Abstract
Changes in muscle thickness (MT), isometric torque, and arterial occlusion pressure (AOP) were examined following four sets of twenty unilateral elbow flexion exercise. Participants performed four sets of maximal voluntary contractions with no external load throughout a full range of motion of a bicep curl with and without the application of blood flow restriction (BFR). For torque there was an interaction (p = 0.012). The BFR condition had lower torque following exercise (56.07 ± 17.78 Nm) compared to the control condition (58.67 ± 19.06 Nm). For MT, there was a main effect for time (p < 0.001). MT increased from pre (3.52 ± .78cm) to post (3.68 ± 81cm) exercise and remained increased above baseline 15 min post-exercise. For AOP, there was an interaction (p = 0.027). The change in AOP was greater in the BFR condition (16.6 ± 13.42mmHg) compared to the control (11.1 ± 11.84 mmHg). NO LOAD exercise with BFR let to greater reductions in torque and an exaggerated cardiovascular response compared to exercise alone. There were no differences in swelling. These results suggest that the application of BFR to NO LOAD exercise may result in greater fatigue.
Keywords: Muscle swelling, isometric strength, muscle thickness, blood flow restriction
INTRODUCTION
When training for skeletal muscle growth, it is recommended that loads corresponding to 1–12 repetition maximum (RM) be used in a periodized fashion with a training load that is at least 70% of one’s one-repetition maximum (1RM)(1). However, emerging data suggest that muscle growth can be achieved across a wide range of exercise loads. For example, low load (e.g., 30% 1RM) training performed to volitional failure produces similar skeletal muscle growth as traditional high load training (e.g., 70–80% 1RM) (20, 27). In addition, acute work has demonstrated that high load and low load training result in similar muscle fiber activation, based on muscle fiber glycogen depletion, when loads are lifted to task failure (23). A review by Ozaki et al. speculates that skeletal muscle growth is achieved through a combination of external load or metabolic induced motor unit recruitment or both (29). In situations of lower mechanical stress, metabolites may accumulate within the muscle. Blood flow restriction (BFR) has emerged as a technique that may help to facilitate fatigue, particularly when combined with low load resistance exercise (16).
Low load training in combination with BFR has been shown to result in similar adaptations for muscle size and strength compared to traditional high load training (18, 36). Blood Flow Restriction is a training method that partially restricts arterial inflow while largely restricting venous outflow in working musculature during exercise (33). Farup et al. demonstrated that the addition of BFR to low load training resulted in fewer repetitions per set compared to unrestricted low load training, while resulting in similar changes in muscle volume, presumably due to a faster rate of metabolic fatigue (8). Interestingly, there does not appear to be a point where BFR is ever necessary to induce skeletal muscle adaptation if repetitions are performed to volitional failure and the external load is sufficient for adaptation. For example, a recent study demonstrated that low-load (15% 1RM) resistance exercise in the lower body with and without BFR resulted in similar increases in muscle thickness compared to high-load training (70% 1RM) (10). However, Buckner et al. demonstrated that eight-week of biceps curl training at 15% 1RM without BFR was not sufficient to induce similar skeletal muscle growth as traditional high load resistance training. Further, the addition of BFR did not make up for this lack of external stimulus (3). Together these findings suggest that BFR is not necessary if exercise is performed to volitional failure at a sufficient external load, and that BFR cannot make up for an insufficient external load. Indeed, most applications of BFR are in combination with low external loads; however, a study by Laurentino et al. compared high load (60–80% 1RM) resistance exercise with and without BFR (13). The authors observed no difference in muscle growth between groups, suggesting that when performing resistance exercise to muscle fatigue with high loads, BFR cannot produce greater hypertrophy. It is reasonable to hypothesize that muscle fatigue is crucial for muscle hypertrophy, and BFR could be a viable tool to lower training volume by increasing metabolic fatigue (10). However, there is no current scenario where BFR is recommended in combination with high force contractions (30).
