Abstract
This study aimed at assessing the performance of 177Lu-DOTATATE-based peptide receptor radionuclide therapy (PRRT) in de-differentiated thyroid carcinoma thyroglobulin-elevated negative iodine scintigraphy (TENIS) in terms of clinical efficacy and outcome. This is a retrospective analysis of patients of TENIS who had undergone PRRT in a tertiary care setting. The selected patients were analyzed for the following parameters: (i) the patient characteristics, (ii) the metastatic burden, (iii) study of PRRT cycles and activity, (iv) response assessment (undertaken by three-parameter scale: symptomatic including Karnofsky/Lansky Performance scoring, biochemical and scan features) employing predefined criteria (detailed in methods), and (v) Grade III/IV hematological or renal toxicity. According to the qualitative uptake of the tracer in somatostatin receptor (SSTR)-based imaging (with either 99mTc-HYNIC-TOC/68Ga-DOTATATE), the lesions were divided into the following four categories: Grade 0: no uptake, Grade I: uptake less than the liver but more than background, Grade II: uptake equal to the liver, and Grade III: uptake more than the liver. A total of eight patients of TENIS who had undergone 177Lu-DOTATATE were retrieved. Among those eight patients, the follow-up duration (from the time of the 1st PRRT cycle) at the time of analysis ranged from 7 to 52 months, with an average of 34 months. At the time of assessment, two (25%) out of the eight patients had expired due to extensive metastatic disease and 6 (75%) were alive. On symptomatic response, complete disappearance of symptoms was found in one patient (12.5%), whereas three patients (37.5%) showed partial improvement in symptoms after PRRT and four patients (50%) showed worsening of and appearance of new symptoms. On biochemical response, reduction in serum thyroglobulin (TG) was found in three patients (37.5%) after PRRT and increase in serum TG was noticed in the rest of five patients (62.5%). Imaging response showed stable scan in two patients (25%) and progressive disease (PD) in six patients (75%), following a progression-free survival ranging from 7 to 16 months, when they were considered for tyrosine kinase inhibitors in view of PD. There was no obvious evidence of Grade III/IV hematological or renal toxicity in any of the patients, suggesting that the therapy in this group of patients is well tolerated. In addition, we also observed that most patients of TENIS showed low-grade uptake on SSTR-based imaging (Grade II as per our semi-quantitative scale), with only one patient showing Grade III uptake. 177Lu-DOTATATE PRRT demonstrates modest response in SSTR-positive metastatic TENIS patients: (i) low SSTR expression and tracer avidity, and correspondingly lesser degree of targeting by the therapeutic agent and (ii) the fact that most of the TENIS patients usually have fluorodeoxyglucose (FDG)-avid disease, where high FDG avidity is commensurate with aggressive biology and could be the reason for the relatively less response documented. Larger prospective data need to be accrued in this domain in view of its well tolerability and nonavailability of better efficacious and less toxic treatment at present; however, this needs to be tried in receptor-positive cases with high-grade uptake (Score III/IV) for a definitive conclusion.
Keywords: 177Lu-DOTATATE, peptide receptor radionuclide therapy, thyroglobulin-elevated negative iodine scintigraphy
INTRODUCTION
With the evolution and wider availability of 177Lu-DOTATATE, peptide receptor radionuclide therapy (PRRT) has gained popularity in the treatment of metastatic/advanced neuroendocrine tumors (NETs) over recent years.[1,2] Most of the peer-reviewed literature and clinical application of this therapeutic modality (with either 177Lu or with90 Y labeled somatostatin analogs) have been on metastatic gastroenteropancreatic (GEP) NETs, where now it has an established role in Grade I and Grade II diseases, with substantially better health-related quality of life, symptomatic improvement, and disease stabilization.[3,4,5,6,7,8,9,10,11,12,13,14] Therapeutic applications of PRRT beyond NET have been postulated to be possible in de-differentiated thyroid carcinoma, especially thyroglobulin-elevated negative iodine scintigraphy (TENIS) form. To date, there is paucity of reported literature on this subject across active treatment PRRT centers. Reservations exist with respect to the efficacy in this group which clearly behaves distinctly compared to the traditional classical indication of GEP-NET. There have been very few reports that have specifically looked into this group of thyroid cancer.[15,16,17] Most of these studies conclude the requirement of further research; hence, analysis of PRRT performance, in terms of efficacy and outcome profile, both in prospective and retrospective settings, is a clear need for defining the appropriate role of this therapeutic approach in this clinical setting.
