Abstract
Swimming is a favorable and ideal modality of exercise for individuals with obesity and arthritis as it encompasses a minimal weight-bearing stress and a reduced heat load. However, the available evidence indicates that regular swimming may not be effective in reducing body weight and body fatness. A current hypothesis is that exercise in cold water stimulates appetite. We determined the effect of swimming training on appetite-related hormones. Thirty-nine adults with obesity and osteoarthritis were randomly assigned to 12 weeks of supervised swimming or cycling training. In the initial few weeks, participants exercised for 20–30 minutes/day, 3 days/week, at an exercise intensity of 40–50% of heart rate reserve (HRR). Subsequently, the intensity and duration of exercise were progressively increased to 40–45 minutes/day, 3 days/week, at an intensity of 60–70% of HRR. Fasting plasma concentrations of ghrelin, insulin, leptin, and peptide YY did not change with the swimming or cycling exercise training (p>0.05). Swimming exercise did not negatively influence appetite-related hormones in adults with obesity and osteoarthritis to impair weight loss.
Keywords: Physical activity, food intake, ghrelin, insulin, leptin, peptide YY
INTRODUCTION
Swimming is a favorable modality of exercise for individuals with obesity and arthritis as it encompasses minimal weight-bearing stress and a reduced heat load (4, 15, 16). Swimming, however, may not be the most effective modality of exercise for weight reduction. For example, six months of swimming was found to be least effective for weight loss when compared with walking and cycling exercise interventions (8). This has been speculated to be due to the effects of immersion in cold water evoking the release of appetite-related hormones when skin is reheated, as postulated in a study comparing exercise with submerged cycling in thermoneutral water (33°C) vs. cold water (20°C) (16, 20). Indeed, food consumption after exercise in cold water increases as much as 44% when compared with the same exercise in neutral water (20).
Among a variety of appetite-related hormones, ghrelin is a gut hormone that regulates energy balance via the stimulation of appetite and is a signal of meal initiation (7). Circulating ghrelin concentrations are sensitive to changes in body weight during exercise programs (12). Additionally, short-term cold exposure upregulates ghrelin and leads to increased food intake (17). Insulin is a hormone involved in long term appetite regulation by stimulating satiety (22) and acts to suppress the role of ghrelin during hyperinsulinemia and inhibit neuropeptide Y to reduce food intake (6). Leptin is a major hormone responsible for energy balance and has been shown to reduce food intake (5). Similarly, peptide YY regulates energy balance by reducing appetite and is modulated by exercise training (10). Previous chronic aerobic exercise studies have demonstrated that ghrelin, insulin, and leptin concentrations decrease while peptide YY levels increase following training (1, 3, 21). However, there is no information available regarding the effects of swimming exercise training on these hormonal concentrations.
Accordingly, we aimed to determine the chronic effects of swimming exercise training on appetite-related hormones in individuals with obesity and osteoarthritis and compare these responses to a cycling training group as a non-weight bearing land-based thermoneutral comparison group.
METHODS
Participants
A total of 39 middle-aged and older sedentary adults (59±1 years) with obesity and osteoarthritis were studied. Exclusion criteria included strenuous physical activity >twice/week, cardiac or pulmonary diseases, joint replacement surgery, intraarticular injection or systemic corticosteroid usage, severe disabling co-morbidity, aquaphobia, and chronic tobacco smoking. Institutional review board approved the study, and participants gave their written informed consent. The participants in this study are a subset of the cohort in our previously-published study (2). This research was carried out fully in accordance to the ethical standards of the International Journal of Exercise Science (14).
Protocol
Participants were randomly assigned to either swimming (n=19; 18 females and 1 male) or cycling (n=20; 18 females and 2 males) supervised exercise training groups. Initially, participants exercised for 20 to 30 minutes/day and 3 days/week at an exercise intensity of 40–50% of heart rate reserve (HRR). As each participant’s level of fitness improved the intensity and duration of exercise increased gradually with the goal of attaining 40–45 minutes/day and 3 days/week at an intensity of 60–70% of HRR, typically by the 6th week. During the 12-week investigation, participants were instructed to maintain their usual lifestyle and dietary habits.