Rennie et al. hypothesized that high levels of localized muscle activation produced from repeated contractions could provide sufficient stimulation of skeletal muscle hypertrophic pathways (31). In other words, increasing internal focus during resistance training could potentially lead to muscle hypertrophy. Internal focus, also known as “muscle-mind connection” under the bodybuilding community, has been popular in the recent decade, but limited research has proven this theory. A recent study by Schoenfeld et al. showed that an internal focus of attention was superior to an external focus of attention when the goal was to maximize hypertrophy of the elbow flexors (32). In order to distinguish two different conditions, participants were cued to “squeeze the muscle” for internal focus and cued to “lift weight up” for external focus. A similar concept was examined by Counts et al., which compared maximal effort elbow flexion exercise with no external load (internal focus coined “NO LOAD”) to traditional high-load (70% 1RM) resistance training in the elbow flexor exercise (6). The authors observed no difference between conditions for changes in muscle thickness. However, the authors noted that NO LOAD training had greater variability in changes in maximal voluntary strength and muscle growth over the 6-week training period. Therefore, it is reasonable to suggest that BFR may provide a means to reduce the variability to NO LOAD training by increasing metabolic fatigue and promoting motor unit recruitment. The purpose of this study was to examine acute changes in muscle thickness, isometric strength, and arterial occlusion pressure following NO LOAD exercise with and without the application of BFR. In addition, ratings of perceived exertion and discomfort were compared across sets.
METHODS
Participants
Forty-one participants, between the ages of 18–35 years, were recruited to participate in this study. Participants had regularly engaged in resistance exercise in the upper body for at least six months. Participants were excluded from the study if they used tobacco products periodically within the previous six months, or if they had an injury that would prevent upper body exercise. Because of the theoretical risk of blood clots, if participants met more than one risk factor associated with deep vein thrombolysis or venous thromboembolism, they were also excluded. The risk factors list consists of body mass index of > 30, Crohn’s disease, past fracture of the hip, pelvis, or femur, major surgery within the last six months, varicose veins, family history of deep vein thrombosis, family history of pulmonary embolism, birth control pills (24). Two participants dropped out of the study before beginning training due to personal reasons. Therefore, the data were analyzed and reported for thirty-nine participants (19 men, 20 women). With and alpha of 0.05, beta of 0.8, and anticipated correlation of 0.9 across repeated measures, our sample size was sufficient to detect an effect size (f) of 0.44. This study was approved by The University of South Florida’s Institutional Review Board. All participants gave written informed consent. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (26).
Protocol
Participants visited the laboratory on two occasions. During the initial visit, height and weight were measured and participants were familiarized with isometric testing and NO LOAD bicep curls. The second visit took place 1–7 days following visit one and consisted of the experimental protocol. One arm was randomized into the control condition and the other into the BFR condition. Participants were seated in a quiet room for ten minutes of rest before the resting arterial occlusion measurement. Upon completion of arterial occlusion pressure, the participant completed four sets of unilateral biceps curls with NO LOAD or with NO LOAD and the application of BFR (in randomized order). Following each exercise set, the researcher asked the participant to provide ratings of perceived exertion and discomfort ratings. Prior to, immediately after, and 15-minutes after the completion of the exercise protocol, muscle thickness was measured using B-mode ultrasound, and isometric strength was measured. In addition to ultrasound and strength, arterial occlusion pressure was measured immediately following exercise. Following the final muscle thickness assessment, the participant rested for 15 minutes before performing the same procedures on the other arm (with its respective conditions: control or BFR).
Muscle thickness was measured using B-mode ultrasound (Mindray DP50, Shenzhen, China) pre, immediately following, and 15 minutes post-exercise for BFR and non-BFR conditions. Ultrasound measurements of muscle size were tested on the anterior of the participant’s upper arm. The probe was coated with gel and held lightly against the participant’s skin. Measurements were taken at 70% of the distance of the upper arm between the acromion process of the scapula and to the lateral epicondyle to the humerus. This site was chosen as Counts et al. has demonstrated acute swelling at a similar site in response to a NO LOAD training protocol (3).
