Abstract
Introduction
In numerous countries, large population testing is impossible due to the limited availability of RT-PCR kits and CT-scans. This study aimed to determine a pre-test probability score for SARS-CoV-2 infection.
Methods
This multicenter retrospective study (4 University Hospitals) included patients with clinical suspicion of SARS-CoV-2 infection. Demographic characteristics, clinical symptoms, and results of blood tests (complete white blood cell count, serum electrolytes and CRP) were collected. A pre-test probability score was derived from univariate analyses of clinical and biological variables between patients and controls, followed by multivariate binary logistic analysis to determine the independent variables associated with SARS-CoV-2 infection.
Results
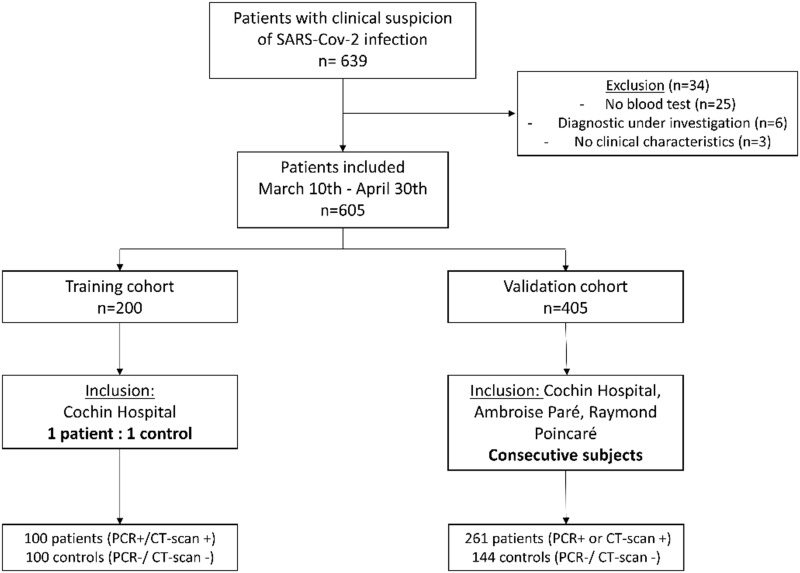
605 patients were included between March 10th and April 30th, 2020 (200 patients for the training cohort, 405 consecutive patients for the validation cohort). In the multivariate analysis, lymphocyte (<1.3 G/L), eosinophil (<0.06 G/L), basophil (<0.04 G/L) and neutrophil counts (<5 G/L) were associated with high probability of SARS-CoV-2 infection but no clinical variable was statistically significant. The score had a good performance in the validation cohort (AUC = 0.918 (CI: [0.891–0.946]; STD = 0.014) with a Positive Predictive Value of high-probability score of 93% (95%CI: [0.89–0.96]). Furthermore, a low-probability score excluded SARS-CoV-2 infection with a Negative Predictive Value of 98% (95%CI: [0.93–0.99]). The performance of the score was stable even during the last period of the study (15-30th April) with more controls than infected patients.
Conclusions
The PARIS score has a good performance to categorize the pre-test probability of SARS-CoV-2 infection based on complete white blood cell count. It could help clinicians adapt testing and for rapid triage of patients before test results.
Introduction
Coronavirus disease 2019 (Covid-19) is a major global threat that has already caused more than 500,000 deaths worldwide. Based on data from patients with laboratory-confirmed Covid-19 from mainland China, admission to intensive care unit (ICU), invasive mechanical ventilation or death occurred in 6.1% [1]. The case-fatality rates (CFR) vary in the different countries, with for example less than 0.5% in South Korea and Germany [2]. These variations might be due to comprehensive screening strategies in the countries with lower CFR values allowing the identification of a large number of individuals with mild symptoms and lower mortality risk, whereas only patients with severe symptoms are tested in others countries due to the lack of availability of RT-PCR kits to undertake large scale population testing [3].
