Abstract
Aims
To characterize the distribution and severity of sensory neuropathy using a portable quantitative sensory testing (QST) device in diabetic patients (DM) hospitalized with severe COVID-19 infection.
Methods
Four patients with diabetes and severe SARS-CoV-2 requiring non-invasive ventilation for a protracted duration underwent clinical, laboratory and radiologic assessment and detailed evaluation of neuropathic symptoms, neurological assessment, QST on the dorsum of the foot and face using NerveCheck Master with assessment of taste and smell.
Results
All four subjects developed neuropathic symptoms characterized by numbness in the feet with preserved reflexes. QST confirmed symmetrical abnormality of vibration and thermal thresholds in both lower limbs in all patients and an abnormal heat pain threshold on the face of two patients and altered taste and smell.
Conclusions
Severe COVID-19 infection with hypoxemia is associated with neuropathic symptoms and widespread sensory dysfunction in patients with DM.
Keywords: COVID-19, Sensory neuropathy, NerveCheck, Quantitative sensory testing, Diagnostic device, Diabetes
1. Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020. The clinical manifestations of COVID-19 include fever, difficulty breathing, diarrhea, muscle pain, fatigue and loss of smell and/or taste. Neuronal involvement of SARS‐CoV2 has been highlighted with reports of altered taste, smell and hearing as well as neuropathic pain in patients with COVID-19 pneumonia [1], [2], [3]. In a case series of 214 patients, 36% had neurological symptoms which were associated with more severe illness and higher mortality [1]. A wide spectrum of neurological complications have been described and include acute cerebrovascular disease, impairment of consciousness, meningitis and encephalopathy, ataxia, seizures, skeletal muscle injury and neuropathic pain [4], [5] as well as Guillain-Barre syndrome [6].
The propensity for corona viruses (Co-Vs) to affect neurons is established for SARS-CoV1 and MERS‐CoV [7]. Transgenic mice have shown that MERS‐CoV [7] when given intranasally enter the brain via the olfactory nerves and rapidly spread to the thalamus and brainstem. There is also evidence that Co-Vs may first invade peripheral nerve terminals and gain access to the CNS via a synapse‐connected route [8], [9]. Viral antigens have been detected in the brainstem in the nucleus of the solitary tract and nucleus ambiguous which receive sensory information from the mechanoreceptors and chemoreceptors and send efferent fibres to airway smooth muscle and glands in the lung and respiratory tract [10], [11], which could lead to cardiorespiratory dysfunction and death. Diabetic patients with microvascular complications have been shown to develop more serious complications from infection with COVID-19 and may be predisposed to the development or worsening of neuropathy. In the recent CORONADO study, the presence of microvascular complications was independently associated with death within 7 days of admission with severe COVID-19 [12].
Diabetic polyneuropathy (DPN) affects over 50% of the diabetic population [13], [14] and affects large and small nerve fibres [32], [14], [21], [15]. Quantitative sensory testing (QST) allows quantitative evaluation of vibration, cold, warm and heat pain perception thresholds. We have previously used a simple quantitative sensory testing device called NerveCheck Master to quantify small and large nerve fibre dysfunction in patients with diabetic neuropathy [17], [16].
In this preliminary report we describe the detailed neurological presentation and undertake quantitative sensory testing in the feet and face and evaluate taste and smell in 4 patients with diabetes who developed severe COVID-19 disease.
