Abstract
Recently introduced fluorine-18 prostate-specific membrane antigen-1007 (18F-PSMA-1007) for imaging prostate cancer has an intense physiologic liver uptake and biliary excretion. The aim of the present study was to evaluate the effect of different dietary conditions on this physiological uptake. Forty consecutive prostate cancer patients were scanned with 18F-PSMA-1007 positron emission tomography/computed tomography at different dietary conditions. In addition to a blinded read scoring, tracer uptake intensities (standardized uptake values [SUVs]) were measured in the liver and small bowel. There was no significant difference in liver and small-bowel uptake between different patient groups. Wilcoxon signed-rank tests revealed no significant difference of the median mean SUV of the liver or maximum SUV of the horizontal part of the duodenum between different dietary conditions groups. A dietary preparation of patients by fasting or the attempt to clear liver activity by high caloric drinks does not have a significant effect on tracer uptake in the liver or in the small bowel.
Keywords: Positron emission tomography/computed tomography, prostate cancer, prostate-specific membrane antigen-1007
INTRODUCTION
Prostate-specific membrane antigen (PSMA)-targeted positron emission tomographic (PET) imaging has gained a major role in the management of patients with prostate cancer, especially since the introduction of gallium-68 PSMA-11 (68Ga-PSMA-11).[1,2] The recently introduced 18F-labeled PSMA-1007 is getting more attention due to the larger amount of activity produced by cyclotrons leading to better availability of PSMA-targeted imaging compared to limited activities of gallium-68, which is eluted from a 68Ge/68Ga-generator.[3,4,5] Due to the F-18 labeling, it has a longer half-life and a higher spatial resolution compared to 68Gallium. Furthermore, the very low urinary activity in fluorine-18 PSMA-1007 (18F-PSMA-1007) PET/computed tomography (CT) scans is another advantage of this new diagnostic agent, which allows better diagnosis of lymph node metastases in the pelvis by merit of lower urinary activity in the ureter and also allows the differentiation of local relapse from the urinary bladder. 18F-PSMA-1007 has shown a delayed renal excretion but a fast metabolism through the liver and gallbladder as well as biliary transportation through the small bowl. Hence, heterogeneous visualization of the small bowel and parts of the colon increasing over time was observed.[6] Furthermore, a highly variable but generally intense liver uptake has been described for this tracer potentially reducing the sensitivity for visceral metastases. The aim of the present evaluation was to analyze the effects of different dietary conditions on the biodistribution of 18F-PSMA-1007 PET/CT.
MATERIALS AND METHODS
Forty consecutive patients were scanned with 18F-PSMA-1007 PET/CT after different dietary preparations to reduce radiation exposure and to improve image quality of 18F-PSMA-1007 PET/CT in clinical routine. There was no change of standard imaging procedures and no study specific drug intervention. Data analysis for the present study was performed retrospectively. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ten patients were scanned without any preparation (e.g. having breakfast at any time they wished), ten patients were fasting for at least 6 h before injection of 18F-PSMA-1007 and remained so after injection and during scanning, ten patients fasted for 6 h before injection but received a high caloric drink (Fresubin, 1.5 kcal/ml, FRESENIUS KABI, Bad Homburg, Germany) 1 h before the tracer injection, and ten patients who fasted for at least 6 h received a high caloric drink 1 h after the injection for 18F-PSMA-1007. Patients were referred to 18F-PSMA-1007 PET/CT for different indications: biochemical recurrence (n = 21), follow-up after therapy (n = 18), and initial staging (n = 1).
All patients received detailed information about the imaging procedures and signed an informed consent according to the institutional guidelines.
Imaging procedures and preparation of fluorine-18 prostate-specific membrane antigen-1007
18F-PSMA-1007 was produced in a GE TracerLab MX synthesizer according to the one-step procedure described by Cardinale et al. and standard operation procedure described before.[3,7]
Patients received 4 MBq per kg body weight dose with a maximum of 400 MBq per patient (mean injected activity: 333 ± 51 MBq, range: 227–410). Scanning was performed 120 min after injection starting at lower limbs to the skull. Imaging at 120 min was previously described to be the optimal time point for 18F-PSMA-1007 due to a higher contrast (tumor-to-background ratio).[3] Patients were asked to void before the scan. Images were acquired with a scan time of 3 min per bed position on a Siemens mCT scanner (Siemens Healthcare, Knoxville, Tennessee, USA). Image reconstruction was performed using standard manufacturer software. For attenuation correction, a low-dose CT was performed corresponding to PET images.
