Abstract
Metabolic stress is a primary mechanism of muscle hypertrophy and is associated with microvascular oxygenation and muscle activation. Considering that drop-set (DS) and crescent pyramid (CP) resistance training systems are recommended to modulate these mechanisms related to muscle hypertrophy, we aimed to investigate if these resistance training systems produce a different microvascular oxygenation status and muscle activation from those observed in traditional resistance training (TRAD). Twelve volunteers had their legs randomized in an intra-subject cross-over design in TRAD (3 sets of 10 repetitions at 75% 1-RM), DS (3 sets of ∼50–75% 1-RM) and CP (3 sets of 6–10 repetitions at 75–85% 1-RM). Vastus medialis microvascular oxygenation and muscle activation were respectively assessed by non-invasive near-infrared spectroscopy and surface electromyography techniques during the resistance training sessions in the leg-extension exercise. Total hemoglobin area under the curve (AUC) (TRAD: −1653.5 ± 2866.5; DS: −3069.2 ± 3429.4; CP: −1196.6 ± 2675.3) and tissue oxygen saturation (TRAD: 19283.1 ± 6698.0; DS: 23995.5 ± 15604.9; CP: 16109.1 ± 8553.1) increased without differences between protocols (p>0.05). Greater decreases in oxygenated hemoglobin AUC and hemoglobin differentiated AUC were respectively found for DS (−4036.8 ± 2698.1; −5004.4 ± 2722.9) compared with TRAD (−1951.8 ± 1720.0; −2250.3 ± 1305.7) and CP (−1814.4 ± 2634.3; 2432.2 ± 2891.4) (p<0.03). Higher increases of hemoglobin deoxygenated AUC were found for DS (1426.7 ± 1320.7) compared with TRAD (316.0 ± 1164.9) only (p=0.04). No differences were demonstrated in electromyographic amplitudes between TRAD (69.0 ± 34.4), DS (61.3 ± 26.7) and CP (60.9 ± 38.8) (p>0.05). Despite DS produced lower microvascular oxygenation levels compared with TRAD and CP, all protocols produced similar muscle activation levels.
Keywords: Drop-set, crescent pyramid, muscle activation, near-infrared spectroscopy, muscle fatigue
INTRODUCTION
Metabolic stress (i.e., accumulation of metabolites, particularly lactate, inorganic phosphate and H+) is a physiological response associated to the exercise-induced changes in oxygenation, which can be assessed through the microvascular oxygenation status (i.e., proxy marker of metabolic stress) (4, 9, 26, 40). Through advances in measurement techniques, various methods have been applied to assess microvascular oxygenation status during RT, such as the near-infrared spectroscopy (NIRS) (14, 27). This technique is used to monitor relative changes in total hemoglobin (tHb), tissue oxygen index (stO2), concentrations of oxygenated hemoglobin (HbO2), concentrations of deoxygenated hemoglobin (HHb), and hemoglobin differentiated (HbDiff) in the muscle tissue during exercise (6). The microvascular oxygenation is considered a responsible mechanism for the resistance training (RT)-induced muscle fatigue (9, 26, 40, 41). It is suggested that muscle fatigue increases muscle activation (i.e., increase of electromyographical signal [EMG]) to maintain muscle function during RT (30, 40). Considering that there is an association between microvascular oxygenation status and muscle activation with muscle hypertrophy (38, 43, 44), coaches and practitioners use several training techniques (i.e., RT systems) emphasizing/modulating different RT variables (e.g., volume or load) in order to change the microvascular oxygenation status and increase muscle activation (10, 16). Albeit RT systems are widely recommended to modulate these mechanisms related to muscle hypertrophy (39), little is known if these RT systems produce a different microvascular oxygenation status and muscle activation from those observed in traditional RT (TRAD; performed with constant load and repetitions).
Drop-set (DS) is a RT system widely recommended and utilized among practitioners. This system is characterized by sets until concentric failure followed by very short pauses enough to quickly reduce total load (e.g., ∼20%) during sets, allowing the execution of a few more repetitions to concentric failure (2, 3). DS involves a large number of repetitions to failure, consequently augmenting total training volume (TTV; sets x repetitions x load [kg]). It is suggested that these characteristics of DS can promote great changes on microvascular oxygenation (39). However, only one study investigated the effects of DS on microvascular oxygenation and muscle activation (17). The authors compared the effects of DS with reverse DS on a microvascular oxygenation parameter (i.e., HbO2) and muscle activation. Despite the results showed a greater change in HbO2 and increases muscle activation for DS, the training protocol was not conducted as preconized (i.e., sets were not performed until concentric failure and the pauses between sets were very long), which decharacterized the DS system (17). Furthermore, the study did not compare the effects of DS with TRAD and assessed only one microvascular oxygenation parameter. Additionally, other studies (15, 16) concluded that a protocol that most closely resembles DS (i.e., a set performed until muscle failure after a short pause at the end of the training session) produced higher increases in indirect indicators of metabolic stress (e.g., lactate and growth hormone [GH]) compared with TRAD. Therefore, despite no study compared the effects of DS on microvascular oxygenation with TRAD, it is conceivable that higher volume inherent to DS would produce higher microvascular oxygenation changes compared with TRAD.