2. Patients and methods
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Barcelona Hospital. 35 patients were admitted and managed at the Barcelona Hospital SCIAS, Barcelona. They underwent testing with a throat swab (AllPlex 2019-nCoV Assay. Seegene) and had confirmed COVID 19 based on reverse transcription polymerase chain reaction identification of the RNA SARS-CoV-2, 3 specific regions, E gene, RdRP gene and N gene. Three subjects with type 2 diabetes and one subject had type 1 diabetes underwent laboratory testing and radiologic assessment according to their clinical care needs. Subjects with communication disorders, cognitive deficits or history of pain conditions or neurological disorders before the development of COVID-19 were excluded. These patients had no prior diagnosis of diabetic neuropathy in their medical records. Informed consent was obtained from all patients. Symptoms were assessed using the short form McGill pain questionnaire. Cranial nerve involvement was assessed by evaluating the oropharyngeal reflex and for the Vernet phenomenon. Taste was evaluated using sweet (0.3 M sucrose), salty (0.15 M NaCl) and neutral (mineral water) solutions. The olfactory capacity was assessed to the aroma of coffee, perfume and acetone (C3H6O). Quantitative sensory testing was performed using the NerveCheck Master device incorporated into protective equipment (Fig. 1 ). Heat-as-pain perception threshold according to the method of limits, cold detection threshold (CDT), vibration (VPT), warm (WPT) according to the JND (levels method) in a quiet environment whilst the patient was attentive and cooperative. The room temperature ranged between 18 and 28 °C and the temperature of the skin was between 34 and 36 °C. The starting (adaptation) temperature was 32 °C and to prevent thermal injury, the highest temperature limit was set at 49 °C and lowest temperature limit was set at 15 °C. VPT was assessed at the 1st metatarsal and thermal thresholds were assessed on the dorsal area of the feet and lateral aspect of the face [17], [16].
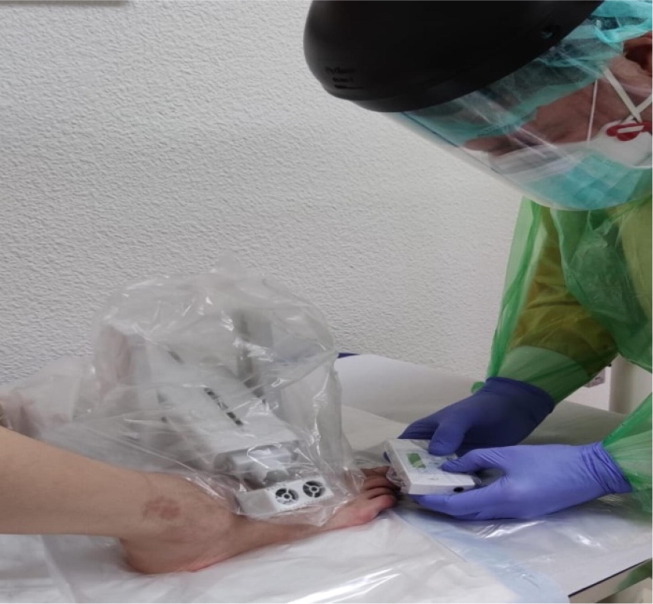
Fig. 1.
Patient undergoing quantitative sensory testing using the NerveCheck Master device incorporated into protective equipment on the foot.
3. Results
Three patients with type 2 diabetes and 1 patient with type 1 diabetes developed neuropathic symptoms and were referred to neurology for further evaluation. The clinical, laboratory, COVID-19 related therapy and detailed neurological examination and QST findings in these 4 patients are presented in Table1, Table 2 . Neurological symptoms developed in each patient approximately 1 to 3 weeks after the onset of COVID-19.
Table1.
Clinical and laboratory data, therapy and neurologic manifestations.