For upper body isometric strength testing, individuals were seated in a preacher curl bench with their elbow flexed at 90° and asked to flex their arm as hard as possible against an immovable object. Isometric strength was measured with a load cell (Ametek Chatillon 50 LBF Digital Force Gauge). Each contraction lasted approximately three seconds, with 60 s rest between each maximal voluntary contractions (MVC). Isometric torque was tested pre, immediately following, and 15 minutes post-exercise for both conditions.
The Borg scale of the rating of perceived exertion (RPE) was used to measure the participants’ perceived effort following each set of unilateral exercise. Immediately following each set of exercise, during the 30-s rest, the participant was asked to rate their perceived exertion on a scale of 6 (none at all) to 20 (maximal effort). In addition, 20-s after each set, the participant was asked to rate their discomfort using the Borg discomfort scale (CR10+) in the exercised arm. In short, the discomfort was rated on a scale of 0 (no discomfort at all) to 10 (maximal discomfort); however, the participant was allowed to exceed ten if discomfort was greater than any he/she had previously experienced. This measurement was taken 20-s into each rest period.
While the participant was standing upright, the researcher applied a pressurized cuff (5 cm) to the uppermost portion of the arm and measured the pressure at which blood flow to the participant’s wrist was no longer present. A bidirectional Doppler probe (Hokanson, Bellevue, WA, USA) was held in place at the radial artery of the right wrist to detect a pulse. Once the Doppler probe can clearly detect a pulse, the cuff was inflated to 50 mmHg. The inflation pressure then was slowly increased until there was no detectable pulse. This measurement was completed on both arms. This was done to ensure that the restriction stimulus was made relative to each individual (14).
NO LOAD training consisted of contracting the musculature of the upper arms as hard as possible through a range of motion replicating that of a unilateral bicep curl exercise without the use of an external load. The participant completed four sets of 20 repetitions with the 30s of rest between sets. The participant was verbally encouraged to maximally contract their bicep throughout the protocol. For example, the participant was encouraged to “squeeze” their muscle as hard as possible during the exercise protocol. The participant had to wear a 5cm BFR cuff at the top of the arm for both conditions. One arm had 40% of resting arterial occlusion pressure, and the other had 0 mmHg pressure. The cuff was inflated for the duration of the protocol including rest periods.
Statistical Analysis
All data analysis was completed on the SPSS 23.0 statistical software package (SPSS Inc. Chicago, IL). A 2 (condition) × 3 (time) repeated measures ANOVA was used to determine differences in muscle swelling, and isometric torque. A 2 (condition) × 2 (time) repeated measures ANOVA was used to determine differences in arterial occlusion pressure. If there was an interaction, paired-samples t-tests were used to find differences across time points within each condition, and between conditions within each time point. If no interaction was found, the main effects were examined. A non-parametric Wilcoxon test was used to determine differences in RPE and discomfort between conditions within each set. All perceptual data are presented as 25th, 50th, and 75th percentiles. Significance was set at p ≤ 0.05 for all tests.
RESULTS
A total of 39 resistance-trained males (n = 19) and females (n = 20) [mean ± SD; age: 24 ± 2.8 years; height: 170.9 ± 8.7 cm; body mass: 72 ± 10.6 kg; BMI: 24.6 ± 2.8 kg/m2] completed the study.