Besides DS, another RT system employed by resistance-trained subjects is the crescent pyramid (CP), which is characterized by increases in training load simultaneously to decreases in the number of repetitions throughout sets progression (e.g., 1st set: 12 reps at 75% of one repetition maximum [1-RM]; 2nd set: 10 reps at 80% 1-RM; 3rd set: 8 reps at 85% 1-RM) (10). Aiming to investigate the effect of manipulations in training load (e.g., 4 sets at 60% 1-RM vs. 4 sets at 90% 1-RM) a study showed similar microvascular oxygenation status between low- and high-load RT through analysis of HbO2 and HHb (18). Thus, despite no study investigated the effects of the CP on microvascular oxygenation, it is possible to suggest that the load manipulation of the CP would produce similar microvascular oxygenation status compared with TRAD.
Thus, the aim of this study was to use NIRS to compare the effect of DS and CP systems with TRAD on microvascular oxygenation status during these RT protocols. Increases in H+ concentration induced by microvascular oxygenation changes could induce progressive increases in muscle activation (43). Thus, as a secondary objective, we investigated if microvascular oxygenation status could modulate the muscle activation in the same RT protocols. We hypothesized that DS will produce a higher microvascular oxygenation changes and muscle activation than TRAD and CP with no difference between the last two.
METHODS
Participants
The power analysis determined that 12 participants were needed for a power of 0.80, with an effect size of 0.5 and an α = 0.05. Then, twelve young men participated in this study (age: 23 ± 2 years, height: 1.77 ± 0.3 m, body mass: 79.8 ± 5.9 kg). Participants had i) no cardiovascular and neuromuscular disorders to mitigate possible risks inherent of any RT protocol; ii) at least 4 months of RT practice before the study to exclude the possibility of a novel effect of RT protocols on the dependent variables, and iii) a body mass index < 30 kg/m2 to avoid interferences of fat tissue thickness in the quality of NIRS and EMG signals. Participants signed an informed consent, the study was conducted in accordance with the Declaration of Helsinki and ethical approval was granted by the University’s ethics committee. This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (32).
Protocol
Experimental design
This study used an intra-subject cross-over design, in which participants performed all experimental conditions. Initially, participants were familiarized with study procedures and tested and re-tested for their 1-RM with 72h intervals among visits. In the first RT session, each leg randomly performed in counterbalanced fashion an experimental condition (i.e., TRAD, DS or CP). In the 2nd RT session (72h after the first RT session) one of the experimental conditions performed in the previous session was performed again, but this time using the contralateral leg. The remaining condition was used in other leg. For example: in the first RT session one participant performed TRAD using the right leg and DS using the left leg; while in the second visit this participant performed DS using the right leg and CP using the left leg. A 30-min rest (was the time necessary for the volunteers to feel recovered to perform the next protocol and to adjust the equipment [NIRS and EMG] for the next RT protocol) was allowed between protocols in each RT session, and the order in which each experimental condition was applied in the first and second RT sessions were randomized. The legs were allocated in each condition in a way that ensured the same number of dominant legs (n = 8) and non-dominant legs (n = 8) in each experimental condition, and the same number of combinations between experimental conditions performed by subjects in each RT session (8 legs performed TRAD combined with DS; 8 legs performed DS combined with CP; 8 legs performed CP combined with TRAD). Participants were tested for microvascular oxygenation levels through NIRS, and muscle activation by EMG. The NIRS started recording 6 min before the beginning of each RT session (i.e., during rest) and stopped 1 min after the end of protocol. EMG signal started recording immediately before the beginning of each RT session and stopped at the end of the last repetition of the last set.
Maximal dynamic strength test
The unilateral maximum dynamic strength on a leg-extension machine (Effort NKR; Nakagym, SP, Brazil) was assessed using the 1-RM test (7). Briefly, a general warm-up on a cycle ergometer at 20 km·h−1 for 5 min, followed by a couple of specific leg-extension exercise warm-up sets were conducted. In the first set, 8 repetitions were performed with 50% of the estimated 1-RM, obtained during familiarization sessions. In the second set, 3 repetitions were performed at 70% of the estimated 1-RM. A 2-min interval was provided between sets. After a 3-min interval, participants had up to 5 attempts to achieve the 1-RM. A 3-min rest interval was allotted between attempts and the highest load achieved (full eccentric–concentric movement with 90° range of motion) was registered. After 72h, the 1-RM was retested and all participants showed a variation lower than 5% compared to the first test; thus, the score achieved in the first test was used as 1-RM value.
Knee joint angle and trigger
To determine the concentric and eccentric phases during knee angular excursion, an angular potentiometer was placed on participants’ knee with its center of rotation aligned with the lateral intercondilar line of the knee joint. Full extension was defined as “zero degree”. The eccentric phase of movement was defined from the minimum to the maximum value of the knee flexion angle, while the concentric phase was defined from the maximum to the minimum value of the knee flexion angle. The A/D converter of the EMG unit was set at 1000Hz and utilized to synchronize the data acquisition from the angular potentiometer with both NIRS and EMG. To align the data in time, the signal from the trigger was split and sent to both NIRS and EMG A/D converters.