| PATIENTS | ||||
|---|---|---|---|---|
| Gender/age/diabetes duration/type of diabetes | PATIENT 1 Male/57/6/T2DM |
PATIENT 2 Male/58/37/T1DM |
PATIENT 3 FEMALE/73/5/T2DM |
PATIENT 4 FEMALE/73/10/T2DM |
| SYMPTOMS | dyspnoea / asthenia | fever/ nausea/ cough/ dyspnoea | diarrhoea/ abdominal pain/ dyspnoea/ fever | fever/ cough/ dyspnoea |
| CONSOLIDATION ON CHEST X-RAY | + | + | + | + |
| SARS-COV-2-Rt-PCR | + (throat) | + (throat) | + (throat) | + (throat) |
| INTUBATION for O2 | + | + | + | + |
| HYDROXYCHLOROQUINE | + | + | + | + |
| AZITHROMYCIN | + | + | + | + |
| RITONABIR | + | + | – | + |
| LOPINAVIR | + | + | – | + |
| METHYLPREDNISOLONE | + | + | + | + |
| BEMIPARINA | + | + | + | + |
| NEUROLOGIC SYMPTOMS | + | + | + | + |
| LABORATORY STUDY: | ||||
| Haemoglobin | 14.2 g/dL | 12.2 g/dL | 11.9 g/dL | 17.7 g/dL |
| Leucocytes | 6.6 × 10E9/L | 6.8 × 10E9/L | 9.7 × 10E9/L | 6.7 × 10E9/L |
| Lymphocytes | 500 × 10E9/L | 600 × 10E9/L | 700 × 10E9/L | 700 × 10E9/L |
| Platelets | 48 × 10E9/L | 94 × 10E9/L | 176 × 10E9/L | 211 × 10E9 |
| D-dimer | 377 ng/mL | 535–6739 ng/mL | 951 ng/mL | 710–955 ng/mL |
| Fibrinogen | 7.9 g/L | 8.8 g/L | 7.7 g/L | 7.9 g/L |
| Random glucose | 8.9 mmol/L | 10.4 mmol/L | 9.9 mmol/L | 9.6 mmol/L |
| *HbA1c | 8.5% | 7.9% | 7.6% | 8.2% |
| Procalcitonin | 0.18 ng/mL | ND | ND | 0.11 ng/mL |
| CRP | 0 mg/L | 171 mg/L | 4 mg/L | 97 mg/L |
| AST | 41 IU/L | 35 IU/L | 16 IU/L | 35 IU/L |
| ALT | 30 IU/L | 25 IU/L | 12 IU/L | 23 IU/L |
| Urea | 5.8 mmol/L | 11.5 mmol/L | 8.8 mmol/L | 17.1 mmol/L |
| Creatinine | 78 mmol/L | 91 mmol/L | 122 mmol/L | 198 mmol/L |
| Sodium | 132 mmol/L | 136 mmol/L | 128 mmol/L | 136 mmol/L |
| Potassium | 4.3 mmol/L | 3.9 mmol/L | 4.0 mmol/L | 3.7 mmol/L |
| Ferritin | 1744 ng/ml | 735 ng/ml | 235 ng/ml | 930 ng/ml |
| Nt proBNP | ND | ND | ND | 3076 pg/ml |
| Troponin I | 31.2 pg/ml | 28.3 pg/ml | 38.8 pg/ml | 74.7 pg/ml |
| Interleukin-6 | ND | 111.2 pg/mL | 48.6 pg/ml | ND |
| Gamma Glutamyl Transferase | 59 IU/L | 35 IU/L | 25 IU/L | 47 IU/L |
| Alkaline Phosphatase | ND | 46 IU/L | 124 IU/L | ND |
| Lactate Dehydrogenase | 220 IU/L | 319 IU/L | 200 IU/L | 276 IU/L |
| Creatine Phosphokinase | ND | 55 IU/L | 17 IU/L | ND |
ND: Not done, Present: +, CRP: C-reactive protein, ALT: Alanine Transaminase, AST: Aspartate Aminotransferase.
HbA1c prior to hospitalization.
Table 2.
Neurologic tests and QST results.
