Abstract
Nanotechnology in medicine—nanomedicine—is extensively employed to diagnose, treat, and prevent pulmonary diseases. Over the last few years, this brave new world has made remarkable progress, offering opportunities to address historical clinical challenges in pulmonary diseases including multidrug resistance, adverse side effects of conventional therapeutic agents, novel imaging, and earlier disease detection. Nanomedicine is also being applied to tackle the new emerging infectious diseases, including severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East Respiratory Syndrome Coronavirus (MERS-CoV), influenza A virus subtype H1N1 (A/H1N1), and more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In this review we provide both a historical overview of the application of nanomedicine to respiratory diseases and more recent cutting-edge approaches such as nanoparticle-mediated combination therapies, novel double-targeted nondrug delivery system for targeting, stimuli-responsive nanoparticles, and theranostic imaging in the diagnosis and treatment of pulmonary diseases.
Keywords: Nanomedicine, Nano carrier, Respiratory disease, Lung cancer, Pneumonia, COVID- 19, Cystic fibrosis, Asthma, Tuberculosis
Introduction
Pulmonary diseases impose a significant financial and emotional burden on patients and their families [1], with predictions that an estimated 20% of all deaths globally will be respiratory-related by 2030 [2]. There is a significant global clinical unmet need to define new classes of safe and effective therapies to enhance delivery to the target organ, namely the lung [3]. Nanomedicine and the application of nanoscience to medicine offer a unique opportunity to develop unique nano-packaged therapeutics in an aerosolized format for delivery directly to the lung. This promising field opens up a whole new vista of both enhanced drug delivery and detection platforms targeting not only for historical lung diseases such as cancer, cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and asthma [4].∗ More recently, it includes, newer, emerging infectious diseases such as SARS-CoV [5], but also Middle East Respiratory Syndrome-Related Coronavirus (MERS-C) [6], influenza A/H1N1 [7], and SARS-CoV-2 [8,9].∗ Over recent years, our understanding of the mechanisms of delivery of nano-packaged payloads has become more refined not only in the therapeutic area but also in medical imaging, in the context of systemic disease detection and diagnosis [4].
Therapeutic agent delivery in infectious and noninfectious disease
In 1965, in seminal work by Banham and colleagues, it was first postulated that nanoparticles (NPs) have the capacity to act as an effective delivery system, transporting compounds across biological membranes [10].∗ Since then an expanding body of work has shown that nanoparticles have the capacity to encapsulate, conjugate, and trap a wide variety of both hydrophobic and hydrophilic compounds and biomolecules such as peptides, DNAs, and RNAs. This ability provides an excellent opportunity to develop nano-based platforms that optimize drug delivery to target organs, while minimizing systemic drug toxicities [11]. In addition, nano-based drug delivery platforms offer the opportunity to enhance drug stability and bioavailability by maximizing the half-life of the delivered nano-packaged drugs to the target organ [12]. Consequently nano-packaged therapeutics are today becoming increasingly part of our therapeutic armory in respiratory diseases such as CF [13], Mycobacterium tuberculosis (M. tb) infection [14], asthma [15], COPD [16], and lung cancer [17].∗
We are in challenging times and with the emergence of novel infectious agents, including most notably COVID-19, there is increased attention on the potential capability of nanopackaging specific humanized antibodies which target key components of both viral entry and replication within cells for vaccine development [18]. In the context of corona viruses, it has recently been shown that targeting the spike protein, which mediates viral entry into cells, with a specific nanobody in MERS-CoV resulted in protection in in-vivo models [19]. Following on from this work, Lin et al. more recently further engineered a virus-like hollow nanoparticle that was impregnated with a spike protein antigen on the surface, which was co-packaged with agonists specific for the cytosolic sensor Stimulator of interferon genes (STING), which is also a key adaptor molecule involved in anti-viral immune signaling. This combined antigen conjugation strategy and nanocarrier morphology was shown to abrogate MERS-CoV infection in an in-vivo model [6]. Over the last 5 months, there have been numerous attempts to employ nanotechnology for the detection of COVID-19, which is discussed in detail elsewhere [20,21].