MATERIALS AND METHODS
This was a retrospective analysis of patients of metastatic TENIS who had undergone PRRT with 177Lu-DOTATATE at a large tertiary care center. The pretreatment work-up and therapeutic protocol followed for these patients were according to our standard procedure similar with metastatic GEP-NETs,[18] and included pretreatment somatostatin receptor (SSTR)-targeted imaging, renal and hematological parameter evaluation to determine the patient's suitability. Before undertaking the 177Lu-DOTATATE PRRT, informed verbal and written consent was obtained from all patients selected for such therapy, which was based on discussion with the patients and relatives with respect to this treatment option versus tyrosine kinase inhibitor (TKI)-based treatment. The patients selected for the study fulfilled the following criterion: patients of metastatic TENIS who demonstrated uptake in the metastatic lesions on initial diagnostic study (68Ga-DOTANOC/TATE or the 99mTc-HYNIC-TOC) and had received at least one cycle of PRRT with 177Lu-DOTATATE. The selected patients were analyzed under the following parameters: (i) the patient characteristics, (ii) the metastatic burden, (iii) study of PRRT cycles and activity and other treatment administered, (iv) response (using a three-parameter assessment: symptomatic including Karnofsky/Lansky Performance scoring and biochemical and scan features), and (v) any incidence of Grade III/IV hematological or renal toxicity in any of the patients. According to the qualitative uptake of the tracer in SSTR-based imaging (with either99m Tc-HYNIC-TOC/68 Ga-DOTATATE), the lesions were divided into the following four categories: Grade 0: no uptake, Grade I: uptake less than the liver but more than background, Grade II: uptake equal to the liver, and Grade III: uptake more than the liver.
Response evaluation
Response was assessed post-PRRT under the following three headings – clinical/symptomatic response (subjective response), biochemical response (tumor marker serum thyroglobulin [TG]), and radiological/molecular imaging response (18F-fluorodeoxyglucose [FDG] positron emission tomography [PET]/computed tomography [CT] and SSTR imaging).
Response scales and definition of categories
Symptomatic response evaluation
An improvement in tumor-related symptoms based on the patient's subjective report relative to baseline symptoms was defined as symptomatic response. The patients were asked at follow-up with direct questioning on a scale of 0%–100% as to whether tumor-related symptoms had “disappeared:” 100% improvement (complete response [CR]), or “improved:” 30%-–75% improvement (partial response [PR]), or were “stable:” <30% improvement (stable disease [SD]), or “worse:” >30% increase in symptoms or new symptoms of PD compared to baseline.
Biochemical response evaluation
Biochemical response was assessed by the serum TG levels. The baseline values of these biochemical markers before the start of PRRT were measured, and the percentage change after each cycle of PRRT was analyzed. More than a 75% reduction in biochemical marker was considered as CR, 30%–75% reduction as PR, <30% reduction or <30% increase as SD, and >30% increase in biochemical marker as PD.
Objective scan response evaluation: Anatomical imaging and molecular imaging
The Response Evaluation Criteria in Solid Tumor (RECIST 1.1) was used to evaluate anatomical imaging response in these TENIS cases. Molecular imaging response evaluation was undertaken by using PET-CT scan (68Ga-DOTATATE and 18F-FDG) with help of PET Response Criteria in Solid Tumors (PERCIST), and molecular imaging responses were categorized into responders (CR, PR and SD) and non-responders (PD). The response evaluation scans were undertaken before each cycle of PRRT.