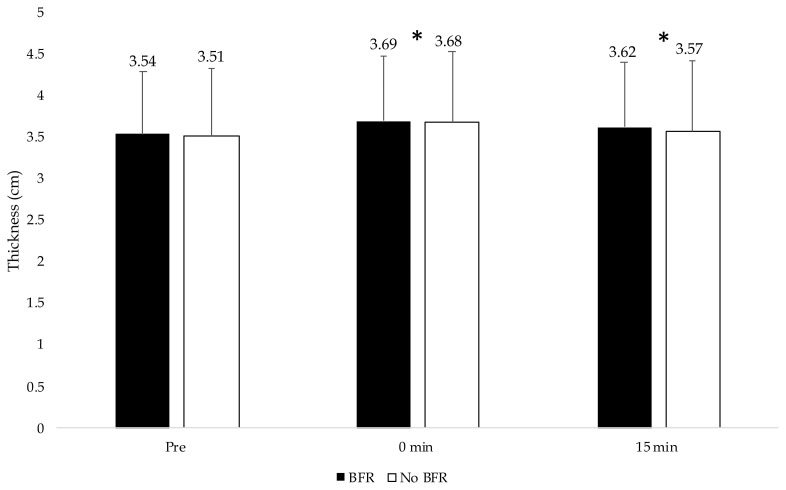
For muscle thickness, there was no interaction (p = 0.343), and there was no main effect for condition (p = 0.417) (Figure 1). However, there was a main effect for time (p < 0.001). Muscle thickness increased from pre to post-exercise [mean difference 0.158 (0.130–0.187) cm, p < 0.001] and remained increased above baseline at 15 min post][mean difference 0.075 (0.056–0.096) cm, p ≤ 0.001].
Figure 1.
Muscle thickness values for muscle thickness for both conditions across time. An asterisk* indicates significantly different than pre-values.
The BFR condition had lower isometric torque immediately following exercise (56.07 ± 17.78 Nm) compared to the control group (58.67 ± 19.06 Nm). In addition, both the BFR and control conditions demonstrated a decrease in torque immediately following exercise [mean change = 4.5 ± 4.5 and 1.82 ± 4.5 Nm for BFR and control conditions respectively], which remained decreased below baseline 15 minutes post-exercise (mean change 2.39 ± 5.5 and 2.28 ± 3.19 Nm for BFR and control conditions respectively).
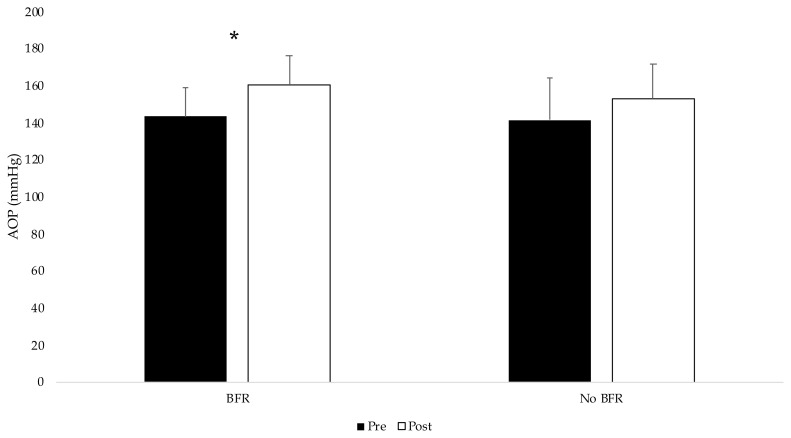
For arterial occlusion pressure, there was a group x time interaction (p = 0.027) (Figure 2). The change in AOP was greater in the BFR group (16.6 ± 13.42) compared to the control group 11.1 ± 11.84)
Figure 2.
Arterial occlusion values for both conditions pre and post exercise. An asterisk* indicates an interaction between conditions.
Rating of perceived exertion and discomfort data are displayed in Tables 1 and 2 respectively. For RPE, perceived exertion was higher in the BFR condition compared to the control condition for sets 1 (p = 0.048), set 3 (p = 0.005) and set 4 (p = 0.034). For discomfort, values were higher in the BFR condition for set 1 (p = 0.006), set 2 (p = 0.001), set 3 (p < 0.001) and set 4 (p = 0.001).
Table 1.
Ratings of perceived exertion across sets.
| BFR | 25th | 50th | 75th | No BFR | 25th | 50th | 75th |
|---|---|---|---|---|---|---|---|
| Pre RPE | 6 | 6 | 7 | 6 | 6 | 7 | |
| Set 1 RPE | 7 | 11* | 14 | 8 | 9 | 13 | |
| Set 2 RPE | 9 | 12* | 15 | 8 | 12 | 14 | |
| Set 3 RPE | 11 | 13* | 16 | 9 | 13 | 15 | |
| Set 4 RPE | 13 | 14* | 17 | 9 | 14 | 16 |
Values are displayed for 25th, 50th, and 75th percentile.
