Abstract
BACKGROUND
Inflammation plays an important role in cardiovascular disease (CVD) development. Diet modulates inflammation; however, it remains unknown whether dietary patterns with higher inflammatory potential are associated with long-term CVD risk.
OBJECTIVES
This study sought to examine whether proinflammatory diets are associated with increased CVD risk.
METHODS
We prospectively followed 74,578 women from the Nurses’ Health Study (NHS) (1984–2016), 91,656 women from the NHSII (1991–2015), and 43,911 men from the Health Professionals Follow-up Study (1986–2016) who were free of CVD and cancer at baseline. Diet was assessed by food frequency questionnaires every 4 years. The inflammatory potential of diet was evaluated using a food-based empirical dietary inflammatory pattern (EDIP) score that was pre-defined based on levels of 3 systemic inflammatory biomarkers.
RESULTS
During 5,291,518 person-years of follow-up, we documented 15,837 incident CVD cases, including 9,794 coronary heart disease (CHD) cases and 6,174 strokes. In pooled analyses of the 3 cohorts, after adjustment for medication use and CVD risk factors including body mass index, a higher dietary inflammatory potential, as indicated by higher EDIP scores, was associated with an increased risk of CVD (hazard ratio [HR] comparing the highest to lowest quintiles: 1.38; 95% confidence interval [CI]: 1.31 to 1.46; p-trend < 0.001), CHD (HR: 1.46; 95% CI: 1.36 to 1.56; p-trend < 0.001), and stroke (HR: 1.28; 95% CI: 1.17- to 1.39; p-trend < 0.001). These associations were consistent across cohorts and between sexes, and they remained significant after further adjustment for other dietary quality indices. In a subset of study participants (n = 33,719), a higher EDIP was associated with a higher circulating profile of proinflammatory biomarkers, lower levels of adiponectin, and an unfavorable blood lipid profile (p < 0.001).
CONCLUSIONS
Dietary patterns with a higher proinflammatory potential were associated with higher CVD risk. Reducing the inflammatory potential of the diet may potentially provide an effective strategy for CVD prevention.
Keywords: cardiovascular disease, chronic inflammation, coronary heart disease, dietary patterns, predictive biomarkers, stroke
Cardiovascular disease (CVD) imposes an enormous burden on health care systems and causes one third of deaths in the United States (1). Accumulating evidence indicates that the activation of the immune system and chronic inflammation contribute substantially to CVD pathogenesis (2–4). Circulating concentrations of inflammatory biomarkers, including interleukin 6, C-reactive protein (CRP), tumor necrosis factor-α receptor 2 (TNFα-R2), and soluble intercellular adhesion molecule (sICAM) 1, have been associated with incident CVD in prospective cohort studies (5,6). Randomized trials and Mendelian randomization studies also support the causal roles of several inflammatory cytokines (i.e., interleukin 1β and interleukin 6) in CVD development (7,8).
As an important modifiable exposure, diet has been implicated in CVD etiology and has been shown to influence inflammation (9). Intakes of foods that are rich in antioxidants, fiber, and long-chain n-3 polyunsaturated fatty acids have been associated with lower levels of proinflammatory biomarkers (9–13). Compared to individual foods/nutrients, dietary patterns integrate the potential interacting and joint effects of multiple dietary components and, thus, may be more informative in reflecting individuals’ habitual diets (14). A few healthy dietary patterns, including the Mediterranean diet, have been associated with lower concentrations of some inflammatory biomarkers (9,15) and a reduced CVD risk (16,17). However, because most dietary patterns and indices were not designed to comprehensively characterize the overall inflammatory potential of the diet, it remains unknown whether long-term adherence to proinflammatory diets is associated with increased CVD incidence.
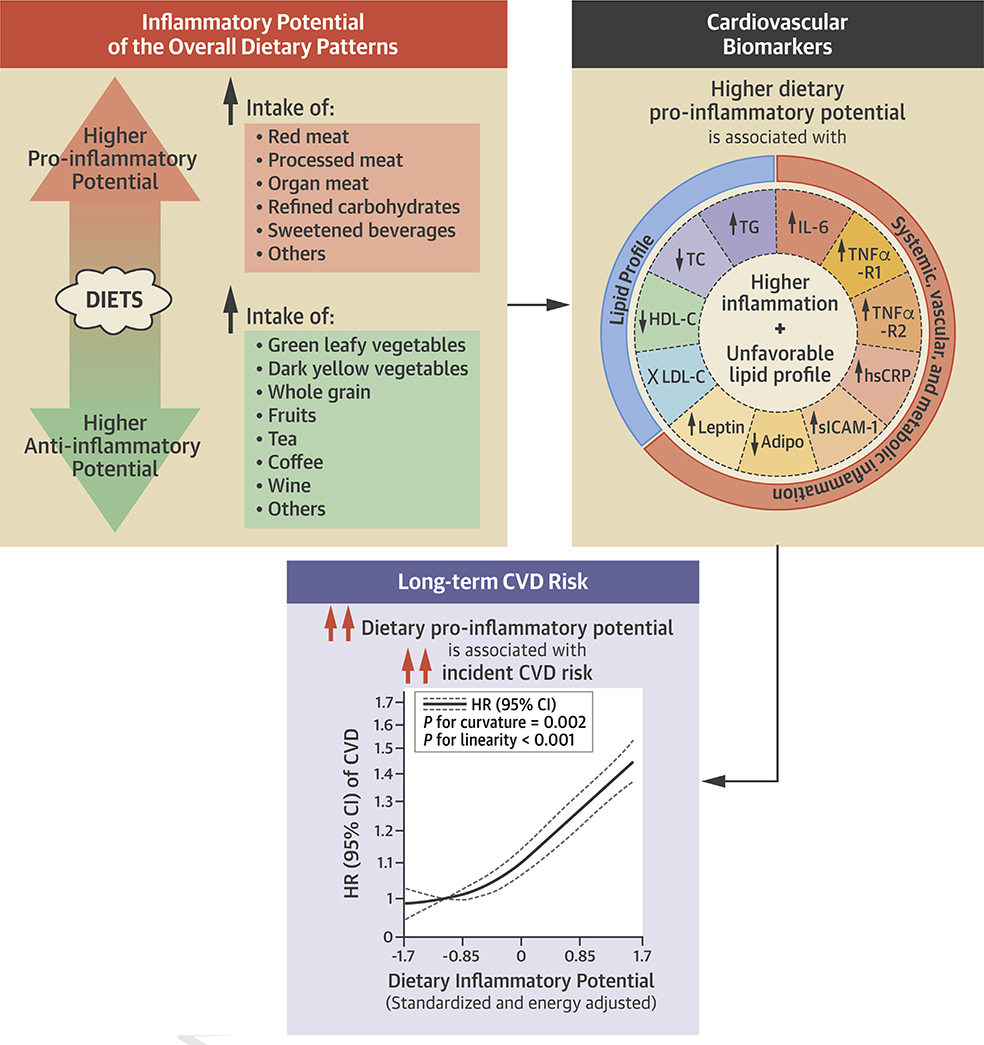
We therefore assessed the overall inflammatory potential of diets using a food-based index that was empirically developed based on circulating concentrations of 3 systemic inflammatory biomarkers (18–20) and examined its association with incident CVD and CVD subtypes in 3 large prospective cohorts. We further examined associations between dietary inflammatory potential with plasma profiles of inflammatory biomarkers and blood lipids in a subpopulation of the study cohorts (Central Illustration).