Reverse Transcription Polymerase Chain Reaction (RT-PCR) is currently the test of reference to diagnose patients with SARS-CoV-2 infection [4]. Computed Tomography (CT)-scan has a reported high sensitivity [5] but radiological signs can be delayed after disease onset, with up to 56% CT negativity in the first 3 days of symptomatic infection [6]. Moreover, performing large scale CT scanning during the current pandemic is made difficult by the need to apply rigorous disinfection protocols between patients [7] without mentioning that it is problematic to induce radiation exposure for asymptomatic/paucisymptomatic patients. The Fleischner Society recently published a Multinational Consensus Statement on the use of thoracic imaging based on different scenarios [8]. Imaging is indicated in case of moderate-to-severe disease manifestations, but not indicated in case of mild symptoms consistent with COVID-19 and no risk factor for disease progression. Even though CT-scan has a high sensitivity (71–95% for PCR and 94–98% for CT), radiologists may still experience some difficulties in differentiating COVID-19 from non-COVID pneumonia [9–11]. False-negativity of PCR or CT as well as COVID-19 pneumonia mimickers on CT may lead to inaccurate diagnoses. Pre-test probability combining clinical and biological features has proven to be a very useful tool, already used in clinical practice for the management of patients with a suspicion of pulmonary embolism [12]. Therefore, we aimed to derive a new prediction score based on clinical and biological variables, independently from subjective clinical evaluation by assessing a retrospective cohort of patients admitted to the emergency department (ED) of our institution for a clinical suspicion of SARS-Cov-2 infection. We validated this Pre-test probability for SARS-Cov-2 Infection based on Scoring (PARIS score) in a distinct multicenter cohort of patients admitted for the same reason in ED or infectious disease departments of three other hospitals.
Material and methods
This retrospective observational study has been approved by our local ethics committee (Institutional Review Board: “Comité local d’éthique pour les publications de l’hôpital Cochin” CLEP N° AAA-2020-08014), which waived the need for patient consent. Patients were recruited from the ED, internal medicine and infectious disease departments (consultations) of four different hospitals (Cochin Hospital, Paris; Hotel-Dieu Hospital, Paris; Ambroise Paré Hospital, Boulogne; Raymond Poincaré Hospital, Garches) between March 10th and April 30th, 2020. The retrospective data used for this study were obtained between March 15th and May 15th, 2020.
Derivation cohort
We developed a diagnostic strategy for SARS-Cov-2 infection based on clinical and biological features to determine pre-test probability before RT-PCR and/or CT-scan. We recruited a retrospective cohort of outpatients that had both RT-PCR and CT-scan results available with a 1:1 patient:control inclusion ratio, who had been evaluated at the ED of Cochin Hospital (Paris, France) for a suspicion of SARS-Cov-2 infection between March 10th and April 8th, 2020. Exclusion criteria were: 1) diagnosis still under investigation; 2) lack of blood test including complete white blood cell count and serum electrolytes; 3) absence of reported clinical characteristics. We chose to include one hundred consecutive patients with RT-PCR positivity and 100 consecutive controls in this cohort (controls were patients with clinical suspicion of SARS-Cov-2 for whom RT-PCR and CT were negative). Final diagnoses of controls are presented in S1 Table. Clinical examination was standardized at the ED. Demographic characteristics and comorbidities including hypertension, respiratory diseases (asthma, restrictive or chronic obstructive pulmonary disease (COPD)), immunodeficiency, renal insufficiency were recorded. Furthermore, clinical symptoms such as cough, fever, headache, diarrhea, anosmia, ageusia, oxygen desaturation were evaluated. Finally, biological tests including results of complete white blood cell count, serum electrolytes and C Reactive Protein (CRP) were analyzed. A final diagnosis of SARS-Cov-2 infection was retained if the patient had a confirmed diagnosis with positive RT-PCR and/or CT-scan showing signs of COVID-19 pneumonia (ground-glass opacities with subpleural predominance with or without focal consolidation). If the RT-PCR was negative, patients were considered as COVID+ if CT-scan images evaluated by a senior radiologist specialized in thoracic imaging (>10 years of experience) were typical for COVID-19 pneumonia. Thus, controls were patients with both negative RT-PCR results and negative CT-scans. The diagnoses of controls were recorded, but PCR tests of other viruses than SARS-Cov-2 (Influenza, Rhinovirus…) were not always performed (performed in only 10 of the 144 controls). We evaluated clinical and biological variables to find the ones associated with SARS-Cov-2 infection. We only included basic biological tests, widely available and affordable, which are adapted for a pre-test probability score.
Validation cohort
Our pre-test diagnostic score was validated between March 10th and April 30th, 2020 on 405 consecutive outpatients suspected of SARS-Cov-2 infection (different from those of the derivation cohort) with both RT-PCR and CT-scan results available. Patients were confirmed to have SARS-Cov-2 infection based on RT-PCR positivity. If the RT-PCR was negative, patients were considered as COVID+ in this cohort if CT-scan images evaluated by a senior radiologist specialized in thoracic imaging (>10 years of experience) were typical for COVID-19 pneumonia (N = 19). Patients with unavailability of initial complete white blood cell count were excluded.