Significant differences between conditions.
Differences are noted on the 50th percentile; BFR, blood flow restriction; RPE, rating of perceived exertion.
Table 2.
Discomfort across sets.
| BFR | 25th | 50th | 75th | No BFR | 25th | 50th | 75th |
|---|---|---|---|---|---|---|---|
| Discomfort Pre | 0 | 0 | 0.5 | 0 | 0 | 0.5 | |
| Discomfort Set 1 | 0.5 | 1* | 3 | 0 | 0.5 | 2 | |
| Discomfort Set 2 | 1 | 2 | 4 | 0.5 | 1 | 3 | |
| Discomfort Set 3 | 1 | 3* | 5 | 0.5 | 2 | 3 | |
| Discomfort Set 4 | 1 | 3* | 6 | 0.5 | 3 | 4 |
Values are displayed for 25th, 50th, and 75th percentile.
Significant differences between conditions.
Differences are noted on the 50th percentile; BFR, blood flow restriction.
DISCUSSION
The primary findings of the present study were as follows: 1) both conditions increased muscle thickness following exercise. This response was the greatest immediately following exercise and remained elevated above baseline at 15 min post-exercise, 2) Torque decreased in both groups from pre to post-exercise; however, the BFR condition observed a greater decrease following exercise, 3) Arterial occlusion pressure increased in both groups from pre to post-exercise, with a larger increase observed in the BFR condition, 4) The BFR condition resulted in higher levels of RPE and discomfort compared to the non-BFR condition.
Similar acute muscle swelling has been noted in response to resistance exercise in the upper body (2, 6, 38), as well as across a variety of protocols in the lower body (15). Notably, this acute response is highly repeatable and, in line with the hypotheses of Haussinger et al., is believed to be an indicator of anabolic potential (9). To provide some support, Yasuda et al. observed that concentric exercise in combination with BFR resulted in both a greater acute muscle swelling response and a greater increase in muscle size over 6 weeks compared to a group performing the eccentric exercise in combination with BFR (38). The authors suggest that the greater growth response may be explained by the greater degree of acute swelling seen with the exercise protocol. Although it is not known if the response itself is anabolic, it may provide important information on the robustness of an acute exercise bout. Our data demonstrate that both conditions increased muscle thickness significantly, with no difference between conditions. Although acute changes in muscle thickness have often been observed to understand an exercise stimulus better, recent data brings into question the relevance of acute swelling. For example, a recent study by Jessee et al. showed that low-load (15% 1RM) exercise in combination with BFR observed greater acute muscle swelling compared to high-load (70% 1RM) without BFR in the lower body (11). However, chronic training data (8-weeks) showed that similar muscle growth for all conditions (10). Similar to this, a study in the upper body demonstrated that BFR with low load-exercise (15% 1RM) resulted in greater acute muscle swelling compared to traditional high-load resistance training (4). Interestingly, the same conditions across 8-weeks of resistance training demonstrated that there was a greater hypertrophic effect in the high-load condition compared to the low-load BFR condition (3). Thus, acute swelling does not appear predictive of skeletal muscle growth. However, it does appear to accompany most exercise protocols that produce a growth response.
In line with previous investigations, the addition of BFR lead to greater decrements in isometric torque compared to training on its own without BFR (7, 11). This may suggest that the addition of BFR may help individuals to recruit motor units, achieving a higher level of fatigue across the same four sets of 20 repetitions. Moritani et al. examined motor unit recruitment and lactate concentrations during intermittent isometric contractions of handgrip muscles with or without blood flow (22). The authors observed an increase in motor unit recruitment and firing rate while under arterial occlusion, suggesting that the metabolic state may have played an important role in this increased recruitment (22). Other studies have observed similar increases in muscle activation with the application of BFR, attributing such increases to reduced oxygen and metabolic accumulation within the working muscle (21, 35, 37).