CENTRAL ILLUSTRATION. Adherence to Proinflammatory Dietary Patterns and Cardiovascular Disease Incidence.
Dietary patterns with a higher inflammatory potential were significantly associated with a higher incidence of cardiovascular disease and subtypes, including coronary heart disease and stroke, in 3 prospective cohorts including 210,145 U.S. women and men and followed up for up to 32 years. Secondary analyses further showed that higher dietary inflammatory potential was significantly associated with biomarkers indicating higher systemic, vascular, and metabolic inflammation and an unfavorable lipid profile. The axis for hazard ratio is nature log-scaled with original labels.
METHODS
STUDY POPULATION.
The Nurses’ Health Study (NHS) started in 1976, recruiting 121,701 female nurses ages 30 to 55 years; the NHSII was initiated in 1989 with 116,429 female nurses ages 25 to 42 years; and the Health Professionals Follow-up Study (HPFS) started in 1986, enrolling 51,529 male health professionals ages 40 to 75 years (21,22). Participants were followed up biennially on lifestyles, medical history, and health-related information (response rate: ~90%). Among participants alive at study baseline (1984 for NHS, 1991 for NHSII, and 1986 for HPFS), we excluded those who were lost to follow-up; had cancer, stroke, or coronary heart disease (CHD); did not complete food frequency questionnaires (FFQs), or met the exclusion criteria of FFQs (missing a certain amount of data; reported an energy intake of <600 or >3,500 kcal/day for women or <800 or >4,200 kcal/day for men) (Supplemental Table 1). The final analyses included 74,578 women from NHS, 91,656 women from NHSII, and 43,911 men from HPFS. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, with participants’ consent implied by the return of the questionnaires.
ASSESSMENT OF DIET AND DIETARY INFLAMMATORY POTENTIAL.
Starting from 1984 and 1986 in NHS, 1991 in NHSII, and 1986 in HPFS, an FFQ was administered every 4 years to ascertain dietary intakes. The FFQ was validated by comparisons with 24-hour recalls and multiweek weighted dietary records and was found to have reasonably high validity in measuring most food and nutrient intakes (23–25). The empirical dietary inflammatory pattern (EDIP) score was previously developed in 5,230 NHS women (19). Briefly, plasma levels of interleukin 6, TNFα-R2, and CRP were regressed on 39 pre-defined food groups by using reduced-rank regressions and stepwise linear regressions, selecting 18 food groups most predictive of these biomarkers. The EDIP was calculated as the weighted sum of these 18 food groups (average intakes are shown in Supplemental Table 2), with weights (i.e., the contributions of each food to the overall score) equal to the coefficients from the stepwise regression. A higher score indicates pro-inflammatory diets, and a lower score indicates anti-inflammatory diets. EDIP has been validated in other cohorts, showing stronger associations with plasma TNFα-R2, adiponectin, and CRP when compared to a literature-derived nutrient-based dietary inflammatory index (DII) (18,20).
We calculated EDIP scores using updated dietary data in each of the 4-year FFQ cycles and adjusted the scores for total energy intake using a residual method (26). We computed cumulatively averaged EDIP scores at each cycle (i.e., averaging scores from all prior cycles up to the then-latest cycle) to best present long-term diet and reduce random measurement errors. Most participants (>97.5%) were independent of the NHS subsample in which the EDIP was developed. The alternate healthy eating index (AHEI), Dietary Approaches to Stop Hypertension (DASH), and Alternate Mediterranean Diet Score (AMED) were derived as described previously (27).
ASCERTAINMENT OF CVD.
CVD is defined as the composite of incident nonfatal myocardial infarction (MI), fatal CHD, and fatal and nonfatal stroke. Nonfatal MI was confirmed by physicians according to the World Health Organization criteria (28); nonfatal stoke was confirmed according to the National Survey of Stroke criteria (29). Death was identified from next of kin, postal authorities, or the National Death Index (>98% follow-up rate) (30). Cause of death was defined according to International Classification of Diseases-8th Revision. Fatal CHD and stroke were confirmed by autopsy records or death certificate with an evidence of prior CVD.
ASSESSMENT OF PLASMA BIOMARKERS.
Blood samples were collected in subpopulations of the NHS (n = 32,862) during 1989 and 1990, NHSII (n = 29,611) from 1996 to 1999, and HPFS (n = 18,019) from 1993 to 1995 (>95% follow-up rate) (6). Plasma concentrations of interleukin 6, TNFα-R1, TNFα-R2, CRP, sICAM-1, adiponectin, leptin, triglyceride, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol, were previously measured in multiple substudies within cohorts (Supplemental Table 3). We combined data from these substudies and corrected for batch effects using an average batch correction method (31). After excluding outliers, duplicates, or individuals missing diet or having cancer or CVD at blood draw, 33,719 participants were included in biomarker analyses (n = 4,385 to 21,546 for individual biomarkers).
ASSESSMENT OF COVARIATES.
We obtained updated information on participants’ medical history, family history, lifestyles, body mass index (BMI), reproductive factors, and medication use through biennial questionnaires. Fasting status and steroid use at blood draw were obtained from blood-collecting questionnaires.
STATISTICAL ANALYSIS.
We calculated person-years of follow-up from the return date of the first FFQ until the date of CVD diagnosis, death, or the end of follow-up (June 2016 in NHS, June 2015 in NHSII, and January 2016 in HPFS), whichever came first. We used Cox regressions with time-varying covariates to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of CVD risk, comparing higher to the lowest EDIP quintiles. To quantify a linear trend, we assigned the median value of EDIP scores within each quintile and modeled as a continuous variable. All analyses were stratified by age and follow-up intervals, and multivariable models were further adjusted for race, BMI, smoking, physical activity, multivitamin use, regular aspirin use, other anti-inflammatory medication use, menopausal status and post-menopausal hormone use (in women), hypertension, diabetes, dyslipidemia, and family history of premature CHD. Analyses were performed separately in each cohort and combined using a fixed-effect meta-analysis.
Several sensitivity analyses were performed. First, we additionally adjusted for amounts of alcohol consumption, smoking pack-years, regular use of lipid-lowering and antihypertensive medications, or sodium intake and systolic and diastolic blood pressures. Second, we replaced cumulatively averaged EDIP scores with EDIP scores from a single preceding FFQ (i.e., the most recent diet). Third, because conditions like diabetes, hypertension, dyslipidemia, and cancer are potentially associated with CVD and their diagnoses may change a person’s diet, we stopped updating EDIP scores when participants reported such conditions. Finally, to examine the added value of EDIP over other well-studied dietary indices in CVD prevention, we additionally adjusted for AHEI, DASH, or AMED. We conducted stratified analyses by subgroups of cardiovascular risk factors, such as BMI and smoking, and examined association differences across subgroups.