The swimming training was performed in 25-yard long therapeutic swimming pools, in which water temperature was held constant at 27–28°C. The cycling training was performed on a stationary cycle ergometer in the indoor gymnasium in which the room temperature was maintained at 24–26°C. Each participant received instructions to exercise continuously except during the time needed for checking a target intensity by heart rate monitor (Polar, Lake Success, NY, USA).
A 10-mL sample of whole blood was obtained before the exercise intervention (pre) and 48 hours after their last exercise training session (post) from an antecubital vein using standardized protocol, centrifuged for 15 minutes, and aliquoted for plasma. Blood collection was performed in the same order and at the same time of day on each participant after having refrained from food, alcohol, caffeine, and exercise for at least 8 hours before their arrival. The fasting blood samples were analyzed for ghrelin, insulin, leptin, and peptide YY concentrations using Millipore multiplex array (HMHEMAG-34K, Millipore Sigma, Burlington, MA, USA) following manufactures instructions in a Bio-Plex 200 analyzer (Bio-Rad, Hercules, CA, USA).
Statistical Analysis
Data analyses were performed using the SPSS version 22.0. Data were first tested for the normal distribution. Independent sample t-tests were used to analyze group differences in baseline variables and percent change values. A 2-way repeated measures ANOVA was performed to evaluate changes in body weight, body mass index (BMI), and hormonal concentrations. A post hoc power analysis was conducted using the program G*Power (version 3.1.9.2). Considering the difference between the mean and standard deviation (pre to post) with an α-level of 0.05 for appetite-related hormones in response to the exercise interventions, the overall sample size of 39 participants in this study achieved an adequate power of >80%. A p-value <0.05 was used to determine statistical significance.
RESULTS
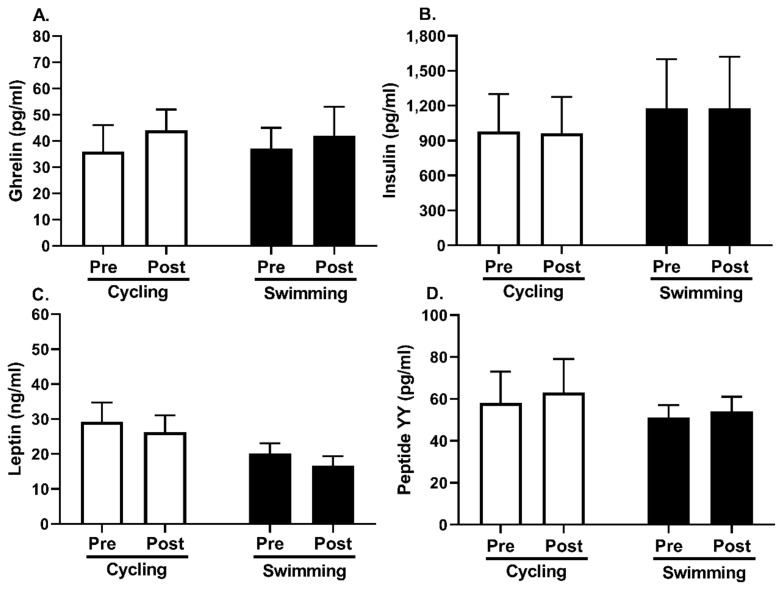
There were no significant differences in height, body weight, BMI, or appetite-related hormones prior to the exercise interventions between the cycling and swimming exercise groups, although we observed decreased body weight and BMI with both groups following exercise training (Table 1). As shown in Figure 1, fasting plasma concentrations of ghrelin (F[1, 37] = 0.785, p = 0.381), insulin (F[1, 37] = 0.049, p = 0.827), leptin (F[1, 37] = 2.921, p = 0.096), and peptide YY (F[1, 35] = 1.154, p = 0.290) did not change with either the swimming or cycling exercise interventions. Moreover, there were no significant differences in percent change values of ghrelin (14% vs. 22%, p = 0.825), insulin (0% vs. −1%, p = 0.937), leptin (−18% vs. −10%, p = 0.256), and peptide YY (6% vs. 9%, p = 0.588) between the swimming and cycling exercise interventions, respectively.
Table 1.