Interestingly, Counts et al. compared EMG amplitude between NO LOAD and traditional high load resistance exercise, finding that high load training produced greater EMG amplitudes during the last three repetitions of exercise (87% MVC) compared to NO LOAD training (52% MVC) (6). Despite this, the authors observed similar decreases in isometric torque from pre to post-exercise. Although it is reasonable to assume that NO LOAD training may produce maximal activation, the ability to maximally activate relies on the individual’s capacity to maximally contract the muscle without the aid of an external load. Counts et al. observed similar changes in muscle size between NO LOAD training and traditional high load training following 8-weeks of exercise (6). However, the authors noted greater variability in response to NO LOAD training when comparing exercise programs. It seems reasonable to suggest that the variability was driven by different ability for individuals to maximally contract the muscle throughout a full range of motion. The application of BFR may help to rectify this issue. The results of the present study may suggest that the incorporation of BFR has facilitated a higher level of metabolic induced fatigue.
The present study showed a significantly greater increase in arterial occlusion pressure following four sets of unilateral NO LOAD biceps curls with BFR compared NO LAOD without BFR. This result could potentially be explained by exercise pressor reflex (34). However, in the present study, arterial occlusion pressure was examined immediately after and not during the exercise sets. Blood pressures during high load resistance training have been reported to reach above 200 mmHg during single-arm biceps curls with 95% 1RM (17). In the present study, the application of BFR only resulted in a small increase in arterial occlusion pressure immediately post-exercise above that of NO LOAD training without BFR (~5 mmHg). Overall, the pressure response observed in the present study is similar to what has been reported previously following both high load and low load BFR protocols. Mouser et al. noted a similar increase in arterial occlusion pressure (~10 mmHg) following four sets of high load training, low load training, or low load training with the application of BFR (25). Further work is necessary to understand the blood pressure response during NO LOAD training with and without the application of BFR.
The present study showed that the BFR condition had higher RPE and discomfort scores following each set of unilateral bicep curls compared to the non-BFR condition. Previous studies have demonstrated that BFR with high pressure (80% AOP) leads to greater discomfort and higher RPE than moderate pressure (40% AOP) during the last set of an exercise (12). Thus, the addition of restrictive pressure tends to increase the level of discomfort associated with training. Interestingly, Mattocks et al. showed that both RPE and discomfort decrease significantly across training when utilizing BFR (19). Therefore, chronic exposure to the blood flow restrictive stimulus may attenuate the higher perceptual values noted in a single exposure.
The present study is not without limitations. Firstly, our results represent acute changes and provide limited information on the chronic adaptations; thus, future research is necessary to understand long term adaptations. Secondly, since no external load exercise is a unique stimulus, surface EMG amplitude could be used to monitor internal focus muscle activation, even though we know that surface EMG cannot be used to determine actual motor unit recruitment. Another limitation in the present study is the lack of a non-exercise control, which would have helped to understand variability in our measurements over time. In addition, the within-subject design lends to the possibility that systemic fatigue may have influenced responses observed in the arm that trained second. However, the randomized nature of the design helps to limit any influence this may have on the present findings. Finally, changes of arterial occlusion pressure during exercise should be monitored in the future study to provide a comprehensive understanding of exercise pressor reflex effect. Despite these limitations, our findings provide an important addition to the BFR literature, using a within the subject model to further develop our understanding of the efficacy of BFR when no external load is utilized.
Conclusions
To our knowledge, this is the first study to examine the acute responses to NO LOAD exercise with the application of BFR. Previous studies have demonstrated that low loads may have implications for clinical, injured populations who have limited ability to perform traditional resistance exercise (5, 28). Our findings demonstrate that no external load exercise with BFR could increase more muscle fatigue compared to NO LOAD exercise alone. In addition, the application of BFR resulted in a slightly higher cardiovascular response. Although the long-term implications of these responses are presently unknown, our results would suggest that if no external load exercises are implemented, BFR may help be a helpful tool for facilitating fatigue.