We pooled individual-level data from the 3 cohorts together to examine the combined effect of smoking and EDIP on CVD risk and to evaluate the dose-response relationship between EDIP and CVD risk using restricted cubic splines. Regression models were additionally stratified by cohorts.
Linear regressions were used to analyze associations of EDIP with levels of inflammatory biomarkers and lipids. We used the average EDIP scores from the 2 FFQs administrated several years before blood collection to reduce concerns of reverse causality (in NHS, 1984 to 1986 FFQs; in NHSII, 1991 to 1995 FFQs; in HPFS, 1986 to 1990 FFQs). Multivariable models were adjusted for all aforementioned covariates; fasting status, steroid use, study cohort, case-control status in original substudies; and lipid-lowering medications (for blood lipids).
All statistical analyses were performed by using SAS, version 9.4 (Cary, North Carolina). In CVD analyses, a 2-sided p value of 0.5 (for CVD analyses) or 0.0045 (for biomarkers analyses; Bonferroni correlations for 11 biomarkers) was considered as statistically significant.
RESULTS
Our study included 210,145 U.S. men and women. At baseline and through follow-up, compared with participants consuming anti-inflammatory diets (i.e., in lower EDIP quintiles), those who consumed prion-flammatory diets (i.e., in higher EDIP quintiles) reported higher BMI and lower physical activity and were more likely to have a family history of CHD. They were also less likely to use multivitamins and consumed fewer fruits, vegetables, and whole grains (Table 1 and Supplemental Tables 2 and 4).
TABLE 1.
Baseline Characteristics According to Quintiles of EDIP Scores in the NHS (1984), NHSII (1991), and HPFS (1986)
| Quintiles of EDIP Scores |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| NHS (1984) |
NHSII (1991) |
HPFS (1986) |
|||||||
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| Total number | 15,014 | 14,928 | 14,816 | 18,393 | 18,352 | 18,180 | 9,011 | 8,692 | 8,697 |
| Median EDIP score | −1.19 | 0.04 | 1.14 | −1.15 | −0.01 | 1.15 | −1.20 | 0.06 | 1.12 |
| Age, yrs | 50.9 ± 6.9 | 51.0 ± 7.2 | 49.8 ± 7.2 | 37.1 ± 4.5 | 36.5 ± 4.6 | 36.2 ± 4.7 | 52.8 ± 9.0 | 54.2 ± 9.7 | 53.6 ± 9.8 |
| White, % | 98.8 | 98.1 | 96.0 | 95.8 | 94.1 | 91.5 | 96.7 | 95.2 | 92.2 |
| Smoking status, % | |||||||||
| Current smoker | 29.4 | 22.5 | 21.7 | 10.3 | 11.4 | 15.5 | 11.6 | 9.0 | 9.3 |
| Past smokers | 36.6 | 31.6 | 27.7 | 26.5 | 21.7 | 19.3 | 48.2 | 39.5 | 34.7 |
| Never smokers | 34.0 | 46.0 | 50.6 | 63.1 | 66.8 | 65.2 | 40.2 | 51.5 | 56.0 |
| Individuals who reported alcohol consumption, % | 84.1 | 69.5 | 53.4 | 70.2 | 56.0 | 46.7 | 90.8 | 77.0 | 60.7 |
| Alcohol intake among consumers, servings/week | 8.2 ± 8.0 | 3.9 ± 4.8 | 3.6 ± 5.3 | 4.3 ± 5.2 | 2.2 ± 2.6 | 2.0 ± 2.6 | 12.0 ± 10.3 | 5.9 ± 6.3 | 5.2 ± 6.7 |
| Hypercholesterolemia, % | 7.7 | 8.1 | 9.7 | 12.9 | 14.4 | 17.2 | 10.8 | 10.6 | 10.9 |
| Hypertension, % | 18.2 | 21.8 | 31.1 | 5.5 | 6.0 | 10.5 | 18.5 | 19.4 | 23.6 |
| Diabetes, % | 1.7 | 2.6 | 5.6 | 0.5 | 0.7 | 2.0 | 1.7 | 2.2 | 4.0 |
| Family history of myocardial infarction, % | 20.9 | 21.2 | 22.1 | 20.4 | 21.1 | 24.3 | 14.5 | 15.5 | 14.9 |
| Body mass index, kg/m2 | 23.8 ± 3.7 | 24.8 ± 4.4 | 26.7 ± 5.7 | 23.7 ± 4.5 | 24.3 ± 4.9 | 26.2 ± 6.2 | 25.2 ± 3.0 | 25.3 ± 3.1 | 26.0 ± 3.7 |
| Post-menopausal women, % | 57.2 | 58.5 | 60.5 | 2.6 | 3.1 | 3.5 | – | – | – |
| Post-menopausal hormone therapy, % | 22.9 | 23.9 | 21.1 | 85.1 | 84.3 | 81.6 | – | – | – |
| Physical activity, MET-h/week | 16.1 ± 23.8 | 14.2 ± 20.6 | 12.6 ± 17.0 | 24.5 ± 31.4 | 19.7 ± 25.1 | 19.1 ± 26.7 | 21.4 ± 25.8 | 19.3 ± 25.9 | 17.9 ± 24.8 |
| Total energy intake, kcal/d | 1,825 ± 526 | 1,681 ± 510 | 1,828 ± 567 | 1,909 ± 546 | 1,681 ± 517 | 1,903 ± 589 | 2,106 ± 623 | 1,896 ± 592 | 2,134 ± 667 |
| Sugar-sweetened beverages, servings/day | 1.1 ± 1.0 | 1.0 ± 0.9 | 1.3 ± 1.3 | 1.2 ± 1.3 | 1.0 ± 1.0 | 1.4 ± 1.6 | 1.2 ± 1.3 | 1.1 ± 0.9 | 1.5 ± 1.3 |
| Vegetable intake, servings/day | 3.3 ± 1.9 | 2.7 ± 1.4 | 2.8 ± 1.6 | 3.6 ± 2.3 | 2.7 ± 1.6 | 2.9 ± 1.8 | 3.3 ± 2.0 | 2.9 ± 1.6 | 3.1 ± 1.9 |
| Fruit intake, servings/day | 1.5 ± 1.2 | 1.4 ± 1.0 | 1.3 ± 1.1 | 1.4 ± 1.1 | 1.1 ± 0.9 | 1.1 ± 0.9 | 1.6 ± 1.5 | 1.6 ± 1.3 | 1.5 ± 1.3 |
| Polyunsaturated fatty acids, % of energy | 6.6 ± 1.9 | 6.5 ± 1.7 | 6.4 ± 1.7 | 5.7 ± 1.5 | 5.6 ± 1.3 | 5.6 ± 1.4 | 5.8 ± 1.7 | 5.8 ± 1.5 | 5.7 ± 1.5 |
| Whole grain intake, g/day | 15.0 ± 13.2 | 14.8 ± 13.6 | 11.0 ± 11.2 | 23.8 ± 17.1 | 20.9 ± 15.5 | 15.8 ± 13.2 | 22.8 ± 19.6 | 23.2 ± 20.2 | 17.5 ± 17.6 |
| Multivitamin supplement use, % | 33.0 | 31.0 | 28.0 | 48.0 | 44.3 | 39.4 | 44.4 | 41.8 | 37.9 |
| Regular aspirin use, % | 71.9 | 71.3 | 69.4 | 11.7 | 11.1 | 11.9 | 29.1 | 26.2 | 26.4 |
Values are mean ± SD or %. All presented variables, except age and median EDIP values, were age-standardized separately in each cohort.