| PATIENT 1 | PATIENT 2 | PATIENT 3 | PATIENT 4 | ||
|---|---|---|---|---|---|
| McGill Pain Questionnaire | Numbness | + | + | + | + |
| Pain | – | + | – | – | |
| Left leg | |||||
| Deep Tendon Reflexes | Patellar | + | + | + | + |
| Achilles | + | – | + | – | |
| Right leg | |||||
| Patellar | + | + | + | + | |
| Achilles | + | + | + | – | |
| Taste | Sweet | – | + | + | – |
| Salt | – | + | + | + | |
| Smell | Perfume | + | – | – | + |
| Coffee Acetone |
+ + |
– – |
– – |
– + |
|
| QST | Left foot | ||||
| VPT= (N 8-12) (ABN 7-0) | 3 ABN | 0 ABN | 0 ABN | 1 ABN | |
| CPT= (N 3-6) (ABN 0-2) | 5 N | 0 ABN | 0 ABN | 6 N | |
| WPT= (N 3-6) (ABN 0-2) | 3 N | 0 ABN | 0 ABN | 0 ABN | |
| HPT (1 °C/sec) | ABN HIGH | ABN HIGH | ABN HIGH | ABN HIGH | |
| Hyperalgesia 32–35.9 °C | |||||
| Normal 36–45.9 °C | |||||
| Normal Moderate 46–46.9 °C | |||||
| Normal Borderline 47–48.9 °C | |||||
| Abnormal High 49–49.7 °C | |||||
| Right foot | |||||
| VPT=(N 8-12) (ABN 7-0) | 0 ABN | 1 ABN | 0 ABN | 3 ABN | |
| CPT=(N 3-6) (ABN 0-2) | 6 N | 0 ABN | 0 ABN | 2 ABN | |
| WPT= (N 3-6) (ABN 0-2) | 0 ABN | 3 N | 0 ABN | 0 ABN | |
| HPT (1 °C/sec) | ABN HIGH | ABN | ABN HIGH | ABN HIGH | |
| Hyperalgesia 32–35.9 °C | |||||
| Normal 36–45.9 °C | |||||
| Normal Moderate 46–46.9 °C | |||||
| Abnormal 47–48.9 °C | |||||
| Abnormal High 49–49.7 °C | |||||
| QST Face |
HPT (1 °C/sec) | NORMAL | ABN | NORMAL | ABN HIGH |
| Hyperalgesia 32–35.9 °C | |||||
| Normal 36–45.9 °C | |||||
| Normal Moderate 46–46.9 °C | |||||
| Abnormal 47–48.9 °C | |||||
| Abnormal High 49–49.7 °C | |||||
| Oropharyngeal Reflex Vernet phenomenon |
– + |
– + |
+ + |
+ + |
QST: Quantitative sensory testing, VPT: Vibration Perception Threshold, CPT: Cold Perception Threshold, WPT: Warm Perception Threshold, HPT: Heat Perception Threshold, ABN: Abnormal, N: Normal, Present: +, Absent.
3.1. Patient 1
A 57-year-old male with T2DM and psoriatic arthropathy was admitted to the hospital on 28th March 2020 with dyspnoea and asthenia over 4 days. He was diagnosed with COVID-19 pneumonia and treated with Lopinavir-Ritonavir, hydroxychloroquine, ceftriaxone, azithromycin, tocilizumab, prednisone, bemiparin and low-flow oxygen. He deteriorated clinically and radiologically and was transferred to the ICU between the 5th to 7th April and again between the 9th and 13th of April for non-invasive mechanical ventilation (NIMV) with positive end expiratory pressure (PEEP). On 14th April thoracic CT-angiography showed the presence of right lower lobar artery pulmonary thromboembolism and upper lobe ground glass opacities with a focus of “crazy-paving”. With further deterioration he was transferred to the ICU on 19th April for non-invasive mechanical ventilation and PEEP. On 26th April he recovered to return to the medical ward and 45 days after admission he was discharged from hospital to home (Table 1).
The patient reported numbness but no pain in the feet. The patellar and Achilles deep tendon reflexes were normal with a bilateral flexor plantar response. On the feet, VPT and HPT were abnormal and CPT was normal. On the face HPT was normal. Taste was absent for sweet and salt, smell was present for acetone, perfume and coffee. There was no evidence of the Vernet phenomenon and the oropharyngeal reflex was absent (Table 2).