Multidrug-loaded nanoparticles against drug-resistant pathogens
A number of life-threatening conditions such as M. tb infection [22], pneumonia [22], HIV [23], and CF [24] are oftentimes complicated by overarching issue of multidrug resistance. Historically, to address this issue, combination therapy, utilizing two or more drugs, has been shown to be of superior efficacy compared to monotherapy strategies [25]. Nanoparticles have the capacity to carry and deliver multiple therapeutic agents to affected organs [4]. A classic example of this is the combination of anticancer agents paclitaxel and cisplatin, which together have a synergistic enhanced anti-lung-cancer effect when dual-loaded in a nano-drug delivery system compared to free drugs at the same concentration [26]. More recently, the combination of etoposide, an anticancer drug, with PIK3CA small-interfering RNA (siPIK3CA) loaded in a peptide nanoparticle has shown a slower release profile, improved pharmacokinetics, and superior anticancer activity compared to free drugs [27]. The ability to administer enhanced concentrations of nano-packaged combination therapies has had the added benefit of allowing the clinicians decrease the frequency of administration from daily to weekly. In the context of M. tb infection, three FDA-approved drugs (rifampicin, pyrazinamide, and isoniazid) were encapsulated in a polymeric nano-drug carrier and administered weekly. Results demonstrated similar efficacy in those receiving weekly administrations compared to those receiving daily administration in this in-vivo study [28]. Oral administration of drugs, which is the main drug delivery route in M. tb infection, has a number of drawbacks including rapid hepatic first-pass metabolism, decreased intestinal drug absorption, and increased systemic exposure. These issues can be tackled through nondrug carriers directly administered to the lung via aerosol. Moreover, this targeted organ strategy requires the administration of lower doses to achieve therapeutic efficacy [4]. Ethionamide (ETH) is an antibiotic which is reserved for use as second-line anti-M. tb drug, mainly due to systemic toxicity and a short half-life. In a murine model of M. tb infection, co-encapsulated ETH and its booster (BDM41906) in a biodegradable polymeric nanoparticle were administrated directly to the lung. They showed that this nebulized treatment regime achieved a 3-log reduction in bacterial burden compared to the oral route [29]. The combination of dual-loaded and inhaled nanoparticle strategies has also achieved promising results in the treatment of chronic lung infections in CF [24]. In a recent study, the inhalation of two drugs encapsulated in a lipid-based nano-drug carrier, lumacaftor and ivacaftor, demonstrated a significant reduction in the volume of tissue destruction compared to untreated mice (Figure 1 ) [30]. These studies highlight the potential of combined therapies, nano-packaged in an inhaled or nebulized format, as highly effective treatments in chronic inflammatory pulmonary diseases.
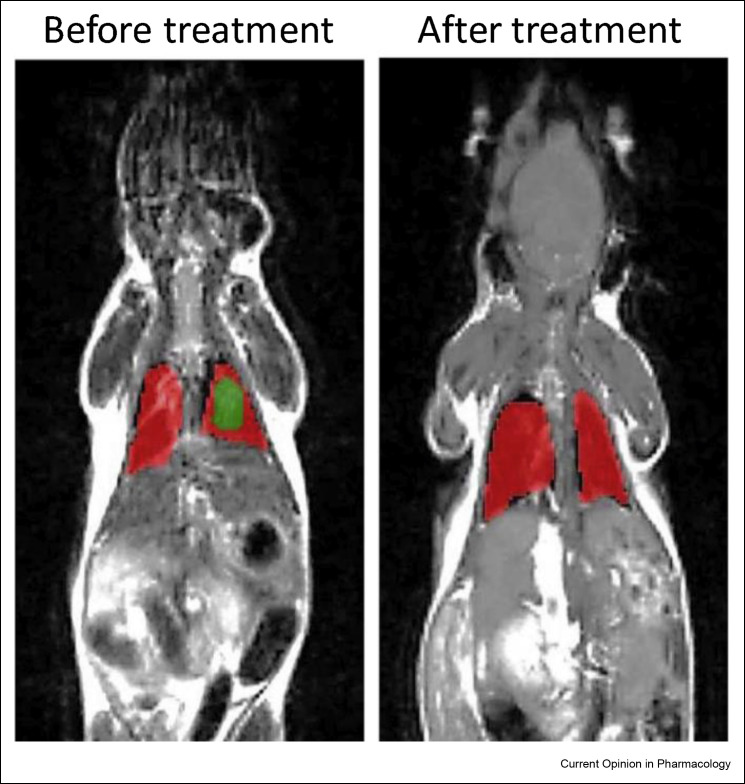
Figure 1.