EDIP = Empirical dietary inflammation pattern; HPFS = Health Professional Follow-up Study; MET = metabolic equivalent of tasks; NHS = Nurses’ Health Study; NHSII = Nurses’ Health Study II.
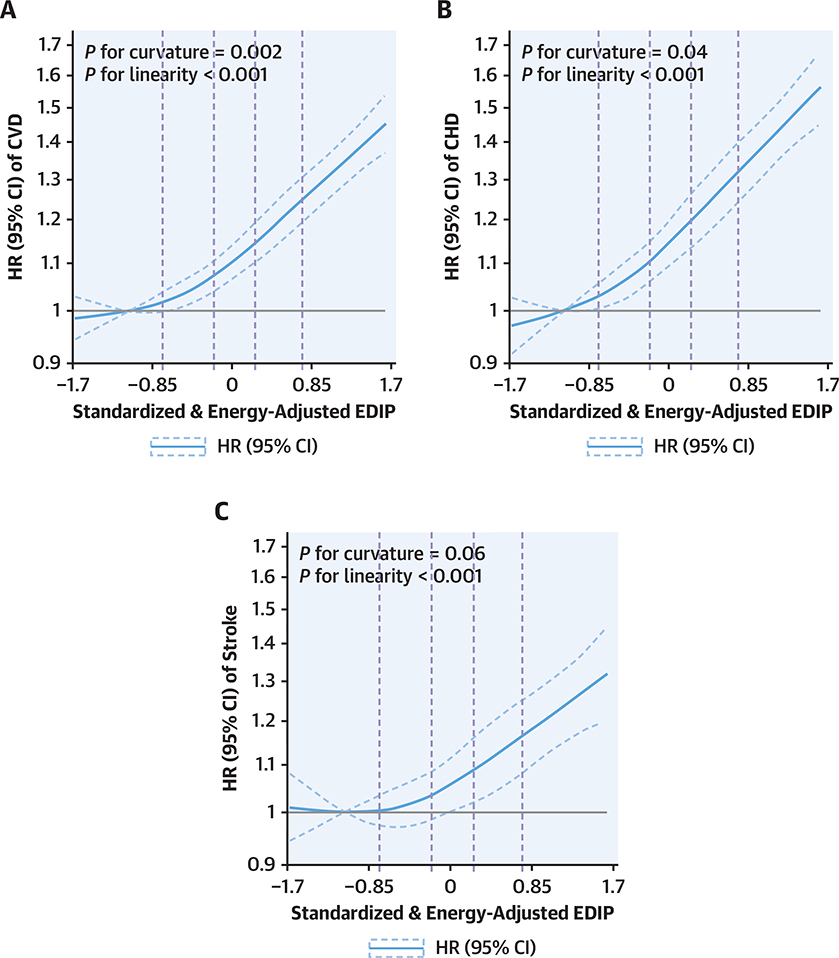
We documented 15,837 incident CVD events (7,226 in NHS, 1,359 in NHSII, and 7,252 in HPFS), including 9,794 incident CHD and 6,174 incident stroke events, during 5,291,518 person-years of follow-up. After adjusting for age, lifestyle factors, and other confounders, higher EDIP scores were significantly associated with a higher CVD risk, without evidence of heterogeneity by sex or cohort (p-difference = 0.98). The associations were slightly attenuated but remained significant after further adjusting for potential mediators including hypertension, dyslipidemia, diabetes, and BMI (Table 2 and Supplemental Table 5). In pooled analyses of the 3 cohorts, the fully adjusted HRs (95% CIs) of total CVD comparing the higher quintiles to the lowest quintile of EDIP scores were 1.08 (1.02 to 1.14), 1.15 (1.09 to 1.22), 1.19 (1.13 to 1.26), and 1.38 (1.31 to 1.46) (p-trend < 0.001). Similar association trends were observed between EDIP and risk of CHD and stroke. The pooled HRs (95% CIs) comparing higher quintiles to the lowest EDIP quintile were 1.10 (1.02 to 1.17), 1.20 (1.12 to 1.28), 1.24 (1.16 to 1.32), and 1.46 (1.36 to 1.56) for CHD (p-trend < 0.001) and were 1.06 (0.97 to 1.15), 1.09 (1.00 to 1.19), 1.13 (1.04 to 1.23), and 1.28 (1.17 to 1.39) for stroke (p-trend < .001) (Table 2). In dose-response analyses, associations of EDIP with CVD appeared to deviate from linearity with an accelerated increase in risk at higher EDIP scores (tests for curvature: p = 0.002 for CVD, p = 0.04 for CHD, and a marginal p = 0.06 for stroke) (Figure 1 and Central Illustration).
TABLE 2.