3.2. Patient 2
A 68-year-old male with T1DM developed fever, nausea and a cough with dyspnoea and weakness on March 26th, 2020. On April 9th (day 14) he was admitted to hospital with hypoxemia, diagnosed with COVID-19 pneumonia and treated with Lopinavir-Ritonavir, hydroxychloroquine, ceftriaxone, azithromycin and on day 3 of admission, tocilizumab. On April 13th (day 18) he was admitted to the ICU to receive NIMV (CPAP) and steroids. After 6 days in ICU, he returned to the ward and was discharged from hospital to home on May 8th (day 43). Serial blood tests showed an IL-6 increased from 111.20 pg/mL to 424 pg/ml on day 16 with an increase in the D-dimer from 535 ng/mL on admission to 6739 ng/mL on day 19 (Table 1).
The patient reported numbness and pain in both feet. The patellar and Achilles deep tendon reflexes were normal except for an absent left Achilles reflex, with a bilateral flexor plantar response. On the feet, VPT and CPT were symmetrically abnormal, whilst WPT and HPT were abnormal on the left and normal on the right. On the face HPT was abnormal. Taste was present for sweet and salt, smell was absent for acetone, perfume and coffee. There was no evidence of the Vernet phenomenon and the oropharyngeal reflex was absent (Table 2).
3.3. Patient 3
A 73-year-old female with T2DM, hypertension, atrial fibrillation developed diarrhoea with diffuse abdominal pain and dyspnoea and had a marked elevation of D-dimer (951 ng/mL) on April 20th, 2020 (day1). She was treated with prednisolone, antibiotics and oxygen. CT imaging showed evidence of enteritis and bilateral basal pulmonary infiltrates and she developed an itchy rash attributed to COVID-19. She did not require respiratory support and was discharged home on May 9th (day 18) (Table 1).
The patient reported numbness but no pain in the feet. The patellar and Achilles deep tendon reflexes were normal, with a bilateral flexor plantar response. On the feet, VPT, CPT, WPT and HPT were symmetrically abnormal. On the face HPT was normal. Taste was present for sweet and salt, smell was absent for acetone, perfume and coffee. There was no evidence of the Vernet phenomenon and the oropharyngeal reflex was normal (Table 2).
3.4. Patient 4
A 73 year old male with T2DM, hypertension, ischemic heart disease with atrial fibrillation and preserved ejection fraction developed fever, cough and respiratory difficulty on March 20th, 2020. On hospital admission (day 5) he had multi-lobar pneumonia with mild respiratory failure and moderate renal impairment. CT thorax showed pulmonary fibrosis and COVID-19 multi-lobar pneumonia. He was treated with Lopinavir, hydroxychloroquine, ceftriaxone and corticosteroids. The respiratory failure worsened and his D dimer was 955 ng/ml. Tocilizumab and bolus corticosteroids (250 mg) were given with respiratory support (Opti flow −50L). On day 15 he was transferred to the critical care unit and NIV was initiated. Pulmonary function improved, renal function normalized and he was transferred to the ward on day 21. On day 24 technical problems with CPAP resulted in worsening oxygenation and rate control of atrial fibrillation which required transfer to the critical care unit and he was treated with BIPAP, CPAP and Opti flow. The patient improved, a CT thorax showed reduced consolidation and fibrosis and he was transferred to the ward 5 days later. He continued with nocturnal CPAP and showed an improvement in pulmonary function which allowed oxygen withdrawal after 10 days. He was discharged home from the hospital on day 56 (Table 1).
The patient reported numbness but no pain in the feet. The patellar deep tendon reflexes were normal but both Achilles reflexes were abnormal, with a bilateral flexor plantar response. On the feet, VPT, CPT, WPT and HPT were abnormal, except for a normal CPT on the right foot. On the face HPT was abnormal. Taste was absent for sweet and present for salt, whilst smell was present for acetone, perfume and absent for coffee. There was no evidence of the Vernet phenomenon and the oropharyngeal reflex was normal (Table 2).