Illustrative magnetic resonance images (MRI) of a transgenic mouse model with CF before and after treatment. Mice were treated twice weekly for 4 weeks with an inhaled lipid-based nano-drug delivery system carrying lumacaftor and ivacaftor. Healthy lung tissues are labeled in red, while fibrotic injured tissues are labeled in green. Reproduced with permission from Refs. [30].
Smart drug delivery vehicles: targeted nanoparticles and stimuli-responsive nanoparticles
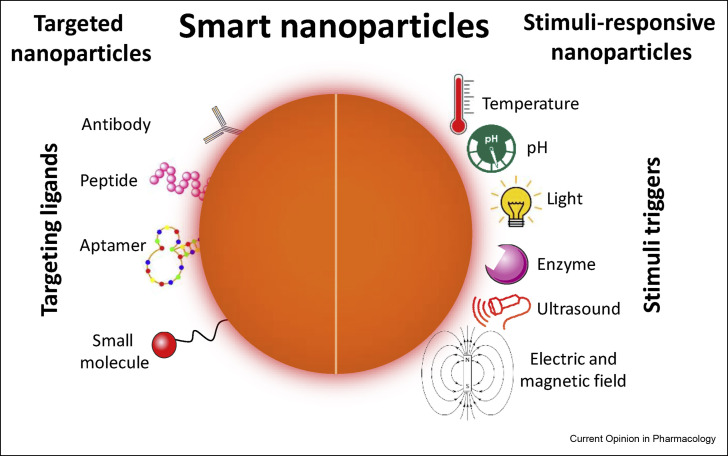
Over the past few years, nanomedicine has evolved from the initial work with single agents with basic nano-drug delivery systems to the so called “smart nano-drug delivery systems” which offer the potential to target specific organs, tumors, or infected sites with therapeutic agents that are efficacious and associated with less associated systemic adverse side effects. Smart drug carrier vehicles are able to deliver medications to a patient in a manner that increases the concentration of therapeutic agents in a specified target site. This has opened a completely new paradigm, with its potential to tackle and overcome historical challenges associated with the more classical therapies and modes of administration. These would include accelerated drug clearance, nonspecific biodistribution, and uncontrolled release of drugs [24]. This field is currently focused on two main areas, targeted nanocarriers and stimuli-responsive nanocarriers. Targeted nanocarriers are surface-coated by specific ligands, for example, antibodies, peptides, and aptamers, which have specific and high affinity to targets on the surface of specific cell types. Stimuli-responsive nanocarriers release their payload in a well-controlled, sustained manner in response to specific stimuli such as pH and ultrasound (Figure 2 ) [4].
Figure 2.
Schematic illustration of smart nano-drug delivery systems.
Smart nanocarriers are fabricated to deliver therapeutic agents precisely to specific organs of interest. This results in both enhanced treatment efficiency and reduced side effects. A targeted nano-drug delivery system involves adding targeting ligands such as antibodies, peptides, small molecules, and aptamers to the surface of the nano-vehicle. This then allows accurate and precise drug delivery to disease effected cells/tissue. Stimuli-responsive nanocarriers have led to the concept of “smart repackaging” where in response to internal or external stimuli (i.e., temperature, pH, light, enzyme activity, ultrasound, electric and magnetic fields) a controlled release of the therapeutic payload occurs at targeted sites.