HR (95% CI) of CVD Risk by Quintile of EDIP Scores in the NHS, NHSII, and HPFS
| HR (95% CI) Comparing Higher to the Lowest Quintiles of EDIP Scores* |
p Trend | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Total CVD: fatal CHD + nonfatal MI + fatal and nonfatal stroke | ||||||
| NHS | ||||||
| Cases/PY | 1,148/426,929 | 1,327/431,816 | 1,527/423,576 | 1,530/413,485 | 1,694/378,490 | |
| Age adjusted | 1 (ref.) | 1.07 (0.99–1.16) | 1.25 (1.16–1.35) | 1.31 (1.21–1.41) | 1.76 (1.63–1.89) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.09 (1.01–1.18) | 1.27 (1.18–1.37) | 1.32 (1.22–1.43) | 1.72 (1.60–1.86) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.06 (0.98–1.15) | 1.18 (1.09–1.27) | 1.17 (1.08–1.27) | 1.40 (1.30–1.52) | <0.001 |
| NHSII | ||||||
| Cases/PY | 190/427,266 | 244/438,049 | 257/439,294 | 291/434,484 | 377/418,622 | |
| Age adjusted | 1 (ref.) | 1.30 (1.07–1.57) | 1.38 (1.15–1.67) | 1.62 (1.35–1.95) | 2.21 (1.86–2.64) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.30 (1.07–1.57) | 1.36 (1.12–1.64) | 1.55 (1.29–1.86) | 1.98 (1.66–2.37) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.26 (1.04–1.53) | 1.27 (1.05–1.54) | 1.36 (1.12–1.63) | 1.55 (1.29–1.86) | <0.001 |
| HPFS | ||||||
| Cases/PY | 1,207/228,292 | 1,392/220,083 | 1,468/210,506 | 1,546/203,806 | 1,639/196,820 | |
| Age adjusted | 1 (ref.) | 1.08 (1.00–1.17) | 1.14 (1.05–1.23) | 1.25 (1.15–1.35) | 1.49 (1.38–1.60) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.08 (1.00–1.17) | 1.13 (1.05–1.22) | 1.23 (1.13–1.32) | 1.42 (1.32–1.54) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.07 (0.99–1.16) | 1.11 (1.03–1.20) | 1.18 (1.09–1.28) | 1.34 (1.24–1.44) | <0.001 |
| Combined | ||||||
| Age adjusted | 1 (ref.) | 1.09 (1.04–1.15) | 1.21 (1.15–1.27) | 1.30 (1.23–1.37) | 1.66 (1.58–1.75) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.10 (1.04–1.16) | 1.21 (1.15–1.28) | 1.29 (1.23–1.36) | 1.60 (1.52–1.68) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.08 (1.02–1.14) | 1.15 (1.09–1.22) | 1.19 (1.13–1.26) | 1.38 (1.31–1.46) | <0.001 |
| Total CHD: fatal CHD + nonfatal MI | ||||||
| NHS | ||||||
| Cases/PY | 566/427,408 | 653/432,382 | 821/424,221 | 804/414,106 | 955/379,116 | |
| Age adjusted | 1 (ref.) | 1.08 (0.97–1.21) | 1.37 (1.23–1.53) | 1.40 (1.26–1.56) | 2.00 (1.80–2.22) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.11 (0.99–1.24) | 1.41 (1.26–1.57) | 1.44 (1.29–1.61) | 1.98 (1.78–2.20) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.07 (0.95–1.20) | 1.28 (1.15–1.42) | 1.24 (1.11–1.38) | 1.53 (1.37–1.71) | <0.001 |
| NHSII | ||||||
| Cases/PY | 96/427,354 | 125/438,165 | 127/439,422 | 145/434,623 | 202/418,795 | |
| Age adjusted | 1 (ref.) | 1.34 (1.02–1.74) | 1.39 (1.06–1.81) | 1.63 (1.26–2.12) | 2.40 (1.88–3.06) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.33 (1.02–1.74) | 1.34 (1.03–1.76) | 1.54 (1.19–2.01) | 2.07 (1.61–2.65) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.29 (0.99–1.69) | 1.25 (0.96–1.64) | 1.31 (1.01–1.71) | 1.53 (1.18–1.97) | 0.002 |
| HPFS | ||||||
| Cases/PY | 852/228,679 | 996/220,495 | 1071/210,867 | 1,147/204,173 | 1,234/197,197 | |
| Age adjusted | 1 (ref.) | 1.11 (1.01–1.21) | 1.17 (1.07–1.29) | 1.31 (1.20–1.44) | 1.59 (1.45–1.74) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.10 (1.00–1.21) | 1.16 (1.06–1.27) | 1.28 (1.17–1.40) | 1.50 (1.37–1.64) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.09 (1.00–1.20) | 1.14 (1.04–1.25) | 1.23 (1.12–1.35) | 1.40 (1.28–1.53) | <0.001 |
| Combined | ||||||
| Age adjusted | 1 (ref.) | 1.11 (1.04–1.19) | 1.26 (1.18–1.35) | 1.36 (1.28–1.46) | 1.79 (1.68–1.91) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.12 (1.04–1.20) | 1.26 (1.18–1.35) | 1.35 (1.27–1.45) | 1.71 (1.60–1.82) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.10 (1.02–1.17) | 1.20 (1.12–1.28) | 1.24 (1.16–1.32) | 1.46 (1.36–1.56) | <0.001 |
| Stroke: fatal and nonfatal stroke | ||||||
| NHS | ||||||
| Cases/PY | 594/427,332 | 688/432,277 | 726/424,135 | 749/413,985 | 756/379,056 | |
| Age adjusted | 1 (ref.) | 1.07 (0.96–1.19) | 1.15 (1.03–1.28) | 1.22 (1.10–1.36) | 1.52 (1.36–1.69) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.07 (0.96–1.19) | 1.15 (1.03–1.28) | 1.22 (1.10–1.36) | 1.48 (1.33–1.65) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.05 (0.94–1.17) | 1.08 (0.97–1.21) | 1.12 (1.01–1.25) | 1.28 (1.14–1.43) | <0.001 |
| NHSII | ||||||
| Cases/PY | 94/427,350 | 119/438,165 | 130/439,391 | 148/434,606 | 177/418,775 | |
| Age adjusted | 1 (ref.) | 1.26 (0.96–1.65) | 1.38 (1.05–1.80) | 1.63 (1.25–2.11) | 2.05 (1.60–2.64) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.26 (0.96–1.65) | 1.36 (1.04–1.78) | 1.58 (1.22–2.05) | 1.91 (1.48–2.47) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.23 (0.94–1.61) | 1.29 (0.99–1.69) | 1.43 (1.10–1.86) | 1.59 (1.22–2.06) | <0.001 |
| HPFS | ||||||
| Cases/PY | 361/228,790 | 401/220,703 | 401/211,118 | 410/204,418 | 420/197,464 | |
| Age adjusted | 1 (ref.) | 1.02 (0.88–1.18) | 1.05 (0.91–1.21) | 1.10 (0.95–1.27) | 1.26 (1.09–1.46) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.02 (0.89–1.18) | 1.06 (0.92–1.23) | 1.11 (0.96–1.28) | 1.26 (1.09–1.46) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.02 (0.88–1.18) | 1.05 (0.91–1.22) | 1.08 (0.93–1.24) | 1.19 (1.03–1.38) | 0.01 |
| Combined | ||||||
| Age adjusted | 1 (ref.) | 1.07 (0.98–1.16) | 1.13 (1.04–1.23) | 1.22 (1.12–1.32) | 1.48 (1.36–1.60) | <0.001 |
| Multivariable model 1† | 1 (ref.) | 1.07 (0.98–1.16) | 1.14 (1.05–1.24) | 1.21 (1.12–1.32) | 1.44 (1.33–1.57) | <0.001 |
| Multivariable model 2‡ | 1 (ref.) | 1.06 (0.97–1.15) | 1.09 (1.00–1.19) | 1.13 (1.04–1.23) | 1.28 (1.17–1.39) | <0.001 |
The EDIP scores were calculated based on cumulatively averaged dietary intakes from all preceding food frequency questionnaires up to each follow-up interval. EDIP scores were adjusted for total energy intake using a residual method. Lower scores indicate anti-inflammatory diets, and higher scores indicate proinflammatory diets.