REFERENCES
- 1.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 2.Buckner SL, Dankel SJ, Mattocks KT, Jessee MB, Mouser JG, Counts BR, et al. Differentiating swelling and hypertrophy through indirect assessment of muscle damage in untrained men following repeated bouts of resistance exercise. Eur J Appl Physiol. 2017;117(1):213–24. doi: 10.1007/s00421-016-3521-9. [DOI] [PubMed] [Google Scholar]
- 3.Buckner SL, Jessee MB, Dankel SJ, Mattocks KT, Mouser JG, Bell ZW, et al. Blood flow restriction does not augment low force contractions taken to or near task failure. Eur J Sport Sci. 2020;20(5):650–9. doi: 10.1080/17461391.2019.1664640. [DOI] [PubMed] [Google Scholar]
- 4.Buckner SL, Jessee MB, Dankel SJ, Mattocks KT, Mouser JG, Bell ZW, et al. Acute skeletal muscle responses to very low-load resistance exercise with and without the application of blood flow restriction in the upper body. Clin Physiol Funct Imaging. 2019;39(3):201–8. doi: 10.1111/cpf.12557. [DOI] [PubMed] [Google Scholar]
- 5.Cook SB, Brown KA, Deruisseau K, Kanaley JA, Ploutz-Snyder LL. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol. 1985;2010;109(2):341–9. doi: 10.1152/japplphysiol.01288.2009. [DOI] [PubMed] [Google Scholar]
- 6.Counts BR, Buckner SL, Dankel SJ, Jessee MB, Mattocks KT, Mouser JG, et al. The acute and chronic effects of “NO LOAD” resistance training. Physiol Behav. 2016;164:345–52. doi: 10.1016/j.physbeh.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Dankel SJ, Jessee MB, Buckner SL, Mouser JG, Mattocks KT, Loenneke JP. Are higher blood flow restriction pressures more beneficial when lower loads are used? Physiol Int. 2017;104(3):247–57. doi: 10.1556/2060.104.2017.3.2. [DOI] [PubMed] [Google Scholar]
- 8.Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sports. 2015;25(6):754–63. doi: 10.1111/sms.12396. [DOI] [PubMed] [Google Scholar]
- 9.Haussinger D, Roth E, Lang F, Gerok W. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993;341(8856):1330–2. doi: 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- 10.Jessee M, Buckner S, Mouser JG, Mattocks K, Dankel S, Abe T, et al. Muscle adaptations to high-load training and very low-load training with and without blood flow restriction. Front Physiol. 2018;9:1448. doi: 10.3389/fphys.2018.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessee MB, Buckner S, Mattocks K, Dankel SJ, Mouser JG, Bell ZW, et al. Blood flow restriction augments the skeletal muscle response during very low-load resistance exercise to volitional failure. Physiol Int. 2019;106(2):180–93. doi: 10.1556/2060.106.2019.15. [DOI] [PubMed] [Google Scholar]
- 12.Jessee MB, Dankel SJ, Buckner SL, Mouser JG, Mattocks KT, Loenneke JP. The cardiovascular and perceptual response to very low load blood flow restricted exercise. Int J Sport Med. 2017;38(08):597–603. doi: 10.1055/s-0043-109555. [DOI] [PubMed] [Google Scholar]
- 13.Laurentino G, Ugrinowitsch C, Aihara A, Fernandes A, Parcell A, Ricard M, et al. Effects of strength training and vascular occlusion. Int J Sport Med. 2008;29(08):664–7. doi: 10.1055/s-2007-989405. [DOI] [PubMed] [Google Scholar]
- 14.Loenneke JP, Fahs CA, Rossow LM, Sherk VD, Thiebaud RS, Abe T, et al. Effects of cuff width on arterial occlusion: Implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;12(8):2903–12. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, et al. The influence of exercise load with and without different levels of blood flow restriction on acute changes in muscle thickness and lactate. Clin Physiol Funct Imaging. 2017;37(6):734–740. doi: 10.1111/cpf.12367. [DOI] [PubMed] [Google Scholar]