Another application developed from the use of advanced smart nanocarriers is theranostics which uses nanoscience strategies to combine diagnostic and therapeutic in a single agent to a targeted disease organ. It represents a classical example of precision medicine facilitating diagnosis, drug delivery, and assessment of response to delivered therapies. Theranostics is extremely relevant to respiratory medicine, with frequent application of theranostics in lung cancer [31, 32, ∗33]. Historically, it is well known that ruthenium has anticancer activity. However, the use of ruthenium as a therapeutic agent has been limited due to significant issues regarding its toxicity profile when administered systemically. To address this issue, a targeted theranostic nano-drug delivery system loaded with ruthenium(II) complex was engineered utilizing an enhanced green fluorescent protein and a specific fusion protein for targeting non-small cell lung carcinoma cells. Real-time fluorescence monitoring revealed a significant accumulation of the nanoparticles in the tumor upon imaging, which was associated with a twofold enhancement in tumor growth inhibition in an in-vivo non-small cell lung cancer model (Figure 3 ) [31]. In another example, three effective strategies in a murine in-vivo model, namely, theranostics, targeted drug delivery, and combination therapy, were employed by encapsulating two anticancer drugs, doxorubicin and vinorelbine, in two peptide-targeted liposomal drug delivery systems which were decorated by superparamagnetic iron oxide nanoparticles as a theranostic agent. MRI analysis showed significant tumor penetration in mice treated with targeted nanoparticles, leading to an improved median overall survival of 40% compared to untargeted nanoparticles [34].∗∗
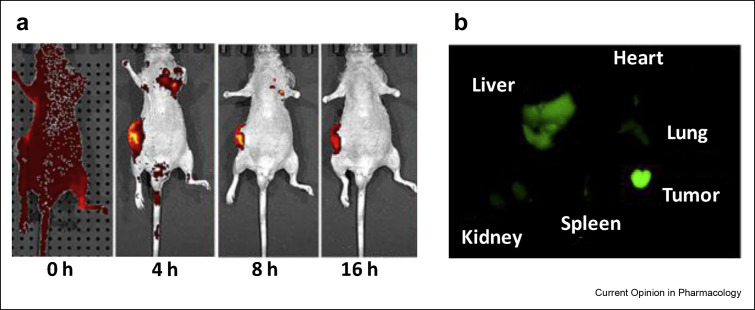
Figure 3.
The biodistribution of a targeted nanoformulation against established subcutaneous H1299 tumor, a human non-small cell lung carcinoma cell in nu/nu mice (a) The biodistribution of targeted nano-drug delivery system from nonspecific distribution in 0 h time point to fully accumulated in the tumor in 16 h time points (b) The fluorescent image demonstrated a significant delivery of the nanoparticles in tumor tissue (green region). Reproduced with permission from Ref. [31].
Smart nanoparticles can be designed to deliver therapeutic agents to targeted intracellular organelles [35]. This advanced approach would allow more efficient drug accumulation in the targeted site [36], precision medicine, and the ability to overcome drug resistance [37]. For example, a mitochondrial drug targeting strategy has shown promising results to overcome multidrug resistance in cancers, including lung cancer, through blocking the overexpression of the adenosine triphosphate (ATP)-driven drug efflux pumps [38]. Supporting this strategy, recent in-vivo work, where taxol, also known as paclitaxel (PTX), encapsulated in a polymeric nanocarrier with a mitochondria-targeting triphenylphosphine (TPP) head group (TPH/PTX), demonstrated a significant inhibition in tumor growth in mice receiving targeted nanoparticles compared to both those receiving untargeted nanoparticles and free drug (Taxol) in a breast cancer lung metastasis mouse model (Figure 4 ) [39].