Statistical analysis
Univariate analyses were performed to select potential independent variables of SARS-Cov-2 infection to include in the multivariate analysis. The Mann Whitney U test was performed for comparison of continuous variables such as hemoglobin, and chi-square test was used for comparison of nominal variables. Exact p-values were computed for the Mann Whitney tests without any assumption of normality or asymptomatic approximation. A p-value less than 0.05 was considered as statistically significant (two-tailed). The statistically significant variables were evaluated to find the optimal cut-offs to differentiate patients and controls based on receiver operating characteristic (ROC) curve analysis (upper left point of the graph, showing the value with the best compromise between sensitivity and specificity) [13] (S2 Table). A binary logistic regression analysis was performed to measure the relationship between the categorial variables associated with the presence or absence of SARS-Cov-2 infection and the potential independent variables. Variables with non-statistical significance in the multivariate models were assessed to determine if they should be included in the final model because of their clinical importance. Odds-Ratio (OR) were used to assign points for the pre-test probability score, 2 points being assigned to the highest values (OR>10). The Hosmer-Lemeshow goodness of fit statistic [14] and Nagelkerke’s R2 were used to assess the model validity [15]. We performed a second ROC curve analysis to determine the area under the curve (AUC) and to choose the cutoff values, in order to determine the low-probability group (<5% probability of SARS-Cov-2 infection), the intermediate probability-group and the high-probability group (>90% of probability of SARS-Cov-2 infection). The proportion of patients with SARS-Cov-2 infection in each group was evaluated to check the accuracy of the PARIS score.
We assessed the ability of the score to differentiate patients and controls in the validation cohort by a ROC curve analysis and computed the ROC curve. The performance of the test according to the time period (with various prevalence of SARS-Cov-2 infection) was evaluated.
SPSS software (version 24, Chicago, IL, USA) was used for statistical analysis.
Results
Derivation cohort
The flow-chart of included patients is shown in Fig 1. The clinical and biological characteristics of the patients and controls included in the derivation cohort are presented in Table 1.
Fig 1. Flow chart of patients included.
Table 1. Characteristics of patients with SARS-CoV-2 infection (confirmed with both PCR and CT) and controls.
| Patients | Controls | p-value | |
|---|---|---|---|
| n | 100 | 100 | |
| Age | 65 [24] | 60 [31] | 0.14 |
| Sex (M:F) | 65:35 | 45:55 | 0.005 |
| Clinical characteristics | |||
| Cough | 79 | 66 | 0.04 |
| Fever | 90 | 63 | <0.001 |
| Temperature at ER | 37.7 [1.4] | 37 [0.7] | 0.28 |
| Shortness of breath | 70 | 69 | 0.88 |
| Saturation | 95% [6] | 96% [4] | 0.03 |
| Diarrhea | 22 | 14 | 0.14 |
| Myalgia | 34 | 17 | 0.006 |
| Headaches | 16 | 18 | 0.71 |
| Anosmia | 11 | 5 | 0.12 |
| Agueusia | 14 | 4 | 0.01 |
| Time from onset | 6d [5] | 5d [5] | 0.12 |
| Comorbidities | |||
| Comorbidities | 61 | 70 | 0.18 |
| Diabetes | 19 | 19 | 1 |
| Hypertension | 32 | 21 | 0.08 |
| Renal failure | 7 | 6 | 0.77 |
| Pulmonary disease¤ | 17 | 43 | <0.001 |
| Immunodeficiency/ autoimmune disease | 13 | 23 | 0.07 |
| Including: HIV | 2 | 5 | 0.25 |
| Treatments | |||
| Steroids | 4 | 7 | 0.35 |
| Chemotherapy | 2 | 3 | 0.65 |
| Blood tests | |||
| Hemoglobin (g/dL) | 13.70 [2.50] | 13.20 [2.10] | 0.31 |
| Lymphocytes (G/L) | 0.87 [0.48] | 1.96. [1.18] | <0.001 |
| Neutrophils (G/L) | 4.29 [3.06] | 6.47 [4.55] | <0.001 |
| Eosinophils (G/L) | 0.00 [0.01] | 0.10 [0.19] | <0.001 |
| Basophils (G/L) | 0.01 [0.03] | 0.04 [0.01] | <0.001 |
| Monocytes (G/L) | 0.44 [0.33] | 0.70 [0.46] | <0.001 |
| Platelets (G/L) | 195.00 [91.75] | 253.00 97.25] | <0.001 |
| Sodium (mmol/L) | 136.00 [5.00] | 137.00 [4.25] | 0.008 |
| Potassium (mmol/L) | 4.0 [0.50] | 4.00 [0.40] | 0.97 |
| Chloride (mmol/L) | 97.00 [4.00] | 98.50 [5.00] | 0.001 |
| Bicarbonate (mmol/L) | 24.00 [3.83] | 24.00 [4.03] | 0.82 |
| Total protein (g/L) | 71.80 [6.80] | 74.00 [7.00] | 0.07 |
| Creatinine (μmol/L) | 82.00 [33.50] | 78.00 [31.50] | 0.39 |
| CRP (mg/L) | 62.2 [78.20] | 11.1 [67.40] | <0.001 |
¤Pulmonary disease: asthma, COPD or restrictive syndrome; Results are presented as median [interquartile range]; G/L = 109/ Liter.