Changes in participant demographics.
| Variable | Cycling | Swimming | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Males/Females (n) | 2M/18F | - | 1M/18F | - |
| Age (years) | 61±1 | - | 57±2 | - |
| Height (cm) | 164±2 | - | 163±1 | - |
| Body weight (kg) | 87.3±5.2 | 85.9±5.0* | 88.9±4.9 | 86.4±4.5* |
| BMI (kg/m2) | 32.5±2.0 | 32.0±2.0* | 33.1±1.6 | 32.2±1.5* |
Values are Means±SEM. BMI, body mass index.
p <0.05 versus Pre.
Figure 1.
Fasting concentrations of appetite-related hormones pre and post exercise interventions. There were no significant changes in the hormones (all p>0.05). Data are presented as means±SEM.
DISCUSSION
The present study was conducted to determine the effect of a swimming exercise intervention on appetite-related hormones in adults with obesity and osteoarthritis and compare these responses to a thermoneutral land-based comparison (cycling) intervention. We demonstrated that neither swimming nor cycling exercise interventions had a significant impact on fasting plasma concentrations of ghrelin, insulin, leptin, and peptide YY following 12 weeks of training. However, we observed a significant decrease in body weight and BMI with both exercise interventions. This is the first randomized swimming exercise intervention study to evaluate changes in appetite-related hormones.
Acute exercise performed on land has been demonstrated to influence appetite-related hormones. For example, regardless of ambient temperature (20 vs. 30 °C), ghrelin was suppressed following running for 1 hour at 65% of peak oxygen uptake (19). Additionally, an acute bout of treadmill exercise reduced ghrelin concentrations accompanied by decreased appetite (18). Cycling at moderate intensity for 1 hour increased mean peptide YY and temporarily decreased hunger sensations, whereas ghrelin did not have an effect (13). This study found that both 3 months of swimming and cycling did not influence appetite-related hormones. These results differ from previous studies using land-based exercises. In participants with obesity and diabetes, high-intensity interval training has been shown to reduce ghrelin concentration while increasing peptide YY levels (1). Furthermore, leptin levels were decreased following 8 weeks of moderate intensity running in previously sedentary females (3). Moreover, insulin levels were decreased 24 hours following a cycling sprint interval training intervention but returned to baseline levels after 72 hours (21). In the present study, in an attempt to avoid the acute effect of exercise, 48 hours transpired after their last exercise training session prior to the blood collection. It could be assumed that if we had assessed the appetite-related hormones sooner, we may have found significant changes. However, we would not be able to tease out chronic effects from the acute effects of exercise. In this context, an acute bout of swimming had no influence on food intake or ghrelin concentrations (11).
Irrespective of modes of exercise, temperature of the exercising medium appears to influence the outcome on body weight. For instance, eight weeks of cycling exercise in cold water did not result in weight loss in obese women (15). In the present study, the water temperature in the swimming pool was relatively mild at 27–28°C as this was a therapeutic pool, which may account for the significant decrease in body weight and BMI observed with our swimming intervention. Although, this water temperature is still below skin temperature (33°C), which was hypothesized to evoke the release of appetite stimulating hormones when the skin is reheated (20). It is possible swimming training in cooler or warmer temperatures may evoke a different response than what we observed in our current study. Additionally, previous research has indicated that acute moderate intensity continuous training and sprint interval training increases peptide YY to a larger extent in males than females (9). In the present study, participants were mostly females. As such, if more males were included in the study, it is possible that we may have observed an increase in appetite-related hormones.
In conclusion, our present results indicate that fasting levels of appetite-related hormones did not change with 12 weeks of swimming exercise intervention in participants with obesity and osteoarthritis and that there were no group differences in changes with these hormones between the swimming and cycling exercise interventions. Importantly, this can be taken as a positive finding, in that appetite-related hormones are not negatively influenced to impair weight loss with swimming exercise interventions for individuals with obesity and osteoarthritis. Regarding exercise prescription for this population, our data supports the use of either swimming or cycling exercise training to be used with other lifestyle modifications (e.g. diet) to promote weight loss. Therefore, individuals with obesity and osteoarthritis are encouraged to participate in either minimal weight-bearing stress modality of aerobic exercise that they find enjoyable.