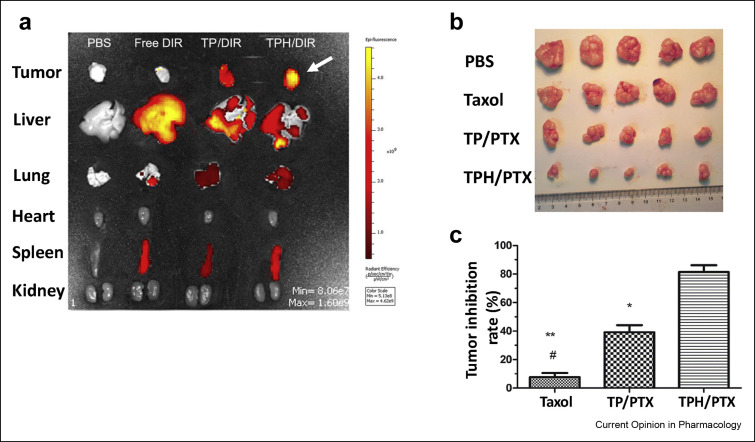
Figure 4.
The smart nanoparticle delivery system targeting intracellular organelle (mitochondria) achieved tumor penetration and inhibition effectively in a drug-resistant breast cancer-bearing mouse model with lung metastasis (a) Ex-vivo fluorescence images showing the distribution of drugs in the tumors and organs isolated from metastatic breast cancer in the lungs. Maximum tumor penetration was achieved by using mitochondria-targeted nanoparticles (white arrow), followed by untargeted nanoparticles, whereas, the free drug accumulated mostly in the liver and spleen (b) Tumor images following untreated (control), free drug (taxol), untargeted nano-drug delivery system (TP/PTX) and targeted nanoparticles (TPH/PTX) treatment arms (c) Tumor growth inhibition after intravenous injection with the three different treatment protocols [39].
More recently, smart nanoparticles have been developed to achieve the controlled release of payloads from the carrier at the selected target (Figure 2). Due to the disordered metabolic profile of some pulmonary diseases, including lung cancer [40], and acute lung injury [41], they exhibit a unique characteristic feature namely, an enhanced acidic (low pH) environment. Numerous recent studies have taken advantage of this distinctive characteristic by designing a wide range of pH-responsive drug delivery systems with the aim of improving drug delivery and therapeutic efficacy [42, 43, 44].
Nanotechnology provides the opportunity to combine different strategies in a single nanoplatform. In an early example, pulmonary aerosol delivery, targeted ligands, and pH stimuli-responsive approaches were applied into a nano-drug delivery system carrying anti-intercellular adhesion molecule-1 (anti-ICAM-1), which was used in the treatment of acute lung injury. The combination of these three different strategies resulted in significant drug delivery to the targeted endothelial cells, with significant decrease in the inflammatory response, supporting this novel smart nanoparticle strategy as a potential anti-inflammatory therapy [43].
Nanoparticles can be also designed in order to respond to multistimuli triggers, thus allowing more refined dosage control through the tailored spatial and temporal drug release [45,46]. In the cancer microenvironment, intracellular glutathione and spermine are overexpressed in numerous cancers, including lung cancer. Recently, Cheng et al. have used pillararene-based polymer as a nanocarrier which has the distinctive property of undergoing changes to its shape and physical property when exposed to high glutathione and spermine concentrations. Using this nanocarrier with paclitaxel as the payload, they demonstrated efficient drug release and accumulation of paclitaxel in neoplastic cells in an in-vitro model of lung cancer [45].
Detection and diagnosis
Early detection of disease gives clinicians the best opportunity to control and cure disease. Rapid methods, simple processes, and accurate tools are critical to the success of an effective diagnostic strategy. Nano-based biosensors are being utilized across a wide range of fields for early detection and diagnosis of disease [47,48]. This method has been employed for rapid diagnosis of various pulmonary diseases, such as lung cancer [49], asthma [50], and bacterial lung infections [51]. In the current COVID-19 pandemic, a key part of the global strategy is detailed contact tracing, which relies on diagnostic assays that are rapid, accurate, and scalable to cope ultimately with nationwide in-depth serial diagnostic screening. Nanoscience and the application of nanotechnological solutions are playing a leading role in this important global role [20,52, ∗53, 54]. An example of this technology at work is a nanobiosensor which was developed by fabricating graphene-based nanosheets. They provided a highly conductive surface which was then coated with a specific antibody against the SARS-CoV-2 spike protein. The detection was based on changes in the electrical current across the graphene sheets which are generated when the trigger, the spike protein, binds to the antibodies. The nanobiosensor analyzed nasopharyngeal swab specimens from COVID-19 patients and showed a high sensitivity to the spike protein at concentrations of 100 fg/mL in clinical transport medium [54].