Resistance training
All protocols consisted of 3 sets of leg-extension exercise with a 2-min rest between sets. TRAD performed 10 repetitions at 75% 1-RM in the 3 sets. In DS, each set started with 75% 1-RM with repetitions until concentric failure, followed by a brief interval (∼15s) sufficient for the load be reduced by ∼20%. Then, more repetitions were performed until a new concentric failure, followed by another ∼15s interval required for a further ∼20% load reduction, allowing a few more repetitions to failure. After the 2-min rest between sets, all the above was repeated for the second and third sets. In CP, sets were performed with 10 repetitions at 75%, 8 repetitions at 80% and 6 repetitions at 85% 1-RM, respectively. The TTV was calculated multiplying sets, repetitions and load (kg).
Microvascular oxygenation
We assessed the oblique fibers of Vastus medialis (VM) (14). Microvascular oxygenation was measured during TRAD, DS and CP protocols by a continuous dual-wave length near-infrared spectroscopy apparatus (NIRS; Oxymon, Artinis Medical Systems, Arnhem, Netherlands). Data was collected at a frequency of 25Hz (20). This device allows estimating: i) tHb (blood volume); ii) stO2 (indicator of tissue oxygenation saturation); iii) HbO2 (amount of oxygenated hemoglobin); iv) HHb (amount of deoxygenated hemoglobin); and v) and HbDiff (indicate O2 consumption through differences between HbO2 and HHb). These variables together provides information about local tissue O2 availability and use, and therefore, the microvascular oxygenation (9, 14, 26). Firstly, thickness of the subcutaneous fat layer at the site of NIRS optodes (i.e., an emitter and a detector) placement was assessed to set the value of laser penetration depth (22). Then, the skin was shaved and cleaned with alcohol to fix the holder of the optodes pair on the skin. The optodes were positioned in the VM, 3cm distal from the EMG electrodes and fixed with black tape to eliminate ambient light (13). NIRS measurements during each RT session were expressed as changes HbO2, HHb, tHb, stO2 and HbDiff from resting values, measured during the last 6 min of rest period before each RT session (8, 23). Data was extracted from the NIRS device and analyzed off-line using Oxisoft (3.0.X; Artinis Medical Systems B.V, Arnhem, Netherlands). Considering the different time frame to perform the RT protocols (DS was performed in a longer period than TRAD and CP), the data analysis was conduct using the area under the curve (AUC) for all protocols including the entire session.
Muscle activation
Muscle activation of VM was assessed by the amplitude of the EMG signal (EMG832C; EMG System do Brazil, SP, Brazil) during the RT sessions. To reduce skin impedance before electrode placement, the skin area was shaved, abraded and cleaned with an isopropyl alcohol pad. Pre-gelled Ag/Ag-CL surface electrodes (EMG System, SP, Brazil) were placed with an interelectrode distance of 2cm over the belly of the VM aligned in parallel with the expected muscle fiber orientation, while a ground electrode was positioned in the ankle at the fibular lateral malleolus according to SENIAM (1). Sampling frequency was set at 1000Hz with a band-pass filter of 20 and 500Hz. There was an input noise below 1μV root mean square (RMS) and an effective common rejection mode of 95dB in the EMG amplifiers. The amplitude of EMG signal was calculated based on the concentric phase (i.e., the maximum and minimum knee joint angle of each repetition). The normalization of RMS values was performed through the RMS value obtained in the maximal voluntary isometric contraction (MVIC), which was calculated over a 250ms interval around the peak torque. MVIC torque was measured by a load cell attached at 90° with the lever arm of the leg extension machine before the exercise protocol. The product of the force values by the length of the shank (i.e., distance from the lateral intercondilar line to the lateral malleolus) determined the MVIC value. Using the A/D converter of the EMG unit, the load cell data was acquired at a frequency of 1000Hz and filtered with a Butterworth filter set a low pass frequency of 20Hz. The normalized RMS values (%MVIC) were presented as mean values of the concentric phase of the entire 3 sets excluding only the first and last repetition of each set performed during the protocols TRAD, DS and CP (28, 36, 37).
Statistical Analysis
After visual inspection, the area under the curve (AUC) analysis for dependent variables (HHb, HbO2, tHb, HbDiff, stO2 and normalized EMG) were performed using the trapezium rule (GraphPad Prism, GraphPad Software, San Diego, CA, United States) in order to characterize the magnitude of the response and the changes over time. A one-way ANOVA was used to compare the TTV and dependent variables between TRAD, DS and CP protocols. In case of significant F-values, a Tukey adjustment was implemented for pairwise comparisons. Statistical analyses were performed in the software SAS 9.2 and significance was set as p < 0.05. To minimize the possibility of a Type II error, the 95% confidence intervals of effect sizes (ES) of the differences between AUC scores (ESCIdiff) were calculated using a non-central t distribution to perform two between-groups analyses (31). Positive and negative confidence intervals [i.e., not crossing zero (0)] were considered as significant. Data are presented as mean ± SD.