4. Conclusions
We report neuropathic symptoms and abnormal quantitative sensory testing in patients with T1DM and T2DM after prolonged admission for COVID-19 pneumonia. All four patients had distal numbness in the lower extremities with preserved deep tendon reflexes in three. In the case series from Wuhan, only 2.3% reported neuropathic pain, but this was 4-fold greater in those with more severe illness [1]. There is clearly a need for more precise and detailed quantification of neuropathic deficits associated with COVID-19 [18]. Indeed, a recent systematic review on behalf of the Infectious Disease Panel of the European Academy of Neurology concluded that there was a need for more careful clinical, diagnostic and epidemiological studies to characterize the manifestations and burden of neurological disease caused by SARS-CoV-2 [19].
We now show a marked abnormality in vibration, thermal and heat pain thresholds in the feet of all patients who developed neuropathic symptoms with severe COVID-19. Although, none of the patients had a history of either symptoms or signs of neuropathy in their medical records prior to hospital admission for COVID-19 infection; neuropathy is often poorly assessed [20], [21], [15], especially in those with T2DM [22], [23]. Indeed, our studies show that the diagnosis of painful diabetic neuropathy [40] and diabetic neuropathy [25] may be missed in approximately 80% of patients.
Multicentre studies using QST in subjects with neuropathic symptoms have identified 3 subgroups: thermal mechanical sensory loss, thermal hyperalgesia and loss of thermal sensation combined with mechanical hyperalgesia [27]. The stratification of these patients according to the sensory phenotype based on the QST profile may allow precision in the selection of treatment to relieve symptoms [28].
Neuropathy and abnormalities in QST could developed or worsen following COVID-19 infection and prolonged stay in ICU with hypoxia and inflammation as well as hyperglycemia exacerbated by high doses of methylprednisolone [30].
We also show elevated heat pain thresholds in the trigeminal sensory distribution on the face in two out of four patients. Indeed, a case of bifacial weakness with paresthesia, without ataxia or other cranial neuropathies has been temporally associated with antecedent COVID-19 [29]. Furthermore, whilst taste was relatively preserved, the sense of smell to irritant and non-irritant stimuli was reduced from presentation until hospital discharge in three out of four patients. We also report an abnormal oropharyngeal gag reflex in two patients, although the Vernet phenomenon was normal in all patients. A recent case report has described a 70-year-old male who developed dysphagia and aspiration pneumonia during recovery from severe COVID-19. Examination revealed altered sense of taste and an absent gag reflex and videoendoscopic, fluorography and high-resolution manometry revealed impaired pharyngolaryngeal sensation and mesopharyngeal contractile dysfunction indicative of glossopharyngeal and vagal neuropathy [31]. Corneal confocal microscopy (CCM) is a non-invasive ophthalmic technique that rapidly images corneal nerve fibres and has been used to quantify neurodegeneration in a range of peripheral and central neurodegenerative diseases [24], especially diabetic neuropathy [33], [34]. We and others have also used CCM to identify corneal nerve fibre loss in patients with burning mouth syndrome [35] and Parkinson’s disease [36], [37], multiple sclerosis [39], [38] and dementia [40]. CCM may therefore be particularly useful for identifying neurodegeneration in patients with COVID-19.
In conclusion a proportion of patients with diabetes and severe COVID-19 may develop or show worsening of peripheral neuropathy characterized by neuropathic symptoms and small and large fibre dysfunction in the feet and face with a loss of smell. Quantitative sensory testing using NerveCheck Master incorporated into protective equipment appears to be a fast and simple procedure to objectively quantify and characterize the type and distribution of these sensory deficits. We acknowledge that lack of a history of diabetic neuropathy and normal deep tendon reflexes cannot exclude prior diabetic neuropathy. Longitudinal cohort studies using objective measures of neuropathy including QST and CCM may provide a better understanding of the development and progression of COVID 19 related neuropathy in patients with and without diabetes.
Acknowledgments
Acknowledgments
We thank the laboratory staff at Hospital de Barcelona SCIAS and the Hospital Director Dr Miquel Gómez whose offered a very professional service to our study subjects and for always being extra helpful.