Conclusion
Nanotechnology applied to pulmonary diseases represents a new vista with regards to its potential application in the diagnosis, treatment, and clinical staging of disease. In this review we have specifically highlighted the ability of the technology to address multidrug resistance, improve targeted organ treatment efficiency, limit systemic adverse effects, and contribute to the earlier diagnosis of lung diseases. Nanomedicine is currently being employed in clinical practice, particularly in lung cancer, and will shortly be an important part of the clinician's armoury in addressing both acute and chronic respiratory disease.
Sir William Olser stated, “The future is today”—which very much applies to the harnessing of nanomedicine and its application to pulmonary medicine. The old adage “Old ways won't open new doors”—nanomedicine is the new way, and there is now a growing body of work which suggests that nano-therapeutics as applied to the lung and other organs will “open new doors” and change the way we practice medicine in the near future.
Credit author statement
Mohammad Doroudian: Conceptulaization, Writing – original draft preparation, Reviewing and Editing. Andrew O’ Neill: Writing, Reviewing and Editing. Ronan Mac Loughlin: Writing, Reviewing and Editing. Adriele Prina-Mello: Writing, Reviewing and Editing. Yuri Volkov: Writing, Reviewing and Editing. Seamas C. Donnelly: Conceptulaization, Writing – original draft preparation, Writing, Reviewing and Editing.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
SCD, AO’N, MD acknowledge the support of Science Foundation Ireland (SFI, The Health Research Board (HRB) of Ireland, Aerogen Ltd, The Laboratory for Biological Characterization of Advanced Materials (LBCAM(and the Irish Lung Foundation.
A.P.-M. would like to thank the partial support from the EC under the H2020 project grant agreement No 760928.
This review comes from a themed issue on Pulmonary
Edited by Paola Rogliani, Mario Cazzola, Luigino Calzetta and Maria Gabriella Matera
References
- 1.Li X., et al. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020;368 doi: 10.1136/bmj.m234. m234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The burden of lung disease: the European Lung white book. 2011. https://www.erswhitebook.org/chapters/the-burden-of-lung-disease/ [cited 2020; Available from. [Google Scholar]
- 3.Barnes P.J., et al. Barriers to new drug development in respiratory disease. Eur Respir J. 2015;45:1197–1207. doi: 10.1183/09031936.00007915. [DOI] [PubMed] [Google Scholar]
- Doroudian M., et al. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74:965. doi: 10.1136/thoraxjnl-2019-213037. [DOI] [PubMed] [Google Scholar]; Comprehensive review discussing recent progress and development in clinical applications of nanotechnology in respiratory diseases. This paper covers a wide range of attempts in clinical trials employing nanotechnology for theraputic agent delivery, vaccination, and detection for the treatment of lung diseases.
- 5.Liu Y.V., et al. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29:6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L.C.W., et al. Viromimetic STING agonist-loaded hollow polymeric nanoparticles for safe and effective vaccination against Middle East respiratory syndrome coronavirus. Adv Funct Mater. 2019;29:1807616. doi: 10.1002/adfm.201807616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., et al. Double-layered m2e-NA protein nanoparticle immunization induces broad cross-protection against different influenza viruses in mice. Advanced healthcare materials. 2020;9:1901176. doi: 10.1002/adhm.201901176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talebian S., et al. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 9.Campos E.V.R., et al. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnol. 2020;18 doi: 10.1186/s12951-020-00685-4. 125-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham A.D., Standish M.M., Watkins J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13 doi: 10.1016/s0022-2836(65)80093-6. 238-IN27. [DOI] [PubMed] [Google Scholar]; The very first report demonstrating the potential of nanoparticles for drug delivery across biological membranes.
