2. As a comparison, for AJCC-7 pathological staging did not change for 237 patients (77%), while 61 patients (20%) were upstaged by 1, 3 patients (1%) were upstaged by 2 and 8 patients (3%) were downstaged by 1.
Discussion:
The HPV epidemic has fundamentally altered the landscape for OPSCC as patients are younger and the disease caries a much improved prognosis.2,3,5,12 Due to the emergence of this clinically distinct entity, the AJCC recently updated the staging system to reflect this improved prognosis. 9 Analysis of our surgical cohort confirmed the improvement of the AJCC-8 pathological stages over the AJCC-7 pathological stages in predicting overall survival and disease free survival by showing a statistically significant difference in the model fit (AIC, p< 0.0001) of the Cox models using the respective guidelines. While the pathologic AJCC-8 stages did have a better model fit than the clinical AJCC-8 stages, this difference was not statistically significant. Our findings help validate the new AJCC-8 staging system for HPV+ OPSCC. Horne et. al. similarly validated the new criteria in a large, registry based analysis.13 While other studies have also individually validated the ICON-S and Haughey criteria, the current study is the largest multi-institutional surgical cohort that has been evaluated against the AJCC-8 criteria.12
The AJCC-8’s system is novel, because the nodal classification between the clinical and pathologic staging are defined differently. The reason for this difference is that the clinical staging system is based on the ICON-S study where patients where staged clinically and treated primarily with CRT, while the pathologic staging system was born from the Haughey et. al. surgical cohort.7–9 The findings from these studies demonstrate that in clinically staged patients treated primarily with CRT, survival was stratified by the size of the largest lymph (cN3 is any node larger than 6 cm) and location of positive nodes (ipsilateral vs contralateral). However, in patients that were treated surgically, the pathologic data suggested that survival was more dependent upon the number of positive lymph nodes, with more than 4 positive nodes carrying a worse prognosis. Interestingly, the external validity of these two studies was insufficient to find a unifying system that was applicable to both cohorts. The AJCC-8, therefore, utilizes both systems, as this is the best data currently available. It is also important to note that both smoking status and the presence of extranodal extension were examined when creating the AJCC-8. For HPV+ patients, prognosis did not stratify strongly enough along these variables for them to be included in the staging system.
Theoretically, pathologic staging would be expected to be more informative than clinical staging; this is often cited as a rationale for primary surgical management of oropharyngeal carcinoma.15 AJCC-8 utilizes separate systems for clinical and pathologic staging for reasons discussed above. When examining overall survival, both clinical and pathologic systems performed equal well. Importantly, the clinical staging for the majority of patients (76%) did not change upon re-staging after surgery. Of the 24% of patients whose stage did change under AJCC-8 criteria, 59% were upstaged and 41% were downstaged. It is important to note, however, that for DFS, the AJCC-8 pathologic outperformed the clinical staging system (AIC, p< 0.0001). Additional studies are needed to further evaluate the AJCC-8 and confirm the utility of having a unique surgical staging system that does not apply to patients treated with primary CRT. Most importantly, clinicians should understand the rationale behind the creation of these different staging schemes.
The AJCC-8 is a staging system that helps stratify patients by prognosis. However, it is important to understand that this staging system does not make any recommendations regarding treatment. Although patients with HPV+ OPSSC do have an improved prognosis, at the time of this writing, the National Comprehensive Cancer Network (NCCN) guidelines for management of HPV+ OPSCC have not changed.16 Studies are currently ongoing to help understand the role of therapy de-escalation to help appropriately treat patients oncologically while minimizing therapeutic toxicity. These include surgical de-escalation with transoral surgery (Eastern Cooperative Oncology Group 3311), response-based, reduced-dose radiotherapy after induction chemotherapy (Eastern Cooperative Oncology Group 1308), and the omission of concurrent chemotherapy (NRG HN-002). Until the results of these and other studies are known, current treatment paradigms for OPSCC should not be changed due to the new staging system.