- 16.Loenneke JP, Wilson JM, Marin PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: A meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–59. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 17.MacDougall J, Tuxen D, Sale D, Moroz J, Sutton J. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785–90. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Hernandez J, Marin PJ, Menendez H, Ferrero C, Loenneke JP, Herrero AJ. Muscular adaptations after two different volumes of blood flow-restricted training. Scand J Med Sci Sports. 2013;23(2):e114–20. doi: 10.1111/sms.12036. [DOI] [PubMed] [Google Scholar]
- 19.Mattocks KT, Mouser JG, Jessee MB, Buckner SL, Dankel SJ, Bell ZW, et al. Perceptual changes to progressive resistance training with and without blood flow restriction. J Sports Sci. 2019;37(16):1–8. doi: 10.1080/02640414.2019.1599315. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, et al. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol. 2012;113(1):71–7. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore DR, Burgomaster KA, Schofield LM, Gibala MJ, Sale DG, Phillips SM. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur J Appl Physiol. 2004;92(4–5):399–406. doi: 10.1007/s00421-004-1072-y. [DOI] [PubMed] [Google Scholar]
- 22.Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M. Oxygen availability and motor unit activity in humans. Eur J Appl Physiol Occup Physiol. 1992;64(6):552–6. doi: 10.1007/BF00843767. [DOI] [PubMed] [Google Scholar]
- 23.Morton RW, Sonne MW, Farias Zuniga A, Mohammad IY, Jones A, McGlory C, et al. Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J Physiol. 2019;597(17):4601–13. doi: 10.1113/JP278056. [DOI] [PubMed] [Google Scholar]
- 24.Motykie GD, Zebala LP, Caprini JA, Lee CE, Arcelus JI, Reyna JJ, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253–62. doi: 10.1023/a:1018770712660. [DOI] [PubMed] [Google Scholar]
- 25.Mouser JG, Mattocks KT, Dankel SJ, Buckner SL, Jessee MB, Bell ZW, et al. Very-low-load resistance exercise in the upper body with and without blood flow restriction: Cardiovascular outcomes. Appl Physiol Nutr Metab. 2018;44(3):288–92. doi: 10.1139/apnm-2018-0325. [DOI] [PubMed] [Google Scholar]
- 26.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogasawara R, Loenneke JP, Thiebaud RS, Abe T. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int J Clin Med. 2013;4(02):114. [Google Scholar]
- 28.Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74(1):62–8. doi: 10.1080/00016470310013680. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki H, Loenneke JP, Buckner SL, Abe T. Muscle growth across a variety of exercise modalities and intensities: Contributions of mechanical and metabolic stimuli. Med Hypotheses. 2016;88:22–6. doi: 10.1016/j.mehy.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Patterson SD, Hughes L, Warmington S, Burr JF, Scott BR, Owens J, et al. Blood flow restriction exercise position stand: Considerations of methodology, application and safety. Front Physiol. 2019;10:533. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld BJ, Vigotsky A, Contreras B, Golden S, Alto A, Larson R, et al. Differential effects of attentional focus strategies during long-term resistance training. Eur J Sport Sci. 2018;18(5):705–12. doi: 10.1080/17461391.2018.1447020. [DOI] [PubMed] [Google Scholar]
- 33.Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45(3):313–25. doi: 10.1007/s40279-014-0288-1. [DOI] [PubMed] [Google Scholar]
- 34.Spranger MD, Krishnan AC, Levy PD, O’Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol. 2015;309(9):H1440–H52. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 1985;2000;88(1):61–5. doi: 10.1152/jappl.2000.88.1.61. [DOI] [PubMed] [Google Scholar]
- 36.Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32(12):2035–9. doi: 10.1097/00005768-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 1985;2000;88(6):2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda T, Loenneke JP, Thiebaud RS, Abe T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PloS one. 2012;7(12):e52843. doi: 10.1371/journal.pone.0052843. [DOI] [PMC free article] [PubMed] [Google Scholar]