Fever, cough, oxygen saturation, agueusia and myalgias were the clinical variables that significantly differed between patients with confirmed SARS-Cov2 infection and controls in the univariate analysis. Patients with confirmed SARS-Cov2 infection had less frequent underlying pulmonary diseases (asthma, COPD or restrictive lung disease). Four percent of patients and 7% of controls were treated with corticosteroids. Among blood test variables, we noted significantly lower lymphocyte, neutrophil, eosinophil, monocyte, and basophil cell counts in patients as compared to controls. Additionally, the platelet count was significantly lower in patients with SARS-Cov-2 infection. Sodium, chlorides and CRP were significantly higher in patients.
All these variables were included in the multivariate binary logistic regression analysis. Only low lymphocyte, basophil, eosinophil, and neutrophil cell counts were significantly associated with SARS-Cov-2 infection (Table 2). The clinical variables were statistically insignificant and the performance of the score decreased when the clinical variables were included, perhaps due to inclusion of study participants with similar presenting symptoms in both cases and controls. ROC curve analysis determined the best cut-offs for sensitivity and specificity (S1 Table).
Table 2. Binary logistic regression using descending wald model.
| Patients | Controls | B | Exp(B)/OR | p-value | |
|---|---|---|---|---|---|
| Basophils<0.04G/L | 92 | 41 | 1.89 | 6.59 | 0.001 |
| Eosinophils <0.06G/L | 91 | 36 | 1.92 | 6.81 | 0.001 |
| Lymphocytes<1.3G/L | 89 | 24 | 2.56 | 12.88 | <0.001 |
| Neutrophils<5G/L | 64 | 30 | 2.00 | 7.73 | <0.001 |
OR: Odd-Ratio; Nagelkerke R2 = 0.70; Hosmer-Lemeshow goodness of fit statistic: p = 0.83.
Points were assigned according to the odds-ratio (Exp (B)): only lymphocytopenia (<1.3G/L) was associated with OR>10 (12.9) and 2 points were consequently assigned. The final score is presented in Table 3. AUC of the score in the derivation cohort was AUC = 0·924; STD = 0·019; CI = [0·887–0·961]. The Nagelkerke R2 was 0.70 and the Hosmer-Lemeshow goodness of fit statistic showed a p = 0.83.
Table 3. Pre-test diagnostic probability of COVID-19 infection: PARIS score.