The multivariable models were adjusted for age (months), race (White, non-White), smoking (never; past; current: 1–14,15–24, ≥25 cigarettes/day), menopausal status and post-menopausal hormone use (premenopausal, never/past users of hormone therapy, and current users of hormone therapy; only in NHS and NHSII), multivitamin use (no, yes), regular aspirin use (no, yes), regular use of other anti-inflammatory medications (no, yes), physical activity (<3, 3–9, 9–18,18–27, 27–42, ≥42 metabolic equivalents/week), and family history of myocardial infarction (no, yes).
Models were further adjusted for hypercholesterolemia (no, yes), hypertension (no, yes), diabetes (no, yes), and body mass index (continuous). Results from the 3 cohorts were pooled using a fixed-effect meta-analysis. In the fully adjusted model, p-heterogeneity testing association difference across the 3 cohorts was 0.98 for CVD, 0.60 for CHD, and 0.69 for stroke.
CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; MI = myocardial infarction; PY = person-years; ref. = reference; other abbreviations as in Table 1.
figure 1. Dose-response relationship of the EDIP scores with risk of (A) CVD, (B) CHD, and (C) stroke.
We combined data from NHS, NHSII, and HPFS for the cubic spline analyses. Models were stratified by cohort, age in months, and follow-up period and were adjusted for race, smoking, menopausal status and post-menopausal hormone use (in women), multivitamin use, regular aspirin use, regular use of other anti-inflammatory medications, physical activity, hypercholesterolemia, hypertension, diabetes, family history of myocardial infarction, and body mass index. Reference levels were set to the median EDIP values of the first quintile. Vertical dashed lines indicate cutoff values of EDIP quintiles (i.e., −0.74, −0.19, 0.24, and 0.75). Solid curves indicate HRs, and dashed curves depict 95% CIs. The axis for HR is nature log-scaled with original labels. CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; EDIP = empirical dietary inflammatory pattern; HPFS = Health Professionals Follow-Up Study; HR = hazard ratio; NHS = Nurses’ Health Study.
In sensitivity analysis, the associations between EDIP and CVD risk remained robust when we further adjusted for alcohol consumption, pack-years of smoking, lipid-lowering or antihypertensive medications, or sodium intake and blood pressures (Supplemental Table 4). The associations did not materially change when we used the most recent EDIP in place of long-term cumulatively averaged EDIP scores or stopped updating EDIP scores when the participants developed intermediate conditions including diabetes, hypercholesterolemia, or hypertension during follow-up (Supplemental Table 5). As expected, EDIP showed only weak correlations with other indices assessing overall dietary quality, including AHEI (r = −0.24 to –0.15 across cohorts), DASH (r = −0.28 to −0.18), and AMED (r = −0.19 to −0.10). Further adjusting for these indices did not materially alter the associations between EDIP and CVD risk (Supplemental Table 6).
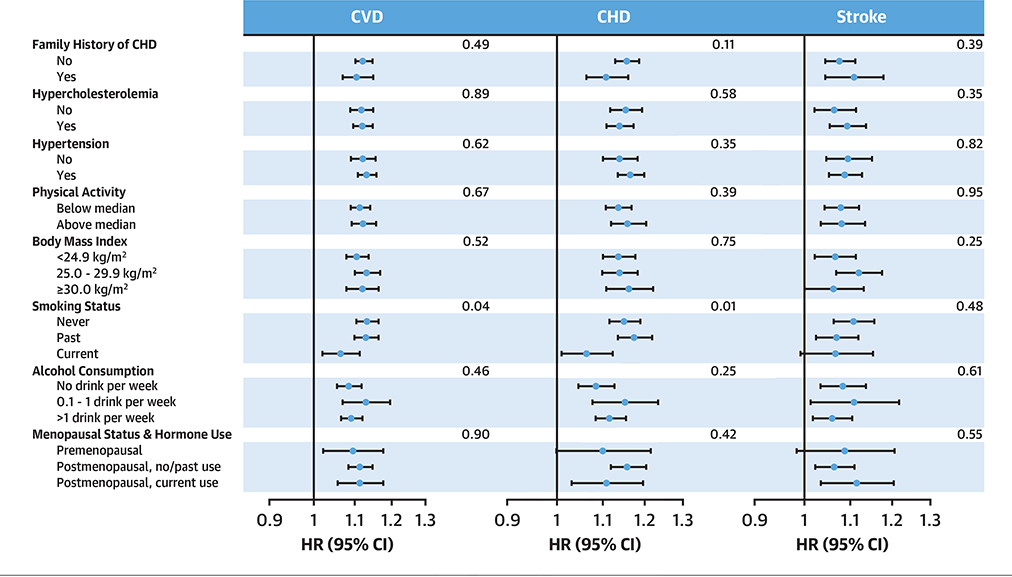
In stratified analyses, the associations were consistent across subgroups of major cardiovascular risk factors including BMI and physical activity (p-difference > 0.05). However, associations between EDIP with risk of total CVD and CHD were significantly stronger among never/past smokers than in current smokers (p-difference = 0.04 for CVD and 0.01 for CHD) (Figure 2), which was likely due to an overall higher CVD risk among smokers (Supplemental Figure 1). Further joint analysis suggested a potential additive effect between EDIP and smoking: compared to never/past smokers in the lowest EDIP quintile, the HR (95% CI) of current smokers in the highest EDIP quintile was 3.06 (2.76 to 3.39) for CVD and 3.44 (3.02 to 3.91) for CHD (Supplemental Figure 1). The association consistency across BMI strata remained among nonsmokers (Supplemental Figure 2).
figure 2. HR (95% CI) for CVD, CHD, and Stroke Associated With a 1-SD Increase in EDIP Scores, Stratified by Pre-selected Cardiovascular Risk Factors.
Multivariable adjusted HRs (indicated by blue dots) and 95% CIs (indicated by black horizonal lines) were calculated separately in each cohort and combined using a fixed-effects meta-analysis. The full model in Table 2 was used, with the exception of not adjusting for a categorical covariate when it was used as strata. The p values for differences across subgroups of risk factors are presented to the right. The axis for HR is nature log-scaled with original labels. Abbreviations as in Figure 1
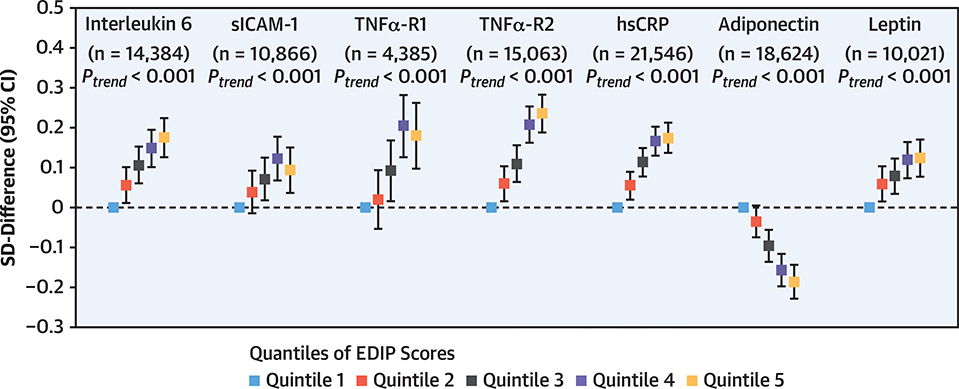
In secondary analyses, we examined associations of EDIP (i.e., averaged scores from 2 FFQ cycles administered before blood collection) with plasma levels of inflammatory biomarkers and lipids in a subpopulation (Central Illustration). We verified a positive association between EDIP and levels of systemic inflammatory biomarkers CRP, interleukin 6, and TNFα-R2 (p < 0.001) in a larger sample than our prior studies (18,19). We further found a strong positive association between EDIP with levels of TNFα-R1, sICAM-1, and leptin and a strong inverse association with levels of adiponectin (i.e., markers not included in EDIP development; p < 0.001) after adjustment for risk factors including BMI (Figure 3). Moreover, a higher EDIP score was associated with increased plasma triglyceride levels, a substantial reduction in high-density lipoprotein cholesterol, and a modest reduction in total cholesterol levels (p < 0.001) (Supplemental Figure 3).