Our study has several limitations. Our cohort contained only surgical patients, we were therefore unable to truly validate the clinical staging system within the AJCC-8. Our surgical cohort is also susceptible to selection bias, as primary chemoradiation therapy could be selected as a treatment modality for higher stage patients. Our study included patients from 1983–2015 in order to optimize long-term survival data and increase statistical power. However, oncologic treatment has changed over the last 30 with improvements in surgical technique via transoral robotic and laser microsurgery. Additionally, radiation therapy techniques have advanced with intensity modulated radiation therapy (IMRT) and stereotactic radiosurgery; and medical oncology has also evolved with new systemic agents like cetuximab and immunotherapy. Our cohort of patients was treated with a heterogeneous combination of these therapies. While this may be a good reflection of real-world practice, the absolute survival figures observed in this study may not fully reflect modern treatment strategies.
Conclusion:
For HPV-related OPSCC, the updated AJCC 8 staging system produces groups that statistically stratifies by overall survival and disease-specific survival, having a better model fit with Cox models than the AJCC-7 stages. While the Cox model for overall survival using the AJCC-8 pathologic stages did have a slightly better model fit than for AJCC-8 clinical, this difference was not statistically significant. However, the AJCC-8 pathologic Cox model for disease-specific survival did produce a significantly better fit than for AJCC-8 clinical. Additional studies are needed to further validate this new prognostic tool as it will be widely utilized in the head and neck oncology community. Disclosure: This project was supported by the National Institutes of Health through Grant Number UL1TR001857. This project received no other support from public, private or non-profit agencies.
Highlights:
The AJCC, 8th edition contains changes to the staging system for HPV+ oropharyngeal SCC.
Our multicenter study validates the AJCC-8’s ability to stratify surgical patients.
AJCC-8 more effectively risk stratifies patients by OS and DFS than AJCC-7.
AJCC-8 pathologic is not more effective than AJCC-8 clinical for OS.
AJCC-8 pathologic is more effective than AJCC-8 clinical for DFS.
Acknowledgments
This project was presented at the American Head and Neck Society (AHNS) Section of the Combined Otolaryngology Spring Meeting (COSM) April, 2018.
Disclosure: This project was supported by the National Institutes of Health through Grant Number UL1TR001857. This project received no other support from public, private or non-profit agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744–754. http://www.ncbi.nlm.nih.gov/pubmed/15599852. [DOI] [PubMed] [Google Scholar]
- 2.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–89. doi: 10.1002/cncr.27727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brizel DM. Different strokes for different folks: new paradigms for staging oropharynx cancer. J Clin Oncol. 2015;33(8):817–818. doi: 10.1200/JCO.2014.60.1757 [DOI] [PubMed] [Google Scholar]
- 6.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33(8):836–845. doi: 10.1200/JCO.2014.58.6412 [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440–451. doi: 10.1016/S1470-2045(15)00560-4 [DOI] [PubMed] [Google Scholar]
- 8.Haughey BH, Sinha P, Kallogjeri D, et al. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral Oncol. 2016;62:11–19. doi: 10.1016/j.oraloncology.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL TA. Pharynx. In: AJCC Cancer Staging Manual. 7th Editio New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 11.Kremers WK. No Title In: Concordance for Survival Time Data: Fixed and Time-Dependent Covariates and Possible Ties in Predictor and Time. Technical Report Series #80. Mayo Clinic, Rochester, Minnesota: Department of Health Sciences Research and The William J. von Liebig Transplant Center; 2007:Technical Report Series #80. [Google Scholar]
- 12.Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol. 2001;13(3):183–188. http://www.ncbi.nlm.nih.gov/pubmed/11307062. [DOI] [PubMed] [Google Scholar]
- 13.Horne ZD, Glaser SM, Vargo JA, et al. Confirmation of proposed human papillomavirus risk-adapted staging according to AJCC/UICC TNM criteria for positive oropharyngeal carcinomas. Cancer. 2016;122(13):2021–2030. doi: 10.1002/cncr.30021 [DOI] [PubMed] [Google Scholar]
- 14.Malm I-J, Fan CJ, Yin LX, et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer. January 2017. doi: 10.1002/cncr.30512 [DOI] [PubMed] [Google Scholar]
- 15.Pitman KT, Johnson JT, Myers EN. Effectiveness of selective neck dissection for management of the clinically negative neck. Arch Otolaryngol Head Neck Surg. 1997;123(9):917–922. http://www.ncbi.nlm.nih.gov/pubmed/9305240. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Melanoma. Accessed 2015 Nov 8.