| Variables | Points |
|---|---|
| Eosinophils < 0.06 G/L | 1 |
| Lymphocytes < 1.3 G/L | 2 |
| Neutrophils <5G/L | 1 |
| Basophils <0.04G/L | 1 |
| Score = 0–1 → Low probability | |
| Score = 2–3 →Intermediate probability | |
| Score ≥4 → High probability | |
Validation cohort
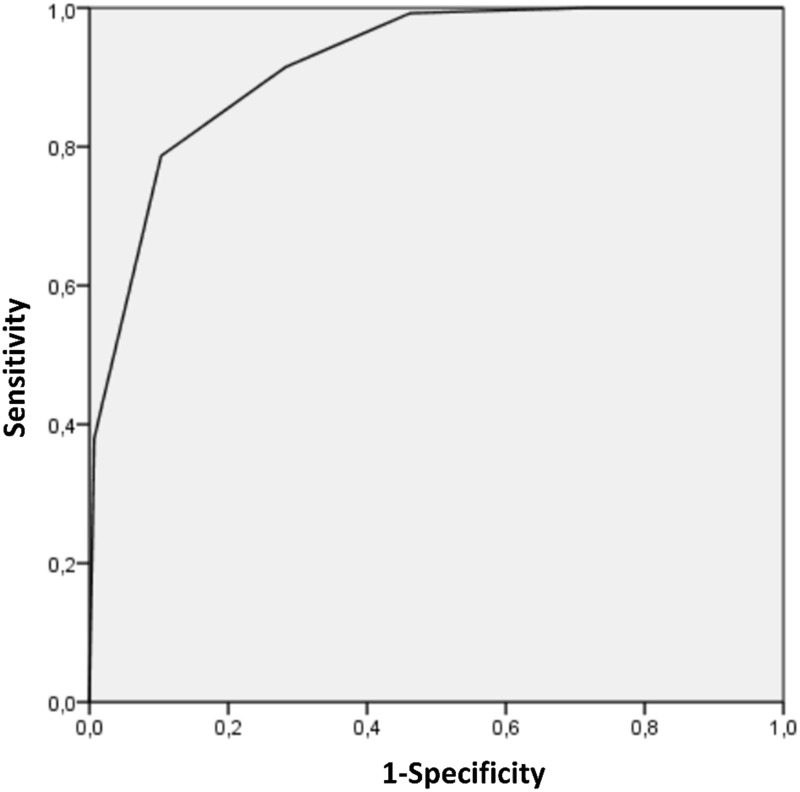
Individuals included in the validation cohort had a mean age of 65 years (significantly older than those of the training cohort; p = 0·007), with 223 of male and 182 of female gender. There were 261 confirmed SARS-Cov-2 infected patients (64%) and 144 controls (36%). The pre-test probability score had a good performance in this cohort (AUC = 0·918 (CI: [0·891–0·946]; STD = 0·014). The sensitivity and specificity of the high-probability score (score 4 or 5) were 79% and 90% respectively (Table 4). Furthermore, the Negative Predictive Value (NPV) of a low-probability score (score 0 or 1) was 98% (CI = 0.93–0.99). A low-probability score was found for 54% of controls (N = 78/144). Many controls (36%) presented with intermediate probability scores (score 2 to 4) but the NPV of combined low- and intermediate-probability scores was only 70% (CI = 0.63–0.77). Fifty-three of the 261 SARS-Cov-2 infected patients (20%) from the cohort of validation had an intermediate score. No patient with SARS-Cov-2 infection had a score equal to 0 and there were only two infected patient with a score of 1. ROC curve for the validation cohort is presented in Fig 2.
Table 4. Performance in the validation cohort depending on the value of the PARIS score (performance for a score ≥ to the value).
| PARIS score | Sensitivity | Specificity | PPV | NPV | N |
|---|---|---|---|---|---|
| 0 | 1 | 0 | 0.64 | NA | 40 |
| 1 | 1 | 0.28 | 0.71 | 1 | 39 |
| 2 | 0.99 | 0.53 | 0.79 | 0.98 | 46 |
| 3 | 0.92 | 0.72 | 0.85 | 0.83 | 60 |
| 4 | 0.79 | 0.90 | 0.93 | 0.70 | 120 |
| 5 | 0.38 | 0.99 | 0.99 | 0.47 | 100 |
PPV = Positive Predictive Value; NPV = Negative Predictive Value; N: Number of Patients.
Fig 2. Receiver operating characteristic (ROC) curve of the PARIS score for the validation cohort.
Area under the curve = 0.919.
Misclassification and reclassification
Twenty-nine controls (training and validation cohorts) presented with a high-probability score (≥4). The explanation for their lymphocytopenia was a septic shock (N = 3), immunodepression induced by autoimmune diseases and/or their treatments (N = 5), cystic fibrosis with lung transplantation (N = 2), pancytopenia due to hematological disorder under chemotherapy (N = 5), typhoid fever (N = 1) (S1 Table). Thirteen controls didn’t have any final diagnosis accounting for their lymphocytopenia and clinical symptoms. Only two patients with a low-probability score of 1 were considered as false-negatives of the pre-test probability score. These two patients had a negative RT-PCR result but positive CT-scan with minimal extent of pneumonia on CT (small ground glass opacities).
The score was also helpful to reclassify patients: Fourteen out of 21 infected patients, based on RT-PCR, with negative or indeterminate CT-scan results had a high-probability score (while the 7 other patients had an intermediate PARIS score). Twenty-one out of 31 patients with only negative PCR (1 to 3 tests) but CT features typical for COVID-19 pneumonia had a high-probability score (8 other patients had an intermediate score and 2 patients had a low score).