Image analysis
Analysis was performed on coregistered images and maximum intensity projection 3D images using the Syngo.via software (version: VB20A, Siemens Healthcare). According to the institutional procedures, all scans were analyzed by two board-certified nuclear medicine physicians and radiologists at an interdisciplinary conference. In addition, a blinded read was performed by an experienced nuclear medicine physician to evaluate and score the amount of activity in the intestine. The abdomen was divided into upper and lower abdomen. Tracer uptake was scored 0 for no activity, and 1 to 3 for low, medium and high tracer uptake respectively within the bowel.
Activity mean standardized uptake value (SUVmean) in the liver and the horizontal part of the duodenum (PHD) were measured. Circular regions of interest were placed in three representative slices of the liver, and the mean value of all three measurements was used for the analysis. For PHD, volumes of interest were placed on the plane with the visually highest uptake and maximum SUV (SUVmax) was measured.
Statistical analysis
Descriptive statistics are absolute and relative frequencies; mean or median and standard deviation or range were used to characterize the study population. Wilcoxon signed-rank test was performed to analyze significance of median SUVmax and SUVmean values. A value of P < 0.05 was considered statistically significant.
RESULTS
There was no significant difference in bowel activity levels between the different patient groups. The results of the blinded read are presented in Table 1. Figure 1 shows representative images of each group of patients. Wilcoxon signed-rank tests revealed no significant difference between the median SUVmean of the liver and SUVmax of PHD in different groups. Table 2 presents the median SUVmean of the liver and SUVmax of PHD in different patient groups.
Table 1.
Bowel activity level in different patient groups depending on dietary preparation
| Group | Upper abdomen | Lower abdomen | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| I | 0 | 1 | 8 | 1 | 1 | 3 | 5 | 1 |
| II | 0 | 3 | 7 | 0 | 1 | 5 | 1 | 3 |
| III | 0 | 4 | 5 | 1 | 1 | 1 | 5 | 3 |
| IV | 0 | 1 | 8 | 1 | 0 | 1 | 4 | 5 |
Group I: Not fasting; Group II: High caloric drink 1 h before injection; Group III: Fasting for at least 6 h; Group IV: High caloric drink 1 h after injection; 0: No bowel activity; 1: Low bowel activity; 2: Intermediate bowel activity; 3: High bowel activity
Figure 1.
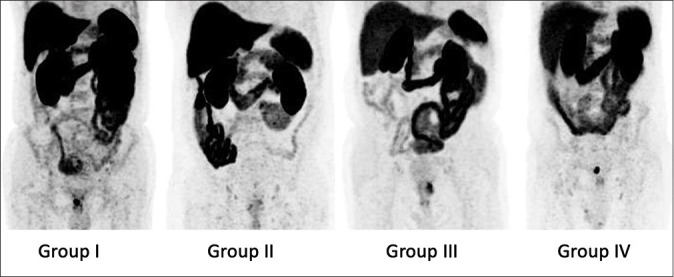
Maximum intensity projections of fluorine-18 prostate-specific membrane antigen-1007 positron emission tomography of the abdomen in different patient groups showing comparable tracer levels in the liver and small bowel through all groups. Group I: not fasting; Group II: high caloric drink 1 h before injection; Group III: fasting for at least 6 h; Group IV: high caloric drink 1 h after injection. For example, local recurrent in the patient in Group IV can clearly be differentiated and is not affected by bowel activity
Table 2.