- 11.Pontes J.F., Grenha A. Multifunctional nanocarriers for lung drug delivery. Nanomaterials. 2020;10:183. doi: 10.3390/nano10020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur P., et al. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J Drug Deliv Sci Technol. 2020;56:101502. [Google Scholar]
- 13.Velino C., et al. Nanomedicine approaches for the pulmonary treatment of cystic fibrosis. Frontiers in Bioengineering Biotechnology. 2019;7 doi: 10.3389/fbioe.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A.K., Singh A., Singh S. In: Diagnosis of tuberculosis: Nanodiagnostics approaches, in NanoBioMedicine. Saxena S.K., Khurana S.M.P., editors. Springer Singapore; Singapore: 2020. pp. 261–283. [Google Scholar]
- 15.Dhayanandamoorthy Y., et al. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: a promising asthma control strategy. Int J Pharm. 2020;591:119958. doi: 10.1016/j.ijpharm.2020.119958. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Liu H., Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J Nanobiotechnol. 2020;18:145. doi: 10.1186/s12951-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W., et al. Nanomedicine in lung cancer: current states of overcoming drug resistance and improving cancer immunotherapy. WIREs Nanomedicine and Nanobiotechnology. 2020:e1654. doi: 10.1002/wnan.1654. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 18.Shin M.D., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhao G., et al. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J Virol. 2018;92 doi: 10.1128/JVI.00837-18. e00837-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan B., et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano. 2020;14:12125–12132. doi: 10.1021/acsnano.0c05657. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava M., et al. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci Total Environ. 2021;754:142363. doi: 10.1016/j.scitotenv.2020.142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabi B., et al. Nano-based anti-tubercular drug delivery: an emerging paradigm for improved therapeutic intervention. Drug Delivery and Translational Research. 2020;10:1111–1121. doi: 10.1007/s13346-020-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surve D.H., Jindal A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J Contr Release. 2020;324:379–404. doi: 10.1016/j.jconrel.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Majumder J., Minko T. Targeted Nanotherapeutics for respiratory diseases: cancer, fibrosis, and coronavirus. Advanced Therapeutics. 2020:2000203. doi: 10.1002/adtp.202000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worthington R.J., Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013;31:177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu R., et al. Combination chemotherapy of lung cancer - Co-delivery of docetaxel prodrug and Cisplatin using aptamer-decorated lipid-polymer hybrid nanoparticles. Drug Des Dev Ther. 2020;14:2249–2261. doi: 10.2147/DDDT.S246574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H.-L., Lin W.J. Dual peptide-modified nanoparticles improve combination chemotherapy of Etoposide and siPIK3CA against drug-resistant small cell lung carcinoma. Pharmaceutics. 2020;12:254. doi: 10.3390/pharmaceutics12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poh W., et al. Active pulmonary targeting against tuberculosis (TB) via triple-encapsulation of Q203, bedaquiline and superparamagnetic iron oxides (SPIOs) in nanoparticle aggregates. Drug Deliv. 2019;26:1039–1048. doi: 10.1080/10717544.2019.1676841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa-Gouveia J., et al. Combination therapy for tuberculosis treatment: pulmonary administration of ethionamide and booster co-loaded nanoparticles. Sci Rep. 2017;7:5390. doi: 10.1038/s41598-017-05453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garbuzenko O.B., et al. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J Contr Release. 2019;296:225–231. doi: 10.1016/j.jconrel.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F., et al. Bioreducible and traceable Ru(III) prodrug-loaded mesoporous silica nanoparticles for sequentially targeted nonsmall cell lung cancer chemotherapy. Applied Materials Today. 2020;19:100558. [Google Scholar]
- 32.Duman F.D., et al. Bypassing pro-survival and resistance mechanisms of autophagy in EGFR-positive lung cancer cells by targeted delivery of 5FU using theranostic Ag2S quantum dots. J Mater Chem B. 2019;7:7363–7376. doi: 10.1039/c9tb01602c. [DOI] [PubMed] [Google Scholar]
- Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–4588. doi: 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]; An illustartive review of theranostic agents to overcome common challenges in lung cancer diagnosis and imaging.