Correlation between the PARIS score and CT-scan extent
The correlation between the PARIS score and the extent of the COVID-19 pneumonia on CT-scan is presented in the S3 Table. The proportion of patients with a high probability score was higher in patients having more than 10% of diseased lung parenchyma on CT while the proportion of patients with an intermediate probability score was higher in patients with minimal or no involvement on CT.
Comparison of performance according to the incidence of the SARS-CoV-2 infections
The performance of the score was stable during the different time periods of the study with AUC ranging from 0.92 to 0.93, even if the proportion of patients and controls markedly changed over time (S4 Table). The prevalence of infection in the included patients was 68% between March 20th and March 31st versus only 31% between April 15th and April 30th.
Discussion
In this study, we established a pre-test probability score (PARIS score) based on simple, affordable, and widely available blood tests (complete white blood cell count). No clinical variable was independently associated with SARS-Cov-2 infection and thus included in this score but four biological variables (lymphocytes<1.3 G/L, eosinophils<0.06 G/L, basophils<0.04 G/L, neutrophils<5 G/L) were associated to the condition. This finding could be particularly useful for countries with a low availability of diagnostic tests. However, this score would need to be validated in other countries due to potential differences in populations, such as different ethnicity/age distributions compared to the population studied.
A low lymphocyte count has been described in patients with SARS-Cov-2 infection, possibly due to a decreased level of T-lymphocytes [16]. Furthermore, the total number of Natural Killer and CD8+ T cells has been reported to be markedly decreased in patients with SARS-Cov-2 infection [17]. The decrease of the absolute number of T lymphocytes is more pronounced in severe cases whereas decreased CD8+ T cells count could be an independent variable associated with the severity of the disease [18–20]. Eosinophils have also been reported to be decreased in previous studies [21, 22]. An increase of the eosinophil count may even be a predictor of improvement in patients with SARS-Cov-2 infection [23]. Even though the eosinopenia associated with COVID-19 is likely to be a secondary phenomenon not directly contributing to the disease course, its mechanism is not yet completely understood [24]. A low proportion of basophils has also been reported in severe cases [16] but no previous study has reported low basophil cell count as a marker of SARS-Cov-2 infection. Furthermore, previous reports demonstrated low levels of neutrophils in SARS-Cov-2 patients [25] but increased neutrophil count in severe cases of COVID-19 pneumonia [26]. Two previous histological studies performed on autopsies of deceased COVID-19 patients showed inflammatory cells including lymphocytes and macrophages in the alveoli, with minimal eosinophilic and neutrophilic infiltration [27, 28]. The decrease in the level of circulating lymphocytes could be due to their mobilization in the pulmonary infiltrates.
The Fleischner Society recently released a report highlighting to the lack of pre-test probability score in patients with mild symptoms consistent with SARS-Cov-2 infection [8]. The probability to be infected is mainly based on background prevalence of the disease and individual’s exposure risk. Committee members disapproved imaging use in case of mild symptoms and negative RT-PCR results. The PARIS score may enhance the proposed Fleischner diagnostic algorithm by improving the pre-test probability. Thus, in case of a high pre-test probability with no comorbidities explaining leukopenia, a second RT-PCR test or an additional CT-scan may be useful to establish the final diagnosis. Furthermore, a low PARIS score may obviate the need for RT-PCR or CT-scan, especially in a period of diagnostic test shortage. Chest radiography is insensitive in mild or early COVID-19 infection [8] but could be useful in combination of PARIS score in a resource-constrained environment.
A previous review indicated that prediction models for diagnosis and prognosis of COVID-19 infection are poorly reported, at high risk of bias, and with probably too optimistic reported performance [29]. We report here a multicenter study assessing diagnostic pre-test probability with a good accuracy, using a study design similar to that of the revised Geneve Score for pulmonary embolism [12].
Clinical symptoms were not included in our model, since most of covid-19 infected patients and controls presented with the same symptomatology, with no specificity of symptoms. Even oxygen saturation was not a discriminative feature. It could be explained by the fact that most patients with SARS-Cov2 infection were not severe, with subnormal oxygen saturation. Furthermore, even if patients with underlying lung diseases were more frequent in the control group, their average oxygen saturation was 96%. Thus, low oxygen saturation may be an additional argument for SARS-Cov-2 infection in patients with no previously diagnosed pulmonary disease. However, the PARIS score appears mostly useful in patients with relatively mild clinical presentation. It could also be more largely used for initial triage of patients presenting at the ED because it is fast, affordable and allows patients with a low probability score to stay apart from those most likely to be infected, avoiding the risk of contamination.