figure 3. Associations Between EDIP Scores and Levels of Inflammatory Biomarkers.
Linear regressions were used to analyze associations between cumulatively averaged EDIP scores (average of 1984 and 1986 in NHS, 1991 and 1995 in NHSII, and 1986 and 1990 in HPFS) and levels of inflammatory biomarkers measured using blood samples collected several years later (1989 and 1990 in NHS, 1996 to 1999 in NHSII, and 1993 to 1995 in HPFS). Multivariable models were adjusted for study cohort, age, fasting status, body mass index, race, smoking, regular aspirin use, regular use of other anti-inflammatory medications, steroid use, multivitamin use, menopausal status and post-menopausal hormone use (in women), physical activity, hypercholesterolemia, diabetes, hypertension, family history of CHD, and case-control status in original substudies. Dots represent SD differences in biomarkers comparing higher to the lowest EDIP quintiles, and vertical lines represent 95% CIs. hsCRP = high-sensitive C-reactive protein; sICAM-1 = soluble intercellular adhesion molecule-1; TNFα-R1 = tumor necrosis factor-a receptor 1; TNFα-R2 = tumor necrosis factor-α receptor 2; other abbreviations as in Figure 1.
DISCUSSION
Using an empirically developed, food-based dietary index to evaluate levels of inflammation induced by diet, we found that a higher inflammatory potential of habitual dietary patterns was associated with an increased incidence of total CVD, CHD, and stroke in U.S. men and women. Compared with individuals consuming anti-inflammatory diets, those consuming proinflammatory diets had a 38% higher risk of developing CVD after adjustment for risk factors and confounders. Consumption of proinflammatory diets was also associated a higher level of systemic, vascular, and metabolic inflammation and an unfavorable lipid profile.
Diet plays an important role in CVD development (16,27,32), and the underlying mechanisms are suggested to partially involve modulation of inflammation (10,32,33). Our study is among the first to link a food-based dietary inflammatory index with CVD incidence. A few prior studies have examined associations of a literature-derived, nutrient-based DII score with CVD risk (34). Results from these studies, as summarized in a meta-analysis, are inconsistent (I2 = 65%) but, in general, suggest a positive association of DII with risk of CVD and MI (comparing extreme categories, a 36% and 43% increased risk, respectively) but not with risk of stroke (34). Given that DII is weighted heavily toward absolute intakes of nutrients and can be influenced by nutritional supplements, its association with CVD may not be completely driven by diet. In contrast, EDIP is based on intakes of food groups and is less affected by nutritional supplements; considering that 31% to 44% of our cohort participants regularly used a multivitamin, EDIP is more accurate in assessing the potential of diet to contribute to inflammation. In addition, food-based indices can overcome some of the limitations of FFQs in assessing absolute intake of individual nutrients and are more translatable to food-based dietary recommendations. Although EDIP and DII are both predictive of inflammatory biomarkers, prior evidence suggested that EDIP exhibited a stronger prediction ability (18). Compared with the results of DII, EDIP showed more robust associations with risk of total CVD, CHD, and stroke, independent of risk factors such as BMI, blood pressure, and family history and confounders such as medications. Although relative risks for total CVD were similar comparing EDIP and DII, because of differences in dietary composition and the moderate correlation (18) between the 2 indices, our results may not be directly comparable to those of DII. Nevertheless, findings from prior studies and our results all suggest that strategies that reduce the inflammatory potential of diets may be of great value in CVD prevention.
Our findings are consistent with prior evidence from prospective cohorts and randomized trials that dietary patterns associated with lower inflammation (e.g., the Mediterranean diet) are associated with reduced CVD risk (10,15,17). Although dietary indices like AHEI, DASH, and AMED assess the general dietary quality, EDIP evaluates specifically the potential of diet to contribute to chronic inflammation. EDIP shares only a few dietary components with other indices (thus explaining their modest correlations) and has a greater emphasis on unique foods associated with inflammation (10,32,33). For instance, it recommends higher intakes of green leafy vegetables, yellow vegetables, whole grains, coffee, tea, and wine, which are rich in anti-inflammatory compounds (e.g., vitamins, carotenoids, flavonoids, and fiber). It also limits intakes of the proinflammatory refined grains and restricts processed, red, and organ meat (vs. others that limit total red/processed meat). We observed a potential synergistic effect between proinflammatory foods, because highly proinflammatory diets were associated with a markedly augmented CVD risk. Of note, the associations between EDIP and CVD risk remained robust after adjustment for AHEI, DASH, or AMED, suggesting that EDIP has unique properties over other traditionally used dietary indices in evaluating dietary quality in CVD prevention.
Smokers had a higher absolute CVD risk compared to nonsmokers, thus explaining the significant smaller relative risk ratios between EDIP and CVD among current smokers compared to never/past smokers. Nevertheless, the additive effect between EDIP and smoking suggested that both smoking and dietary inflammatory potential contribute to CVD development. BMI was not a mediator or an effect modifier for the association between EDIP and CVD risk, suggesting that diets characterized by EDIP may be associated with CVD through inflammatory mechanisms that are not necessarily mediated by obesity.
Inflammation plays a key role in atherosclerosis and thrombosis (2–4). In randomized trials, consumption of some EDIP components (vs. healthy alternatives), including vegetables, coffee, and wine, and red/processed meat, refined grains, and sugary beverages modulated levels of CRP and TNF-α, fibrinogen, and/or adiponectin (11–13,35–37). In our study, EDIP was positively associated CRP, interleukin 6, and TNFα-R2 in larger samples compared to prior studies (18,19), corroborating that EDIP can robustly capture the inflammatory potential of the diet. We further showed that higher EDIP scores were associated with higher circulatory TNFα-R1, indicating activation of pro-inflammatory signaling (38); higher sICAM-1 levels, indicating endothelial activation and vascular inflammation (39); and higher leptin and lower adiponectin levels, indicating metabolic inflammation (40). A higher EDIP was also associated with an unfavorable lipid profile, because most proinflammatory foods have adverse impacts on blood lipids, whereas some anti-inflammatory foods have favorable influences (32). Many EDIP components have been associated CVD risk (32,33). Taken together, our findings suggest that the modulation of systemic, vascular, and metabolic inflammation could be an important mechanism underlying associations between dietary patterns and CVD risk.