RESULTS
Microvascular oxygenation
No significant differences in tHb AUC were detected between TRAD, DS and CP (F[2, 43] = 1.59, p = 0.21) (Fig. 1a). Regarding stO2 AUC, there was also no significant difference between TRAD, DS and CP (F[2, 36] = 1.64, p = 0.20) (Fig. 1b). The one-way ANOVA showed differences between training protocols for HbO2 AUC (F[2, 43] = 4.14, p = 0.02). The post hoc test revealed significant difference between DS and TRAD (DS vs. TRAD: p = 0.04) and CP (DS vs. CP: p = 0.03). No significant difference between TRAD and CP was found (TRAD vs. CP: p = 0.98) (Fig. 1c). There was also significant difference between training protocols for HHb AUC (F[2, 36] = 3.28, p= 0.04). The post hoc test showed significant difference between DS and TRAD only (DS vs. TRAD: p = 0.03). No significant difference between CP and TRAD (CP vs. TRAD: p = 0.45) and CP and DS (CP vs. DS: p = 0.37) was found (Fig. 1d). Finally, the one-way ANOVA showed differences between training protocols for HbDiff AUC (F[2, 43] = 6.29, p = 0.004). The post hoc test revealed significant difference between DS and TRAD (DS vs. TRAD: p = 0.007) and CP (DS vs. CP: p = 0.01). No significant difference was found between TRAD and CP (TRAD vs. CP: p = 0.97) (Fig. 1e). AUC values of NIRS variables are presented in Table 1. All the inferential analysis was confirmed by the CI of the ES (Table 2).
Figure 1.
Total area under the curve (AUC) of the entire session for total hemoglobin (tHb) (a), tissue saturation index (stO2) (b), oxygenated hemoglobin (HbO2) (c), deoxygenated hemoglobin (HHb) (d) and hemoglobin differentiated (HbDiff) (e) for traditional (TRAD), drop-set (DS) and crescent pyramid (CP) resistance training protocols. *Significantly (p = 0.04) different from TRAD. #Significantly (p < 0.03) different from TRAD and CP. Values are presented as mean ± SD.
Table 1.
Values of total hemoglobin (tHb), tissue oxygen index (stO2), concentrations of oxygenated hemoglobin (HbO2), concentrations of deoxygenated hemoglobin (HHb), and hemoglobin differentiated (HbDiff)], normalized electromyography (EMG) amplitude, and total training volume (TTV) for traditional resistance training (TRAD), drop-set (DS) and crescent pyramid (CP).
| Variables | TRAD | DS | CP |
|---|---|---|---|
| tHb (AUC) | −1653.5 ± 2866.5 | −3069.2 ± 3429.4 | −1196.6 ± 2675.3 |
| stO2 (AUC) | 19283.1 ± 6698.0 | 23995.5 ± 15604.9 | 16109.1 ± 8553.1 |
| HbO2 (AUC) | −1951.8 ± 720.0 | −4036.8 ± 698.1# | −1814.4 ± 2634.3 |
| HHb (AUC) | 316.0 ± 1164.9 | 1426.7 ± 320.7* | 831.6 ± 758.2 |
| HbDiff (AUC) | −2250.3 ± 1305.7 | −5004.4 ± 2722.9# | −2432.2 ± 2891.4 |
| EMG (%MVIC) | 69.0 ± 34.4 | 61.3 ± 26.7 | 60.9 ± 38.8 |
| TTV (kg) | 2250.0 ± 714.6 | 4456.6 ± 1569.1# | 1988.0 ± 604.8 |
AUC, area under curve.
%MVIC, percentage of the maximal voluntary isometric contraction.
Values are expressed as mean ± standard deviation.
Significantly (p = 0.04) different from TRAD.
Significantly (p < 0.03) different from TRAD and CP.
Table 2.
Effect size (ES) and confidence interval (CI) comparisons between traditional resistance training (TRAD), drop-set (DS) and crescent pyramid (CP) for total hemoglobin (tHb), tissue oxygen index (stO2), concentrations of oxygenated hemoglobin (HbO2), concentrations of deoxygenated hemoglobin (HHb), and hemoglobin differentiated (HbDiff)], electromyography amplitude (EMG) and total training volume (TTV).
| Variables | TRAD vs. DS | TRAD vs. CP | DS vs. CP | |||
|---|---|---|---|---|---|---|
|
| ||||||
| ES | CI | ES | CI | ES | CI | |
|
|
||||||
| tHb (AUC) | −0.45 | −1.19 to 0.29 | 0.16 | −0.57 to 0.89 | 0.61 | −0.15 to 1.37 |
| stO2 (AUC) | 0.39 | −0.41 to 1.18 | −0.42 | −1.24 to 0.41 | −0.61 | −1.43 to 0.21 |
| HbO2 (AUC) | −0.93* | −1.70 to −0.16 | 0.06 | −0.67 to 0.79 | 0.83* | 0.06 to 1.61 |
| HHb (AUC) | 0.90* | 0.06 to 1.74 | 0.52 | −0.28 to 1.32 | 0.56 | −0.28 to 1.39 |
| HbDiff (AUC) | 1.30* | 0.50 to 2.11 | −0.08 | −0.81 to 0.65 | 0.92* | 0.14 to 1.70 |
| EMG (%MVIC) | −0.25 | −0.99 to 0.50 | −0.22 | −0.95 to 0.51 | −0.01 | −0.74 to 0.72 |
| TTV (kg) | −1.81* | −2.66 to −0.96 | −0.40 | −1.12 to 0.33 | −2.08* | −2.96 to −1.19 |
AUC, area under curve.