RESULTS
A total of eight patients of TENIS were retrieved after analyzing the records of thyroid cancer patients who had undergone PRRT. Out of the eight TENIS patients (male: female = 5:3, age range: 57–83 years), three patients had undergone one cycle of PRRT, two patients had undergone two cycles, another two patients had undergone four cycles of PRRT, and one patient received three cycles of PRRT. The histopathology included classical papillary Carcinoma of thyroid (PCT) (n = 4), follicular variant of PCT (n = 2), poorly differentiated carcinoma (n = 1), and follicular carcinoma thyroid (n = 1). The sites of metastases, the metastatic burden, and the grade of uptake on SSTR-based imaging are detailed in Table 1.
Table 1.
Patients characteristics
| Characteristics | Values |
|---|---|
| Age distribution (years) | 57-83 |
| Sex (male: female) | 5:3 |
| Sites of metastases | Thyroid |
| Bilateral lung (nodules) | |
| Pretracheal and paratracheal nodes | |
| Anterior mediastinum | |
| Right fibula | |
| Right ischium | |
| Multiple vertebrae | |
| Grade of 68Ga- | |
| DOTATATE/99mTc-HYNIC- | |
| TOC uptake (semiquantitative scale) | |
| Grade 0 (no uptake) | 0 |
| Grade I (less than liver) | 1 |
| Grade II (equal to liver) | 6 |
| Grade III (more than liver) | 1 |
The sites of metastases and primary histopathology in the patients of TENIS are summarized in Table 2.
Table 2.
Patient and lesion specific histopathological characteristics
| Patient | Site of metastases | Histopathological characteristics |
|---|---|---|
| Case I | Thyroid, bilateral lung nodules | Metastatic FVPCT |
| Case II | Right supraclavicular, right paratracheal, bilateral lung | Differentiated PCT |
| Case III | Anterior mediastinum | Differentiated PCT |
| Right lung | ||
| Left level III cervical node | ||
| Case IV | Bilateral lung | Differentiated PCT |
| Case V | Retropharyngeal mass | Follicular carcinoma thyroid |
| Case VI | Right fibula | Poorly differentiated Ca thyroid |
| Skull | ||
| Left 6th rib | ||
| Bilateral pelvic bone | ||
| Case VII | Multiple vertebrae | Differentiated PCT |
| Bilateral lung | ||
| Case VIII | Bilateral lung, bony lesions | FVPTC |
Ca: Carcinoma; PCT: Papillary carcinoma thyroid; FVPCT: Follicular variant of papillary carcinoma thyroid
Somatostatin receptor/fluorodeoxyglucose dual-tracer imaging characteristics
The dual-tracer imaging characteristics (with SSTR and FDG) are elaborated in Table 3.
Table 3.
Evaluation of somatostatin receptor based imaging and fluorodeoxyglucose-positron emission tomography at baseline
| Patient | 68Ga-DOTATATE/99mTc-HYNIC-TOC | FDG-PET/CT (SUVmax) |
|---|---|---|
| Case I | Grade I | 13 |
| Case II | Grade II | 15 |
| Case III | Grade II | 8.6 |
| Case IV | Grade II | 7.5 |
| Case V | Grade II | 23.46 |
| Case VI | Grade III | 6.2 |
| Case VII | Grade II | 13 |
| Case VIII | Grade II | 13 |
FDG-PET/CT: Fluorodeoxyglucose-positron emission tomography/computed tomography; SUVmax: Maximum standardized uptake value
The follow-up duration (from the time of the 1st PRRT cycle) at the time of analysis ranged from 7 to 52 months. The number of cycles along with administered activity and duration of follow-up aresummarized in Table 4.
Table 4.
Individual patient specific treatment details
| Patient | Number of cycles of PRRT given (cumulative activity in GBq) | Duration of follow-up from 1st cycle of PRRT (months) |
|---|---|---|
| Case I | 3 cycles (17.24) | 24 |
| Case II | 1 cycle (6.7) | 36 |
| Case III | 1 cycle (5.5) | 12 |
| Case IV | 4 cycles (25.4) | 43 |
| Case V | 2 cycles (10.0) | 52 |
| Case VI | 2 cycles (10.8) | 48 |
| Case VII | 1 cycle (6.7) | 7 |
| Case VIII | 4 cycles (25.4) | 50 |
PRRT: Peptide receptor radionuclide therapy
There was no obvious evidence of Grade III/IV hematological or renal toxicity in any of the patients during the follow-up period.