Author contributions
A.O. researched data and wrote the manuscript. S.O and M.B.O. designed and created the NerveCheck. L.O., L.M., A.T., D.C., S.M., M.P., Y.M., M.P., A.D. researched data. R.A.M reviewed the manuscript. A.O. leads the study and edited the manuscript and is the guarantor of this work.
Conflicts of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to financial contributions to this work. Phi Med Europe S.L. provided the NerveCheck device. S. Odriozola, M.B. Odriozola and A. Odriozola are the owners and inventors of the NerveCheck.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Funding
The Barcelona Hospital contributed to the study, providing the individual protection equipment for COVID-19 and the hospital facilities. Phi Med Europe loaned the NerveCheck equipment free of charge. The study participants did not receive grants or economic rewards of any kind.
References
- 1.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan C.H., Faraji F., Prajapati D.P., et al. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filatov A., Sharma P., Hindi F., et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020 doi: 10.7759/cureus.7352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARScoronavirus-2. Int J Infect Dis. 2020 [Epub ahead of print] [Google Scholar]
- 6.Zhao H., Shen D., Zhou H., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30109-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K., Wohlford-Lenane C., Perlman S., et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y.C., Bai W.Z., Hirano N., et al. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521:203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda K., Park C.H., Sunden Y., et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41:101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- 10.Kalia M., Mesulam M.M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- 11.Hadziefendic S., Haxhiu M.A. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J Auton Nerv Syst. 1999;76:135–145. doi: 10.1016/s0165-1838(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 12.Cariou B., Hadjadj S., Wargny M., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [published correction appears in Diabetologia. 2020 Jul 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pop-Busui R., Boulton A.J., Feldman E.L., et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petropoulos I.N., Ponirakis G., Khan A., Almuhannadi H., Gad H., Malik R.A. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J. 2018;42(4):255–269. doi: 10.4093/dmj.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik R.A. Diabetic neuropathy: a focus on small fibres. Diabetes Metab Res Rev. 2020;36(Suppl 1):e3255. doi: 10.1002/dmrr.3255. [DOI] [PubMed] [Google Scholar]
- 16.Ponirakis G., Odriozola M.N., Odriozola S., et al. NerveCheck: an inexpensive quantitative sensory testing device for patients with diabetic neuropathy. Diabetes Res Clin Pract. 2016;113:101–107. doi: 10.1016/j.diabres.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponirakis G., Odriozola M.N., Odriozola S., et al. NerveCheck for the detection of sensory loss and neuropathic pain in diabetes. Diabetes Technol Ther. 2016;18(12):800–805. doi: 10.1089/dia.2016.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellul MA, Benjamin L, Singh B et al. Neurological associations of COVID-19. Lancet Neurol 2020;S1474-4422(20)30221-0 [published online ahead of print, 2020 Jul 2]. [DOI] [PMC free article] [PubMed]
- 19.Romoli M., Jelcic I., Bernard-Valnet R., et al. A systematic review of neurological manifestations of SARS-CoV-2 infection: the devil is hidden in the details. Eur J Neurol. 2020 doi: 10.1111/ene.14382. [published online ahead of print, 2020 Jun 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik R.A., Aldinc E., Chan S.P., et al. Perceptions of painful diabetic peripheral neuropathy in South-East Asia: results from patient and physician surveys. Adv Ther. 2017;34(6):1426–1437. doi: 10.1007/s12325-017-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik RA, Andag-Silva A, Dejthevaporn C et al. Diagnosing peripheral neuropathy in South-East Asia: a focus on diabetic neuropathy. J Diabetes Investig [published online ahead of print, 2020 Apr 8]. [DOI] [PMC free article] [PubMed]