%MVIC, percentage of the maximal voluntary isometric contraction.
Positive and negative confidence intervals [i.e., not crossing zero (0)] were considered as significant.
Muscle activation
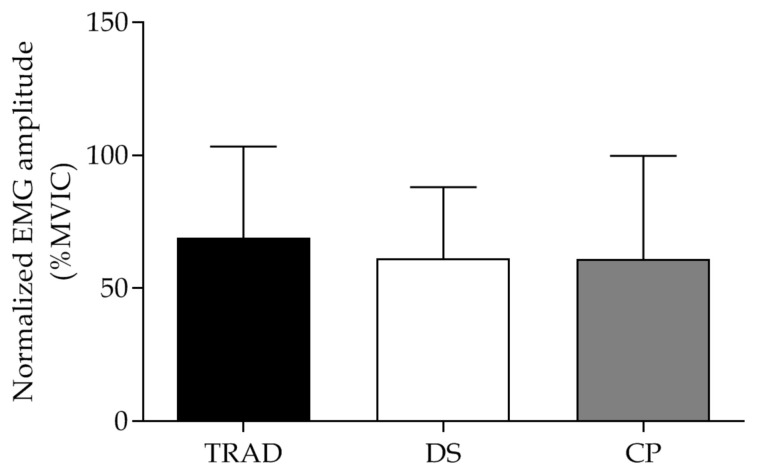
No significant differences in EMG amplitude was detected between TRAD, DS and CP protocols (F[2, 43] = 0.27, p = 0.76) (Fig. 2 and Table 1). The inferential analysis was confirmed by the CI of the ES (Table 2).
Figure 2.
Normalized electromyography (EMG, %MVIC) amplitude of Vastus medialis during traditional (TRAD), drop-set (DS) and crescent pyramid (CP) resistance training protocols. Values are presented as mean ± SD.
Total training volume
One-way ANOVA showed differences between training protocols for TTV (F[2, 45] = 26.43, p < 0.0001). The post hoc test revealed that DS produced higher TTV compared with both TRAD (DS vs. TRAD: p < 0.0001) and CP (DS vs. CP: p < 0.0001). No significant difference was found in TTV between TRAD and CP (TRAD vs. CP: p = 0.76) (Fig. 3 and Table 1). The inferential analysis was confirmed by the CI of the ES (Table 2).
Figure 3.
Total training volume of traditional (TRAD), drop-set (DS) and crescent pyramid (CP) resistance training protocols. #Significant different from TRAD and CP (p = 0.0001). Values are presented as mean ± SD.
DISCUSSION
This is the first study comparing the effects of distinct RT systems (DS and CP) with TRAD on microvascular oxygenation and muscle activation. We observed a higher TTV production for DS compared with TRAD and CP. Although no differences were observed in tHb and stO2 between protocols, DS produced lower levels of HbO2 and HbDiff than TRAD and CP. Additionally, DS resulted in higher levels of HHb compared with TRAD only. Despite the different levels of microvascular oxygenation, no differences on muscle activation was found during protocols.
Microvascular oxygenation is a physiological response to exercise and can be investigated through the analysis of specific NIRS variables. It is established that the tHb indicates a blood perfusion to the microvasculature which occurs due to hyperemia caused by vasodilatation, while stO2 reflects the level of tissue O2 saturation (21, 35). We expected that the higher TTV produced by subsequent sets to failure followed by very short pauses in the DS protocol would result in greater blood perfusion and O2 saturation compared with TRAD and CP, probably due to higher vasodilatation and metabolic demand (21, 38). However, our results showed similar blood perfusion (tHb) and O2 saturation (stO2) between protocols (Figs 1a and 1b, respectively). Despite only the DS required performing sets to failure, all RT protocols was performed within the same load zone (TRAD = 75% 1-RM; DS = ∼65–75% 1-RM; CP = 75–85% 1-RM), possibly leading participants close to concentric failure even in TRAD and CP. Thus, the similar overall effort of exercising to or near to concentric failure may be responsible for similar tHb and stO2 between RT protocols. Accordingly, evidence (45) showed that continuous, long- and short-interval aerobic exercise, but with a similar overall effort, produced analogous mean tHb and stO2. Therefore, regardless of RT protocol performed, overall effort seems to be a pivot variable that modulates blood perfusion and O2 saturation.