At the time of assessment, two patients had expired and six patients were alive. On symptomatic response scale, complete disappearance of symptoms was found in one patient (12.5%), three patients (37.5%) showed partial improvement in symptom after PRRT, whereas four patients (50%) showed worsening of and appearance of new symptoms. On biochemical response, reduction in serum TG was found in three patients (37.5%) after PRRT and increase in serum TG was noticed in the rest of the five patients (62.5%). Imaging response showed stable scan finding in two patients (25%) and PD in six patients(75%), following a progression-free survival ranging from 7 to 16 months, when they were considered for TKIs in view of PD.
DISCUSSION
SSTR-based theranostics has been a recent development in the management of NETs, which incorporates molecular imaging with either gamma camera and single-photon emission CT-based tracers (e.g.,99mTc-HYNIC-TOC/111In-DTPA-octreotide) or more preferably with PET-based tracers such as 68Ga-DOTA-NOC/TATE, which offer the advantages of better resolution and quantification useful for treatment response evaluation. The therapy is undertaken with either 177Lu or with 90Y labeled somatostatin analogs (such as 177Lu-DOTA-TOC/TATE or 90Y-DOTA-TOC), which has been immensely successful in metastatic GEP-NETs. The TENIS has been postulated as a potential indication of PRRT in patients who demonstrate substantial tracer avidity on 68Ga-DOTATATE-positive scan. There is at present relative paucity of literature data on the efficacy of PRRT in patients with TENIS.[15,16,17]
Budiawan et al.[15] studied 16 nonradioiodine-avid and/or radioiodine therapy-refractory thyroid cancer patients that included follicular thyroid carcinoma (n = 4), medullary thyroid carcinoma (n = 8), Hürthle cell thyroid carcinoma (n = 3), and mixed carcinoma (n = 1). These patients were treated with PRRT using (90) Yttrium and/or (177) Lutetium labeled somatostatin analogs. No specific subdivisions were made in this study. There was disease stabilization in four (36.4%) patients, partial remission (PR) in two patients (18.2%), PD in five patients (45.5%).
Versari et al.[16] reported 11 patients treated with PRRT with fractionated injection of 1.5–3.7 GBq 90Y-DOTATOC, of which disease control was achieved in seven patients (two PR and five stabilization), with a duration of response of 3.5–11.5 months. The authors concluded that functional volume over time obtained by PET/CT was the only parameter demonstrating a significant difference between lesions responding and nonresponding to PRRT.
Czepczyński et al.[17] studied 11 patients of radioiodine- refractory differentiated thyroid cancer treated with PRRT. Fractionated treatment protocol with four doses of 90Y-DOTA-TOC in 12-week intervals was employed in these patients, and the activity of each dose administered was 3.7 GBq (100 mCi). Of the 11 patients, 5 died before receiving the fourth course of PRRT. In the remaining six patients, morphological response, evaluated 3 months after the last course using the RECIST criteria, showed PR in one patient, SD in two patients, and PD in three patients. Biochemical response based on TG measurements before and after PRRT showed PR in one patient, SD in four patients, and PD in one patient. The median survival was 21 months from the first course of PRRT.
A comparison was drawn with the findings of previous studies and our findings in the present analysis. In our series, among the eight patients of TENIS, partial biochemical response was noted in three patients (37.5%). On imaging response evaluation, SD was observed in two patients (25%), whereas most demonstrated a PD after 7–16 months of PFS. In addition, we also observed that most patients of TENIS showed low-grade uptake on SSTR-based imaging (Grade 2 as per our semi-quantitative scale), with only one patient showing Grade 3 uptake [Figure 1]. This is similar to what was observed in our previous series.[19]
Figure 1.