Our study presents inherent limitations. First, its retrospective design may introduce a selection bias. We chose to only include 100 patients and 100 controls in the training cohort in order to not delay the score development, so that it could be used in our hospitals as soon as possible during the pandemic. The proportion of controls in the validation cohort was lower, potentially affecting the estimation of the score predictive values. However, the confidence interval for the NPV of a low-probability score remained narrow in the validation cohort, despite this potential limitation. There were no statistically significant differences between demographic characteristics of patients with SARS-Cov-2 infection and controls but the 2 groups were not matched and controls more frequently presented pulmonary diseases and immunodeficiencies. We used only regular blood tests with complete white blood cell count, serum electrolytes and CRP, and did not evaluate LDH or D-dimers, since they seem to be mainly correlated with severity and have been described as risk factors for acute respiratory distress syndrome [30]. Another limitation of this study is the only inclusion of patients from hospital wards, which probably induces a recruitment bias, since they may not be representative of patients with milder symptoms who consult their general practitioner. The evaluation of the performance of our pre-test probability score in a larger population would be of interest. Even though a proportion of patients in our cohort were admitted to Intensive Care Units (ICUs) shortly after presentation, more severe patients directly admitted in ICUs, may be underrepresented. However, the score might not be as useful for severe patients admitted to ICU, since they anyway require admission and urgent care, regardless of RT-PCR positivity. This score was also developed during a pandemic with high prevalence of the disease and might not be as efficient in a situation where SARS-Cov2 infection would be rare, but its diagnostic performance remained stable even when prevalence of infection decreased in April. Numerous diseases or conditions may mimic SARS-Cov-2 biological changes with leukopenia and lymphocytopenia. Clinicians should be aware of the limits of this score due to other causes of variation of the white blood cell count. Lymphocyte count may be high in SARS-Cov-2 patients with leukemia or splenectomy [31, 32]. Conversely, sepsis, myelodysplasia, HIV infection, systemic lupus erythematous but also medications may induce lymphocytopenia in controls leading to a high-probability score [33, 34]. Lymphocytopenia is also a common finding in elderly patients [35], with increased difficulty of ruling out COVID-19 pneumonia in this population based on PARIS score. Another limitation is that most controls did not have a search for other respiratory viruses including influenza. Influenza can also induce lymphocytopenia [36, 37] with potential false positives of the score, but eosinopenia is less frequent in patients with influenza infection [38]. Low eosinophil counts is often related to the use of glucosteroids [39]. Eosinopenia is also considered as a surrogate of inflammation in bacterial infections [40] or myocardial infarction [41]. Basophil counts could be reduced by chronic urticaria or with steroids [42].
In conclusion, the PARIS score shows good performance with a high sensitivity and positive predictive value of intermediate and high probability scores and a high negative predictive value in low probability score individuals. It may help adapt the testing of subjects, especially during a pandemic with difficulties for large scale testing. Furthermore, future studies to evaluate the performance of the combination of PCR/CT and PARIS score could be of interest, in particular to detect COVID-19 patients with negative tests, that play a role in the diffusion of the virus.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge APHP, universities, Inserm for COVID-19 research collaboration. We acknowledge Dr Martin, Dr Alperin, Pr Lee and Pr Allain and all doctors from the Emergency Department, ICU and hospitalization departments at Cochin Hospital.