Other variables related to microvascular oxygenation are HbO2, HHb and HbDiff. HbO2 represents the concentration of oxygenated hemoglobin, while HHb and HbDiff indicate the level of deoxygenated hemoglobin and O2 consumption, respectively (5, 12, 13). Our results showed that HbO2 levels decreased more in DS than in TRAD and CP (Fig. 1c), while the HHb increased to a greater extent in DS compared with TRAD only (Fig. 1d). Additionally, we also found a significant decrease in HbDiff in DS compared with TRAD and CP (Fig. 1e). Comparatively, an important study (14) evaluated microvascular oxygenation using most of the variables analyzed herein (i.e., tHb, stO2, HbO2 and HHb). The authors showed similar changes in tHb, stO2 and HbO2 for a RT protocol to fatigue and another not to fatigue with fewer repetitions (14). Only HHb increased slightly more during RT to fatigue, probably due to the higher number of repetitions and TTV (14). Accordingly, the DS protocol used herein produced a greater number of repetitions than TRAD and CP (i.e., TRAD = 30 ± 0 reps; DS = 64 ± 7 reps; CP = 24 ± 0 reps), allowed by the load “drops” after a very short rest resulting in greater TTV (Fig. 3), what may explain the greater changes not only in HHb, but also in HbO2 and HbDiff. In fact, studies (15, 16) showed higher increases in indirect indicators of metabolic stress (i.e., lactate and growth hormone [GH]) which are speculated be related to changes on microvascular oxygenation status (38) in a protocol that resembles DS (i.e., additional sub-set to concentric failure after reducing load in the last set, resulting in a higher TTV compared with TRAD). Therefore, we suggest that the specificity of the DS protocol (i.e., large number of repetitions resulting in greater TTV than other tested RT protocols) resulted in higher microvascular oxygenation changes compared with TRAD and CP.
We expected that a greater change in microvascular oxygenation promoted by DS would increase muscle activation when compared with TRAD and CP. However, all protocols showed similar muscle activation (Fig. 2). Therefore, it is possible to suggest that muscle activation is not fully dependent on the microvascular oxygenation status. A possible explanation for similar muscle activation, as previously mentioned, is that albeit only in DS participants were instructed to reach concentric failure, TRAD and CP were possibly performed close to concentric failure. Accordingly, to the best of our knowledge, no data support the notion that subjects should reach concentric failure in every set of an RT session for maximize muscle activation (11, 33). In fact, a study compared the effects of RT performed to concentric failure or to volitional interruption (i.e., point in which participants voluntarily interrupted the exercise prior to concentric failure) (34). Results showed that the use of high loads led to similar muscle activation regardless of reaching concentric failure (34). Altogether, microvascular oxygenation status does not seem to be the sole variable to modulate muscle activation, whereas other factors such as RT intensity (19, 34) and level of fatigue (42) may modulate muscle activation.
It was proposed that muscle activation may influence muscle hypertrophy (33, 34, 40). Thus, if a RT protocol is performed to or near to concentric failure, muscle activation will probably be in an optimum level to maximize muscle hypertrophy. A previous study showed similar muscle hypertrophy after 12 weeks of TRAD, DS and CP when matched for TTV (2). It is unknown if a higher TTV and a greater change in microvascular oxygenation found herein for DS RT session would result in greater muscle hypertrophy after an DS-based RT period, as TTV might be an important variable to muscle hypertrophy (24, 25, 29). Future studies could elucidate this issue. In summary, the present study provides by NIRS approach, new insights regarding microvascular oxygenation status induced by TRAD, DS and CP. Our results demonstrated that despite DS produced a greater change in microvascular oxygenation compared with TRAD and CP, all protocols produced similar muscle activation levels. Thus, if the main objective of a RT session is to achieve an optimum level of muscle activation (which is thought to be important for hypertrophic adaptations), the choice of a RT protocol need not be necessarily guided by the microvascular oxygenation status induced by this protocol, since that RT protocol be performed up to or near to concentric muscle failure. Accordingly, the choose of a specific RT system should be made according to the practitioner’s preference/necessity as a strategy to increase the adherence to the RT program.
The present study has some limitations. We did not investigate the effects of TRAD, DS and CP matching the TTV on microvascular oxygenation and muscle activation. Additionally, despite similar muscle activation between protocols, the acute design does not provide information about the long-term effects (e.g., muscle hypertrophy) of TRAD, DS and CP.
ACKNOWLEDGEMENTS
The authors acknowledge the São Paulo Research Foundation (FAPESP) (#2017/05331-6 to V.A., and #2018/13064-0 to F.D.) and National Council for Scientific and Technological Development (CNPq) (to C.A.L. #302801/2018-9). No potential conflict of interest was reported by the authors.