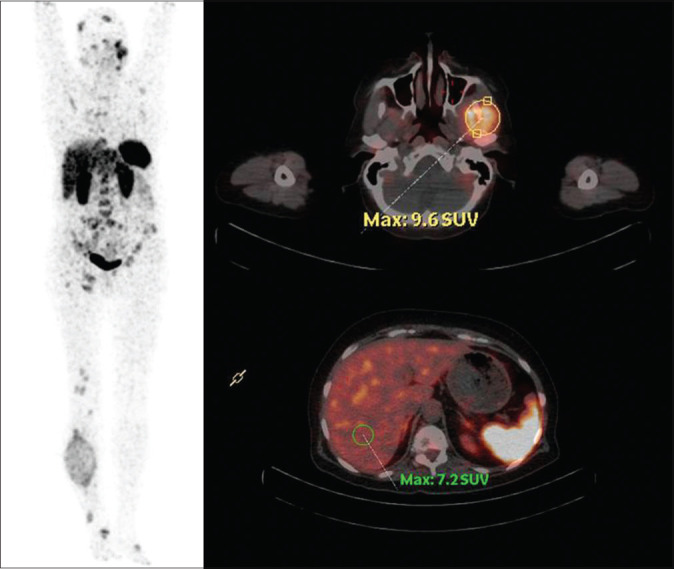
68Ga-DOTATATE positron emission tomography/computed tomography in a patient of thyroglobulin-elevated negative iodine scintigraphy demonstrating Grade III uptake in a number of the skeletal metastatic lesions
Unlike GEP-NET, there is relatively less percentage and obvious objective response to PRRT in thyroid cancer patients, the reason for which this therapy has not been employed widely in this group of patients. Many patients with these malignancies are usually asymptomatic, especially patients with TENIS. Thus, at times, it is difficult to classify them on symptomatic scale, and maintenance of asymptomatic status would usually qualify as SD during response assessment.
From the previously reported studies and the result of this study, it appears that PRRT with 177Lu-DOTATATE is modestly useful in metastatic TENIS and does not produce remarkable improvement akin to that observed in metastatic GEP-NET. A few explanations could be made for the relative inefficacy of PRRT in thyroid tumors as follows: (i) the relatively low-grade SSTR expression and low-grade 68Ga/177Lu-DOTATATE avidity in general in this group of patients is usual and correspondingly the targeting by the therapeutic agent is relatively low compared to GEPNETs and (ii) most of the TENIS patients usually have FDG-avid disease, the high FDG avidity is commensurate with aggressive biology and could be the reason for their relatively low response.
CONCLUSION
In metastatic TENIS patients, PRRT may be considered a therapeutic option with minimal toxicity, depending on SSTR expression in these cases with a fraction of patients showing promising response [Tables 5 and 6]. A larger patient population need to be examined using other radiopeptides with higher affinity to the predominantly expressed SSTR subtypes in thyroid cancer to confirm the benefit of PRRT in TENIS cases.
Table 5.
Patient specific hematological and renal toxicity
| Patient | Hematological toxicity | Renal toxicity | ||||
|---|---|---|---|---|---|---|
| Anemia | WBC | Platelet count | Serum creatinine | GFR | ERPF | |
| Case I | - | - | - | - | - | - |
| Case II | - | - | - | - | - | - |
| Case III | - | - | - | - | - | - |
| Case IV | - | - | - | + (Grade I) | ++ (Grade II) | ++ (Grade II) |
| Case V | - | - | - | - | - | - |
| Case VI | - | - | - | - | - | - |
| Case VII | - | - | - | - | - | - |
| Case VIII | - | - | - | - | - | - |
WBC: White blood cell; GFR: Glomerular filtration rate; ERPF: Effective renal plasma flow; –: No toxicity observed; +: Toxicity with grade in brackets
Table 6.