- 22.Almuhannadi H., Ponirakis G., Khan A., Malik R.A. Diabetic neuropathy and painful diabetic neuropathy: Cinderella complications in South East Asia. J Pak Med Assoc. 2018;68(1):85–89. [PubMed] [Google Scholar]
- 23.Javed S., Hayat T., Menon L., Alam U., Malik R.A. Diabetic peripheral neuropathy in people with type 2 diabetes: too little too late. Diabetic Med. 2020;37(4):573–579. doi: 10.1111/dme.14194. [DOI] [PubMed] [Google Scholar]
- 24.Ponirakis G., Elhadd T., Chinnaiyan S., et al. Prevalence and risk factors for painful diabetic neuropathy in secondary healthcare in Qatar. J Diabetes Investig. 2019;10(6):1558–1564. doi: 10.1111/jdi.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponirakis G., Elhadd T., Chinnaiyan S., et al. Prevalence and management of diabetic neuropathy in secondary care in Qatar. Diabetes Metab Res Rev. 2020;36(4):e3286. doi: 10.1002/dmrr.3286. [DOI] [PubMed] [Google Scholar]
- 27.Vollert J., Magerl W., Baron R. Pathophysiological mechanisms of neuropathic pain: comparison of sensory phenotypes in patients and human surrogate pain models. Pain. 2018;159(6):1090–1102. doi: 10.1097/j.pain.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 28.Rosemberg D., Blechschmidt V., Timmerman H., et al. Challenges of neuropathic pain: focus on diabetic neuropathy. J Neural Transm. 2020;127:586–624. doi: 10.1007/s00702-020-02145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchins K.L., Jansen J.H., Comer A.D., et al. COVID-19-associated bifacial weakness with paresthesia subtype of Guillain-Barré syndrome. AJNR Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6654. [published online ahead of print, 2020 Jun 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chunkiu Zhou, Limin Wu, Fengming Ni. Neural Regen Res 2014 Jan 1;9(1):101–10. [DOI] [PMC free article] [PubMed]
- 31.Aoyagi Y., Ohashi M., Funahashi R., Otaka Y., Saitoh E. Oropharyngeal dysphagia and aspiration pneumonia following coronavirus disease 2019: a case report. Dysphagia. 2020;35(4):545–548. doi: 10.1007/s00455-020-10140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petropoulos I.N., Ponirakis G., Khan A., Gad H., Almuhannadi H., Brines M., et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optom. 2020;103(3):265–277. doi: 10.1111/cxo.12887. [DOI] [PubMed] [Google Scholar]
- 33.Malik R.A., Kallinikos P., Abbott C.A., van Schie C.H., Morgan P., Efron N., et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 34.Petropoulos I.N., Alam U., Fadavi H., Asghar O., Green P., Ponirakis G., et al. Malik RA corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646–3651. doi: 10.2337/dc13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill F., Marshall A., Ferdousi M., Malik R.A. Corneal confocal microscopy detects small-fiber neuropathy in burning mouth syndrome: a cross-sectional study. J Oral Facial Pain Headache. 2019;33(3):337–341. doi: 10.11607/ofph.2338. [DOI] [PubMed] [Google Scholar]
- 36.Kass-Iliyya L., Javed S., Gosal D., Kobylecki C., Marshall A., Petropoulos I.N., et al. Small fiber neuropathy in Parkinson's disease: a clinical, pathological and corneal confocal microscopy study. Parkinsonism & Related Disorders. 2017;21:1454–1460. doi: 10.1016/j.parkreldis.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podgorny P.J., Suchowersky O., Romanchuk K.G., Feasby T.E. Evidence for small fiber neuropathy in early Parkinson's disease. Parkinsonism Relat Disord. 2016;28:94–99. doi: 10.1016/j.parkreldis.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Petropoulos I.N., Kamran S., Li Y., Khan A., Ponirakis G., Akhtar N., et al. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Vis Sci. 2017;58:3677–3681. doi: 10.1167/iovs.17-22050. [DOI] [PubMed] [Google Scholar]
- 39.Bitirgen G., Akpinar Z., Malik R.A., Ozkagnici A. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMAOphthalmol. 2017;135:777–782. doi: 10.1001/jamaophthalmol.2017.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponirakis G., Al Hamad H., Sankaranarayanan A., Khan A., Chandran M., Ramadan M., et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann Clin Transl Neurol. 2019;6:689–697. doi: 10.1002/acn3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]