Liver and duodenal standardized uptake values
| Group | SUVmean liver | SUVmax PHD | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| I | 11.54 | 8.72-17.23 | 19.54 | 10.48-29.50 |
| II | 11.01 | 7.10-16.38 | 20.56 | 11.78-28.38 |
| III | 10.86 | 7.27-14.49 | 17.30 | 12.70-32.80 |
| IV | 11.40 | 8.32-19.79 | 16.50 | 14.64-21.31 |
Group I: Not fasting; Group II: High caloric drink 1 h before injection; Group III: Fasting for at least 6 h; Group IV: High caloric drink 1 h after injection; PHD: Horizontal part of duodenum; SUVmean: Mean standardized uptake value; SUVmax: Maximum standardized uptake
DISCUSSION
The present study analyzed the effects of different dietary conditions on the biodistribution of 18F-PSMA-1007 in PET-CT. The aim was to optimize dietary preparation of the patients to reduce physiological activity in the liver and/or bowel thereby improving the differentiation between tumor lesions and the high background signal derived from the aforementioned organs when using 18F-PSMA-1007. Dietary approaches for improving image quality have already been established for other nuclear medicine procedures: for instance, fatty diet helps to reduce the radioactivity signal of the liver in cardiac scintigraphy with 99mTc-methoxy isobutyl isonitrile.[8]
Our data show that the tracer uptake in the upper and lower abdomen was heterogeneous in general with no difference between the patient groups. A high caloric, fatty drink did not have any effect on the physiological activity of the liver and of the horizontal part of the duodenum. This may indicate that parts of the liver activity are not related to biliary excretion but rather to storage of tracer in the liver parenchyma. In accordance with this assumption, a recent study showed increasing liver activity from images acquired 1 h p. i. and 2 h p. i.[3]
Bowel activity does not interfere with the assessment of prostate cancer since the main paths of lymphatic drainage are retroperitoneal and since the morphological component of PET/CT or PET/magnetic resonance imaging allows to attribute activity to the bowel or to lymph nodes.
In the present small and heterogeneous patient sample, a systematic comparison of accuracies for the detection of prostate cancer and metastasis was not possible. To the authors impression detection rates were comparably high in the groups. As reported in recent studies, the detection rate of 18F-PSMA-1007 PET seems to be higher than for 68Ga-PSMA-11 with 95%[4] and 81%[5] compared to 79.5% by 68Ga-PSMA-11[2] in the largest population reporting results of 1007 patients with biochemical recurrent prostate cancer. Due to the very low renal elimination of 18F-PSMA-1007, lymph nodes or local relapses can be better differentiated from the activity in the ureter or urinary bladder.
Further studies are necessary to investigate if possibly other dietary procedures can help to reduce the signal in the other organs caused by physiological uptake of 18F-PSMA-1007 or alternative PSMA ligands. Until then, other procedures such as application of intravenous or oral contrast medium should be considered to better distinguish between tumor lesions and adjacent/surrounding healthy tissues.
CONCLUSIONS
A dietary preparation of patients with or without fasting or an additional high caloric drink did not have an effect on tracer uptake in the liver and in the small bowl. Therefore, no dietary intervention is recommended before 18F-PSMA-1007 PET/CT.
Financial support and sponsorship
Nil.
Conflicts of interest
The University of Muenster received consulting fees from ABX GmbH, Radeberg, Germany for K.R. and M.B. Additionally, K.R. is scientific consultant/advisor of ABX GmbH. The authors declare no conflict of interest according to the subject and matter of the present manuscript.
Acknowledgments
We thank the radiochemistry group at the department of nuclear medicine for their highly reliable production of 18F-PSMA-1007, as well as the technologists for their support.
REFERENCES
- 1.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68) Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahbar K, Afshar-Oromieh A, Bögemann M, Wagner S, Schäfers M, Stegger L, et al. 18F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: Biodistribution, tumour detection and activity kinetics. Eur J Nucl Med Mol Imaging. 2018;45:1329–34. doi: 10.1007/s00259-018-3989-0. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar K, Afshar-Oromieh A, Seifert R, Wagner S, Schäfers M, Bögemann M, et al. Diagnostic performance of 18F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2055–61. doi: 10.1007/s00259-018-4089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of [18F]PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2018 doi: 10.2967/jnumed.118.212233. pii: jnumed 118212233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinale J, Schäfer M, Benešová M, Bauder-Wüst U, Leotta K, Eder M, et al. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. 2017;58:425–31. doi: 10.2967/jnumed.116.181768. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen AJ, van Rijk PP. Minimizing liver, bowel, and gastric activity in myocardial perfusion SPECT. J Nucl Med. 2000;41:1315–7. [PubMed] [Google Scholar]