- Chi Y.-H., et al. Lung cancer-targeting peptides with multi-subtype indication for combinational drug delivery and molecular imaging. Theranostics. 2017;7:1612–1632. doi: 10.7150/thno.17573. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides an interesting example of nanoparticles ability to perform different strategies simultaneously.
- 35.Liu C.-G., et al. Subcellular performance of nanoparticles in cancer therapy. Int J Nanomed. 2020;15:675–704. doi: 10.2147/IJN.S226186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nag O.K., Delehanty J.B. Active cellular and subcellular targeting of nanoparticles for drug delivery. Pharmaceutics. 2019;11:543. doi: 10.3390/pharmaceutics11100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Z., et al. Nanoparticle delivery systems with cell-specific targeting for pulmonary diseases. Am J Respir Cell Mol Biol. 2020 doi: 10.1165/rcmb.2020-0306TR. [ja)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H., et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat Commun. 2018;9:562. doi: 10.1038/s41467-018-02915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H., et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J Nanobiotechnol. 2020;18:8. doi: 10.1186/s12951-019-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindeman L.R., et al. Differentiating lung cancer and infection based on measurements of extracellular pH with acidoCEST MRI. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-49514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton G.M. A calculated response: control of inflammation by the innate immune system. J Clin Invest. 2008;118:413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Men W., et al. Layer-by-layer pH-sensitive nanoparticles for drug delivery and controlled release with improved therapeutic efficacy in vivo. Drug Deliv. 2020;27:180–190. doi: 10.1080/10717544.2019.1709922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C.Y., et al. pH-responsive nanoparticles targeted to lungs for improved therapy of acute lung inflammation/injury. ACS Appl Mater Interfaces. 2019;11:16380–16390. doi: 10.1021/acsami.9b04051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi M., et al. Stimuli-responsive release and efficient siRNA delivery in non-small cell lung cancer by a poly(l-histidine)-based multifunctional nanoplatform. J Mater Chem B. 2020;8:1616–1628. doi: 10.1039/c9tb02764e. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Q., et al. Dual stimuli-responsive bispillar[5]arene-based nanoparticles for precisely selective drug delivery in cancer cells. Chem Commun. 2019;55:2340–2343. doi: 10.1039/c8cc09432b. [DOI] [PubMed] [Google Scholar]
- 46.Shi H., et al. Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. J Mater Chem B. 2020;8:332–342. doi: 10.1039/c9tb02055a. [DOI] [PubMed] [Google Scholar]
- 47.Kim S.-J., et al. Innovative nanosensor for disease diagnosis. Acc Chem Res. 2017;50:1587–1596. doi: 10.1021/acs.accounts.7b00047. [DOI] [PubMed] [Google Scholar]
- 48.Chan L.W., et al. Engineering synthetic breath biomarkers for respiratory disease. Nat Nanotechnol. 2020;15:792–800. doi: 10.1038/s41565-020-0723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodman C., et al. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Canc Biol. 2020 doi: 10.1016/j.semcancer.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X., et al. Nanoscale fluorescent metal–organic framework composites as a logic platform for potential diagnosis of asthma. Biosens Bioelectron. 2019;130:65–72. doi: 10.1016/j.bios.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Buss C.G., et al. Protease activity sensors noninvasively classify bacterial infections and antibiotic responses. EBioMedicine. 2018;38:248–256. doi: 10.1016/j.ebiom.2018.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu G., et al. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Zhu X., et al. medRxiv; 2020. Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19; p. 2020. 03.17.20037796. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides practical information to fabricate a rapid (60 min) and precise (≈97%) nanoparticles-based biosensor for diagnosis of COVID-19. This method only requires “one-step” and a “single-tube” reaction, which works based on transcription loop-mediated isothermal amplification (RT-LAMP) coupled with a nanoparticle-based biosensor (NBS) assay (RT-LAMP-NBS).
- 54.Seo G., et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]