REFERENCES
- 1.http://seniam.org/sensorlocation.htm
- 2.Angleri V, Ugrinowitsch C, Libardi CA. Crescent pyramid and drop-set systems do not promote greater strength gains, muscle hypertrophy, and changes on muscle architecture compared with traditional resistance training in well-trained men. European journal of applied physiology. 2017;117(2):359–369. doi: 10.1007/s00421-016-3529-1. [DOI] [PubMed] [Google Scholar]
- 3.Bentes CM, Simão R, Bunker T, Rhea MR, Miranda H, Gomes TM, Novaes JDS. Acute effects of dropsets among different resistance training methods in upper body performance. Journal of human kinetics. 2012;34:105–111. doi: 10.2478/v10078-012-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biazon TMPC, Ugrinowitsch C, Soligon SD, Oliveira RM, Bergamasco JG, Borghi-Silva A, Libardi CA. The association between muscle deoxygenation and muscle hypertrophy to blood flow restricted training performed at high and low loads. 2019;10(446) doi: 10.3389/fphys.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (nirs) in health and disease. Scand J Med Sci Sports. 2001;11(4):213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 6.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000;168(4):615–622. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown LE, Weir JP. Asep procedures recommendation i: Accurate assessment of muscular strength and power. J Exerc Physiol Online. 2001;4(3):1–21. [Google Scholar]
- 8.Callewaert M, Boone J, Celie B, De Clercq D, Bourgois J. Quadriceps muscle fatigue in trained and untrained boys. Int J Sports Med. 2013;34(1):14–20. doi: 10.1055/s-0032-1316359. [DOI] [PubMed] [Google Scholar]
- 9.Cayot TE, Lauver JD, Silette CR, Scheuermann BW. Effects of blood flow restriction duration on muscle activation and microvascular oxygenation during low-volume isometric exercise. 2016;36(4):298–305. doi: 10.1111/cpf.12228. [DOI] [PubMed] [Google Scholar]
- 10.Charro MA, Aoki MS, Coutts AJ, Araujo RC, Bacurau RF. Hormonal, metabolic and perceptual responses to different resistance training systems. J Sports Med Phys Fitness. 2010;50(2):229–234. [PubMed] [Google Scholar]
- 11.Davies T, Orr R, Halaki M, Hackett D. Effect of training leading to repetition failure on muscular strength: A systematic review and meta-analysis. Sports medicine (Auckland, NZ) 2016;46(4):487–502. doi: 10.1007/s40279-015-0451-3. [DOI] [PubMed] [Google Scholar]
- 12.De Blasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. Journal of applied physiology (Bethesda, Md. 1985;1994;76(3):1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29(4):463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 14.Ganesan G, Cotter JA, Reuland W, Cerussi AE, Tromberg BJ, Galassetti P. Effect of blood flow restriction on tissue oxygenation during knee extension. Medicine and science in sports and exercise. 2015;47(1):185–193. doi: 10.1249/MSS.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto K, Nagasawa M, Yanagisawa O, Kizuka T, Ishii N, Takamatsu K. Muscular adaptations to combinations of high- and low-intensity resistance exercises. Journal of strength and conditioning research. 2004;18(4):730–737. doi: 10.1519/R-13603.1. [DOI] [PubMed] [Google Scholar]
- 16.Goto K, Sato K, Takamatsu K. A single set of low intensity resistance exercise immediately following high intensity resistance exercise stimulates growth hormone secretion in men. J Sports Med Phys Fitness. 2003;43(2):243–249. [PubMed] [Google Scholar]
- 17.Goto M, Nirengi S, Kurosawa Y, Nagano A, Hamaoka T. Effects of the drop-set and reverse drop-set methods on the muscle activity and intramuscular oxygenation of the triceps brachii among trained and untrained individuals. J Sports Sci Med. 2016;15(4):562–568. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman JR, Im J, Rundell KW, Kang J, Nioka S, Spiering BA, Kime R, Chance B. Effect of muscle oxygenation during resistance exercise on anabolic hormone response. Medicine and science in sports and exercise. 2003;35(11):1929–1934. doi: 10.1249/01.MSS.0000093613.30362.DF. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins ND, Housh TJ, Bergstrom HC, Cochrane KC, Hill EC, Smith CM, Johnson GO, Schmidt RJ, Cramer JT. Muscle activation during three sets to failure at 80 vs. 30% 1rm resistance exercise. European journal of applied physiology. 2015;115(11):2335–2347. doi: 10.1007/s00421-015-3214-9. [DOI] [PubMed] [Google Scholar]
- 20.Kacin A, Strazar K. Frequent low-load ischemic resistance exercise to failure enhances muscle oxygen delivery and endurance capacity. Scand J Med Sci Sports. 2011;21(6):e231–241. doi: 10.1111/j.1600-0838.2010.01260.x. [DOI] [PubMed] [Google Scholar]
- 21.Kime R, Niwayama M, Fujioka M, Shiroishi K, Osawa T, Shimomura K, Osada T, Murase N, Katsumura T. Unchanged muscle deoxygenation heterogeneity during bicycle exercise after 6 weeks of endurance training. Adv Exp Med Biol. 2010;662:353–358. doi: 10.1007/978-1-4419-1241-1_51. [DOI] [PubMed] [Google Scholar]
- 22.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. Journal of applied physiology (Bethesda, Md. 1985;2007;103(6):2049–2056. doi: 10.1152/japplphysiol.00627.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kon M, Ikeda T, Homma T, Akimoto T, Suzuki Y, Kawahara T. Effects of acute hypoxia on metabolic and hormonal responses to resistance exercise. Medicine and science in sports and exercise. 2010;42(7):1279–1285. doi: 10.1249/MSS.0b013e3181ce61a5. [DOI] [PubMed] [Google Scholar]