REFERENCES
- 1.Afrasyabi S, Marandi SM, Kargarfard M. The effects of high intensity interval training on appetite management in individuals with type 2 diabetes: Influenced by participants weight. J Diabetes Metab. 2019;18(1):107–117. doi: 10.1007/s40200-019-00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkatan M, Machin DR, Baker JR, Akkari AS, Park W, Tanaka H. Effects of swimming and cycling exercise intervention on vascular function in patients with osteoarthritis. Am J Cardiol. 2016;117(1):141–145. doi: 10.1016/j.amjcard.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Azizi M. The effect of 8-weeks aerobic exercise training on serum leptin in un-trained females. Soc Behav Sci. 2011;15:1630–1634. [Google Scholar]
- 4.Becker BE, Cole AJ. Swimming onward: The future of aquatic rehabilitation. Back Musculoskelet Rehabil. 1994;4(4):319–320. doi: 10.3233/BMR-1994-4412. [DOI] [PubMed] [Google Scholar]
- 5.Campfield L, Smith F, Guisez Y, Devos R, Burn P. Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endoc Metab. 2003;284(2):E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- 7.Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: A hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96(2):201–226. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- 8.Gwinup G. Weight loss without dietary restriction: Efficacy of different forms of aerobic exercise. Am J Sport Med. 1987;15(3):275–279. doi: 10.1177/036354658701500317. [DOI] [PubMed] [Google Scholar]
- 9.Hazell TJ, Townsend LK, Hallworth JR, Doan J, Copeland JL. Sex differences in the response of total pyy and glp-1 to moderate-intensity continuous and sprint interval cycling exercise. Eur J Appl Physiol. 2017;117(3):431–440. doi: 10.1007/s00421-017-3547-7. [DOI] [PubMed] [Google Scholar]
- 10.Jones TE, Basilio J, Brophy P, McCammon M, Hickner R. Long-term exercise training in overweight adolescents improves plasma peptide yy and resistin. Obesity. 2009;17(6):1189–1195. doi: 10.1038/oby.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King JA, Wasse LK, Stensel DJ. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J Obes. 2010;2011 doi: 10.1155/2011/351628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab. 2004;89(6):2659–2664. doi: 10.1210/jc.2003-031471. [DOI] [PubMed] [Google Scholar]
- 13.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol. 2007;193(2):251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 14.Navalta JW, Stone WJ, Lyons S. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheldahl L, Buskirk E, Loomis J, Hodgson J, Mendez J. Effects of exercise in cool water on body weight loss. Int J Obes. 1982;6(1):29–42. [PubMed] [Google Scholar]
- 16.Tanaka H. Swimming exercise. Sports Medicine: Impact of Aquatic Exercise on Cardiovascular Health. Sports Med. 2009;39(5):377–387. doi: 10.2165/00007256-200939050-00004. [DOI] [PubMed] [Google Scholar]
- 17.Tomasik P, Sztefko K, Pizon M. The effect of short-term cold and hot exposure on total plasma ghrelin concentrations in humans. Horm Metab Res. 2005;37(03):189–190. doi: 10.1055/s-2005-861296. [DOI] [PubMed] [Google Scholar]
- 18.Vatansever-Ozen S, Tiryaki-Sonmez G, Bugdayci G, Ozen G. The effects of exercise on food intake and hunger: Relationship with acylated ghrelin and leptin. J Sports Sci Med. 2011;10(2):283. [PMC free article] [PubMed] [Google Scholar]
- 19.Wasse LK, King JA, Stensel DJ, Sunderland C. Effect of ambient temperature during acute aerobic exercise on short-term appetite, energy intake, and plasma acylated ghrelin in recreationally active males. Appl Physiol Nutr Metab. 2013;38(8):905–909. doi: 10.1139/apnm-2013-0008. [DOI] [PubMed] [Google Scholar]
- 20.White LJ, Dressendorfer RH, Holland E, McCoy SC, Ferguson MA. Increased caloric intake soon after exercise in cold water. Int J Sport Nutr Exerc Metab. 2005;15(1):38–47. doi: 10.1123/ijsnem.15.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Woods SC, Lotter EC, McKay LD, Porte D. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282(5738):503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]