Response assessment
| Response | Symptomatic (%) | Biochemical (%) | Scan (%) |
|---|---|---|---|
| CR | 1 (12.5) | 0 | 0 |
| PR | 3 (37.5) | 3 (37.5) | 0 |
| SD | 0 | 0 | 2 (25) |
| PD | 4 (50) | 5 (62.5) | 6 (75) |
CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2010;40:78–88. doi: 10.1053/j.semnuclmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Thapa P, Parghane R, Basu S. 177Lu-DOTATATE peptide receptor radionuclide therapy in metastatic or advanced and inoperable primary neuroendocrine tumors of rare sites. World J Nucl Med. 2017;16:223–8. doi: 10.4103/1450-1147.207283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 5.Bombardieri E, Maccauro M, De Deckere E, Savelli G, Chiti A. Nuclear medicine imaging of neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S51–61. doi: 10.1093/annonc/12.suppl_2.s51. [DOI] [PubMed] [Google Scholar]
- 6.Olsen JO, Pozderac RV, Hinkle G, Hill T, O'Dorisio TM, Schirmer WJ, et al. Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan) Semin Nucl Med. 1995;25:251–61. doi: 10.1016/s0001-2998(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 7.Briganti V, Sestini R, Orlando C, Bernini G, La Cava G, Tamburini A, et al. Imaging of somatostatin receptors by indium-111-pentetreotide correlates with quantitative determination of somatostatin receptor type 2 gene expression in neuroblastoma tumors. Clin Cancer Res. 1997;3:2385–91. [PubMed] [Google Scholar]
- 8.Chiti A, Briganti V, Fanti S, Monetti N, Masi R, Bombardieri E, et al. Results and potential of somatostatin receptor imaging in gastroenteropancreatic tract tumours. Q J Nucl Med. 2000;44:42–9. [PubMed] [Google Scholar]
- 9.Chiti A, Fanti S, Savelli G, Romeo A, Bellanova B, Rodari M, et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-entero-pancreatic tumours. Eur J Nucl Med. 1998;25:1396–403. doi: 10.1007/s002590050314. [DOI] [PubMed] [Google Scholar]
- 10.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-phe1]- and [123I-tyr3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 11.Seregni E, Chiti A, Bombardieri E. Radionuclide imaging of neuroendocrine tumours: Biological basis and diagnostic results. Eur J Nucl Med. 1998;25:639–58. doi: 10.1007/s002590050267. [DOI] [PubMed] [Google Scholar]
- 12.Jamar F, Fiasse R, Leners N, Pauwels S. Somatostatin receptor imaging with indium-111-pentetreotide in gastroenteropancreatic neuroendocrine tumors: Safety, efficacy and impact on patient management. J Nucl Med. 1995;36:542–9. [PubMed] [Google Scholar]
- 13.Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853–8. [PubMed] [Google Scholar]
- 14.Kowalski J, Henze M, Schuhmacher J, Mäcke HR, Hofmann M, Haberkorn U, et al. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D phe(1)-tyr(3)-octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol. 2003;5:42–8. doi: 10.1016/s1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 15.Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90)Yttrium and (177)Lutetium labeled somatostatin analogs: Toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. 2013;4:39–52. [PMC free article] [PubMed] [Google Scholar]
- 16.Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, et al. Differentiated thyroid cancer: A new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid. 2014;24:715–26. doi: 10.1089/thy.2013.0225. [DOI] [PubMed] [Google Scholar]
- 17.Czepczyński R, Matysiak-Grześ M, Gryczyńska M, Bączyk M, Wyszomirska A, Stajgis M, et al. Peptide receptor radionuclide therapy of differentiated thyroid cancer: Efficacy and toxicity. Arch Immunol Ther Exp (Warsz) 2015;63:147–54. doi: 10.1007/s00005-014-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parghane RV, Talole S, Prabhash K, Basu S. Clinical response profile of metastatic/Advanced pulmonary neuroendocrine tumors to peptide receptor radionuclide therapy with 177Lu-DOTATATE. Clin Nucl Med. 2017;42:428–35. doi: 10.1097/RLU.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 19.Jois B, Asopa R, Basu S. Somatostatin receptor imaging in non-(131) I-avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with (177) Lu-DOTATATE: Low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of chromogranin A level-positive neuroendocrine differentiation. Clin Nucl Med. 2014;39:505–10. doi: 10.1097/RLU.0000000000000429. [DOI] [PubMed] [Google Scholar]