Our study was strengthened by highly consistent findings from 3 well-characterized large prospective cohorts. We used the validated, empirically developed, food-based EDIP score, which robustly assesses dietary inflammatory potential with less bias due to nutritional supplements or measurement errors in absolute nutrient intakes. We repeatedly collected diet every 4 years and lifestyles every 2 years; this allowed us to evaluate between-person variations in long-term diets, capture within-person changes in diet and risk factors, associate diets at most relevant time periods during follow-up to subsequent diseases, and reduce measurement errors. Up to 24 to 30 years of prospective follow-up enabled us to examine long-term CVD incidence, reducing concerns of reverse causality. The comprehensive biomarker data (in blood collected after 2 to 3 FFQ cycles) also allowed us to link proinflammatory diets with levels of systemic, vascular, and metabolic inflammation.
STUDY LIMITATIONS.
First, because of the observational nature, our study cannot conclude causality. Second, although our FFQ was validated to have a reasonably high validity (23–25) and we used repeated assessments to reduce random errors, measurement errors may still exist in self-reported diet and covariates. However, such measurement errors usually attenuate true associations. Third, residual and unmeasured confounding cannot be completely excluded, although we have controlled for extensive demographic, lifestyle, anthropometric, and clinical factors. Finally, our study was performed among nurses and health professionals who are mostly White; therefore, the generalization of our findings requires validation in other populations with diverse socioeconomic and racial/ethnic backgrounds. The relative homogeneity of our participants, however, enhanced the internal validity by possibly reducing potential for confounding and reverse causation.
CONCLUSIONS
Findings from 3 large U.S. prospective cohort studies indicate that dietary patterns with higher inflammatory potential were significantly associated with a higher incidence of CVD, CHD, and stroke. Our study suggests that modulation of chronic inflammation may be a potential mechanism linking dietary patterns with CVD. Future studies are warranted to replicate our findings, to confirm the causal relationship, and to examine the detailed inflammatory mechanisms through which diet/specific foods are associated with CVD risk. Reducing the inflammatory potential of diets may provide an effective strategy for CVD prevention.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
In both men and women in the United States, diets with a higher inflammatory potential are associated with an increased risk of cardiovascular disease, including coronary artery disease, and stroke, independent of other risk factors and dietary quality indices.
TRANSLATIONAL OUTLOOK:
Further studies are needed to determine whether the association between dietary inflammatory potential and cardiovascular risk extends to other populations and whether dietary changes that reduce chronic inflammation reduce cardiovascular risk.
ACKNOWLEDGMENTS
We thank the participants and staff of the NHSs and the HPFS for their enthusiastic participation, valuable contributions, and long-term commitment to scientific research.
The Nurses’ Health Studies and Health Professional Follow-up Studies are supported by National Institutes of Health grants U01 CA186107, R01 CA49449, R01 HL034594, R01 HL088521, U01 CA176726, R01 CA49449, U01 CA167552, R01 HL60712, and R01 HL35464. Dr. Li was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (K99 DK122128) and Boston Nutrition Obesity Research Center (2P30DK046200-26). Dr. Tabung was supported by R00 CA207736.
ABBREVIATIONS AND ACRONYMS
- AHEI
Alternate Healthy Eating Index
- AMED
Alternate Mediterranean Diet score
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence intervals
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DASH
Dietary Approaches to Stop Hypertension
- DII
Dietary Inflammatory Index
- EDIP
empirical dietary inflammatory pattern
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- MI
myocardial infarction
- NHS
Nurses’ Health Studies
- sICAM
soluble intercellular adhesion molecule
- TNFα-R1
tumor necrosis factor-α receptor 1
- TNFα-R2
tumor necrosis factor-α receptor 2
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental tables and figures, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
REFERENCES
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352: 1685–95. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83: 456S–60S. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. [DOI] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 6.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351: 2599–610. [DOI] [PubMed] [Google Scholar]
- 7.Swerdlow DI, Holmes MV, Kuchenbaecker KB, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379: 1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridker PM, Everett BM, Thuren T, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377: 1119–31. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol 2006;48: 677–85. [DOI] [PubMed] [Google Scholar]
- 10.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol 2006;26:995–1001. [DOI] [PubMed] [Google Scholar]
- 11.Hajihashemi P, Haghighatdoost F. Effects of whole-grain consumption on selected biomarkers of systematic inflammation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr 2019;38:275–85. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini B, Berthon BS, Saedisomeolia A, et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr 2018;108:136–55. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinpour-Niazi S, Mirmiran P, Fallah-Ghohroudi A, Azizi F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: a randomised cross-over clinical trial. Br J Nutr 2015; 114:213–9. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 15.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis 2014;24:929–39. [DOI] [PubMed] [Google Scholar]
- 16.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extravirgin olive oil or nuts. N Engl J Med 2018;378: e34. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res 2019;124:779–98. [DOI] [PubMed] [Google Scholar]
- 18.Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr 2017;147: 1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr 2016;146: 1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabung FK, Giovannucci EL, Giulianini F, et al. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of post-menopausal women in the United States. J Nutr 2018;148:771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338: 464–8. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 25.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol 2018;187:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 27.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation 2015;132:2212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction: 2008–09 revision. Int J Epidemiol 2011;40:139–46. [DOI] [PubMed] [Google Scholar]
- 29.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981;12:I13–44. [PubMed] [Google Scholar]
- 30.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984;119:837–9. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Rice MS, Huang T, et al. Circulating prolactin concentrations and risk of type 2 diabetes in US women. Diabetologia 2018;61:2549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand SS, Hawkes C, de Souza RJ, et al. Food Consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol 2015;66:1590–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res 2007;73:326–40. [DOI] [PubMed] [Google Scholar]
- 34.Shivappa N, Godos J, Hébert JR, et al. Dietary inflammatory index and cardiovascular risk and mortality—a meta-analysis. Nutrients 2018;10: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 2011;342: d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011; 94:479–85. [DOI] [PubMed] [Google Scholar]
- 37.Hematdar Z, Ghasemifard N, Phishdad G, Faghih S. Substitution of red meat with soybean but not non- soy legumes improves inflammation in patients with type 2 diabetes; a randomized clinical trial. J Diabetes Metab Disord 2018;17:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- 39.Gross MD, Bielinski SJ, Suarez-Lopez JR, et al. Circulating soluble intercellular adhesion molecule 1 and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults Study. Clin Chem 2012;58:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fantuzzi G Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115: 911–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.