Data Availability
All relevant data are within the manuscript and its Supporting information files (in particular in the S5 Table).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. 30;382(18):1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis [Internet]. 2020. February 19 [cited 2020 May 5]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7159018/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA [Internet]. 2020. March 23 [cited 2020 May 5]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2763667 [DOI] [PubMed] [Google Scholar]
- 4.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3). 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020. February 26;200642 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020. February 20;200463 10.1148/radiol.2020200463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima K, Kato H, Yamashiro T, Izumi T, Takeuchi I, Nakajima H, et al. COVID-19 pneumonia: infection control protocol inside computed tomography suites. Jpn J Radiol. 2020. March 17 10.1007/s11604-020-00948-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology. 2020. April 7;201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020. March 10;200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020. February 19;200432 10.1148/radiol.2020200432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase-Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology. 2020. April 17;201343 10.1148/radiol.2020201343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gal G, Righini M, Roy P-M, Sanchez O, Aujesky D, Bounameaux H, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006. February 7;144(3):165–71. 10.7326/0003-4819-144-3-200602070-00004 [DOI] [PubMed] [Google Scholar]
- 13.Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006. June;27(8):861–74. [Google Scholar]
- 14.Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Communications in Statistics—Theory and Methods. 1980. January 1;9(10):1043–69. [Google Scholar]
- 15.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991. September 1;78(3):691–2. [Google Scholar]
- 16.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. March 12 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020. March 19 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020. March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020. March 30 10.1093/infdis/jiaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, et al. Prediction for Progression Risk in Patients with COVID-19 Pneumonia: the CALL Score. Clin Infect Dis [Internet]. [cited 2020 Apr 12]; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa414/5818317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YX, Wu W, Yang T, Zhou W, Fu YM, Feng QM, et al. [Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19]. Zhonghua Nei Ke Za Zhi. 2020. March 1;59(0):E003 doi: 3760.10/cma.j.cn112138-20200221-00114 [PubMed] [Google Scholar]
- 22.Zhang J-J, Dong X, Cao Y-Y, Yuan Y-D, Yang Y-B, Yan Y-Q, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. February 19 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020. March 12 10.1016/j.ijid.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil Responses During COVID-19 Infections and Coronavirus Vaccination. Journal of Allergy and Clinical Immunology [Internet]. 2020. April 25 [cited 2020 May 5];0(0). Available from: https://www.jacionline.org/article/S0091-6749(20)30569-8/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020. March 1;63(3):364–74. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. March 16 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020. April 10 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020. March 15;49(0):E009. [DOI] [PubMed] [Google Scholar]
- 29.Wynants L, Van Calster B, Bonten MMJ, Collins GS, Debray TPA, De Vos M, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020. 7;369:m1328 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020. March 13 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juneja S, Januszewicz E, Wolf M, Cooper I. Post-splenectomy lymphocytosis. Clin Lab Haematol. 1995. December;17(4):335–7. [PubMed] [Google Scholar]
- 32.Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015. July 23;126(4):454–62. 10.1182/blood-2015-02-585059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cloyd MW, Chen JJ, Adeqboyega P, Wang L. How does HIV cause depletion of CD4 lymphocytes? A mechanism involving virus signaling through its cellular receptors. Curr Mol Med. 2001. November;1(5):545–50. 10.2174/1566524013363320 [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Guffroy A, Argemi X, Martin T. [Systemic lupus erythematosus and lymphopenia: Clinical and pathophysiological features]. Rev Med Interne. 2017. September;38(9):603–13. 10.1016/j.revmed.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 35.Brass D, Mckay P, Scott F. Investigating an incidental finding of lymphopenia. BMJ [Internet]. 2014. March 3 [cited 2020 May 5];348 Available from: https://www.bmj.com/content/348/bmj.g1721 [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Liu Y, Zhang T, Jiang Y, Yang S, Xu Y, et al. Differentiating Between 2019 Novel Coronavirus Pneumonia and Influenza Using a Nonspecific Laboratory Marker-Based Dynamic Nomogram. Open Forum Infect Dis. 2020. May;7(5):ofaa169 10.1093/ofid/ofaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin Z, Kang Z, Yang D, Ding S, Luo H, Xiao E. A Comparison of Clinical and Chest CT Findings in Patients With Influenza A (H1N1) Virus Infection and Coronavirus Disease (COVID-19). AJR Am J Roentgenol. 2020. May 26;1–7. [DOI] [PubMed] [Google Scholar]
- 38.Tanni F, Akker E, Zaman MM, Figueroa N, Tharian B, Hupart KH. Eosinopenia and COVID-19. J Am Osteopath Assoc. 2020. July 16 10.7556/jaoa.2020.091 [DOI] [PubMed] [Google Scholar]
- 39.Khoury P, Stokes K, Gadkari M, Makiya MA, Legrand F, Hu Z, et al. Glucocorticoid-induced eosinopenia in humans can be linked to early transcriptional events. Allergy. 2018;73(10):2076–9. 10.1111/all.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil H, Bouldoires B, Bailly B, Meaux Ruault N, Humbert S, Magy-Bertrand N. L’éosinopénie en 2018. La Revue de Médecine Interne. 2019. March 1;40(3):173–7. 10.1016/j.revmed.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 41.Alkhalil M, Kearney A, Hegarty M, Stewart C, Devlin P, Owens CG, et al. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting with Acute Myocardial Infarction. Am J Med. 2019;132(12):e827–34. 10.1016/j.amjmed.2019.05.021 [DOI] [PubMed] [Google Scholar]
- 42.Grattan CEH, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clinical & Experimental Allergy. 2003;33(3):337–41. 10.1046/j.1365-2222.2003.01589.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files (in particular in the S5 Table).