- 24.Krieger JW. Single versus multiple sets of resistance exercise: A meta-regression. Journal of strength and conditioning research. 2009;23(6):1890–1901. doi: 10.1519/JSC.0b013e3181b370be. [DOI] [PubMed] [Google Scholar]
- 25.Krieger JW. Single vs. Multiple sets of resistance exercise for muscle hypertrophy: A meta-analysis. Journal of strength and conditioning research. 2010;24(4):1150–1159. doi: 10.1519/JSC.0b013e3181d4d436. [DOI] [PubMed] [Google Scholar]
- 26.Lauver JD, Cayot TE, Rotarius T, Scheuermann BW. The effect of eccentric exercise with blood flow restriction on neuromuscular activation, microvascular oxygenation, and the repeated bout effect. European journal of applied physiology. 2017;117(5):1005–1015. doi: 10.1007/s00421-017-3589-x. [DOI] [PubMed] [Google Scholar]
- 27.Lin TY, Lin LL, Ho TC, Chen JJ. Investigating the adaptation of muscle oxygenation to resistance training for elders and young men using near-infrared spectroscopy. European journal of applied physiology. 2014;114(1):187–196. doi: 10.1007/s00421-013-2763-z. [DOI] [PubMed] [Google Scholar]
- 28.Marshall PW, Desai I. Electromyographic analysis of upper body, lower body, and abdominal muscles during advanced swiss ball exercises. Journal of strength and conditioning research. 2010;24(6):1537–1545. doi: 10.1519/JSC.0b013e3181dc4440. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of applied physiology (Bethesda, Md. 1985;2012;113(1):71–77. doi: 10.1152/japplphysiol.00307.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M. Oxygen availability and motor unit activity in humans. Eur J Appl Physiol Occup Physiol. 1992;64(6):552–556. doi: 10.1007/BF00843767. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 32.Navalta JW, Stone WJ, Lyons S. Ethical issues relating to scientific discovery in exercise science. International Journal of Exercise Science. 2019;12(1) doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nóbrega SR, Libardi CA. Is resistance training to muscular failure necessary? Front Physiol. 2016;7:10. doi: 10.3389/fphys.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobrega SR, Ugrinowitsch C, Pintanel L, Barcelos C, Libardi CA. Effect of resistance training to muscle failure vs. Volitional interruption at high- and low-intensities on muscle mass and strength. Journal of strength and conditioning research. 2018;32(1):162–169. doi: 10.1519/JSC.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 35.Okushima D, Poole DC, Rossiter HB. Muscle deoxygenation in the quadriceps during ramp incremental cycling: Deep vs. Superficial heterogeneity. 2015;119(11):1313–1319. doi: 10.1152/japplphysiol.00574.2015. [DOI] [PubMed] [Google Scholar]
- 36.Paoli A, Mancin L, Saoncella M, Grigoletto D, Pacelli FQ, Zamparo P, Schoenfeld BJ, Marcolin G. Mind-muscle connection: Effects of verbal instructions on muscle activity during bench press exercise. Eur J Transl Myol. 2019;29(2):8250–8250. doi: 10.4081/ejtm.2019.8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paoli A, Marcolin G, Petrone N. The effect of stance width on the electromyographical activity of eight superficial thigh muscles during back squat with different bar loads. Journal of strength and conditioning research. 2009;23(1):246–250. doi: 10.1519/jsc.0b013e3181876811. [DOI] [PubMed] [Google Scholar]
- 38.Schoenfeld Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports medicine (Auckland, NZ) 2013;43(3):179–194. doi: 10.1007/s40279-013-0017-1. [DOI] [PubMed] [Google Scholar]
- 39.Schoenfeld BJ. The use of specialized training techniques to maximize muscle hypertrophy. Strength & Conditioning Journal. 2011;33(4):60–65. [Google Scholar]
- 40.Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports medicine (Auckland, NZ) 2013;43(3):179–194. doi: 10.1007/s40279-013-0017-1. [DOI] [PubMed] [Google Scholar]
- 41.Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Takahashi T, Omokawa M, Kinugawa S, Tsutsui H. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. Journal of applied physiology (Bethesda, Md. 1985;2009;106(4):1119–1124. doi: 10.1152/japplphysiol.90368.2008. [DOI] [PubMed] [Google Scholar]
- 42.Sundstrup E, Jakobsen MD, Andersen CH, Zebis MK, Mortensen OS, Andersen LL. Muscle activation strategies during strength training with heavy loading vs. Repetitions to failure. Journal of strength and conditioning research. 2012;26(7):1897–1903. doi: 10.1519/JSC.0b013e318239c38e. [DOI] [PubMed] [Google Scholar]
- 43.Takada S, Okita K, Suga T, Omokawa M, Kadoguchi T, Sato T, Takahashi M, Yokota T, Hirabayashi K, Morita N, Horiuchi M, Kinugawa S, Tsutsui H. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. Journal of applied physiology (Bethesda, Md. 1985;2012;113(2):199–205. doi: 10.1152/japplphysiol.00149.2012. [DOI] [PubMed] [Google Scholar]
- 44.Wakahara T, Miyamoto N, Sugisaki N, Murata K, Kanehisa H, Kawakami Y, Fukunaga T, Yanai T. Association between regional differences in muscle activation in one session of resistance exercise and in muscle hypertrophy after resistance training. European journal of applied physiology. 2012;112(4):1569–1576. doi: 10.1007/s00421-011-2121-y. [DOI] [PubMed] [Google Scholar]
- 45.Zafeiridis A, Kounoupis A, Dipla K, Kyparos A, Nikolaidis MG, Smilios I, Vrabas IS. Oxygen delivery and muscle deoxygenation during continuous, long- and short-interval exercise. Int J Sports Med. 2015;36(11):872–880. doi: 10.1055/s-0035-1554634. [DOI] [PubMed] [Google Scholar]