Abstract
A wide range of cutaneous signs are attributed to COVID-19 infection. This retrospective study assesses the presence and impact of dermatologic manifestations related to the spread of COVID-19 in Lombardy, the geographic district with the first outbreak in Italy. A cohort of 345 patients with laboratory confirmed COVID-19 was collected from February 1, 2020 to May 31, 2020. Cutaneous signs and dermatologic diagnoses were recorded on admission, and during the course of the disease. Of the 345 patients included in the study, 52 (15%) had new-onset dermatologic conditions related to COVID-19. We observed seven major cutaneous clinical patterns, merged under 3 main groups: Exanthems, vascular lesions, and other cutaneous manifestations. Each subset was detailed with prevalence, age, duration, prognosis, and histology. Cutaneous findings can lead to suspect COVID-19 infection and identify potentially contagious cases with indolent course.
Introduction
In December 2019, the first cases of pneumonia with unknown cause were reported in Wuhan, China.1 The new pathogen, a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was isolated from samples from the lower respiratory tract of infected patients,2 and the resulting disease was coined as Coronavirus Disease 2019 (COVID-19). Since then, SARS-CoV-2 has rapidly spread, reaching the level of a pandemic disease. COVID-19 infection is characterized mainly by interstitial pneumonia, which may progress to acute respiratory distress syndrome. SARS-CoV-2 may also damage the heart, liver, kidneys, and other organ systems, including the blood and the immune system. Many affected patients have died from respiratory failure, shock, heart failure, and arrhythmias, as well as renal or multiple organ failures.2 Due to the extraordinary outbreak and a physician shortage, several medical specialties, including dermatologists, were involved in this global fight against SARS-CoV-2.
Recent reports from several countries have indicated that this novel coronavirus may be associated with cutaneous manifestations. These may be useful in identifying otherwise asymptomatic COVID-19 carriers to slow down viral transmission; however, the identification and characteristics of these dermatologic symptoms remain controversial. We report our retrospective cohort study, which assesses and characterizes the cutaneous involvement during COVID-19. The data are taken from Lombardy, Italy, one of the regions with the earliest outbreaks.
Methods
Our study investigates the epidemiologic, clinical, and histopathologic features of COVID-19 cutaneous manifestations observed between February 1, 2020, and May 31, 2020. The data were uniformly collected by experienced dermatologists at Lecco Hospital, in Lombardy, Italy. Some of our observations have been previously reported.3, 4, 5, 6, 7, 8, 9 Data from both the inpatient (n = 37) and outpatient services (n = 15) were included. Patients were included only if there was laboratory confirmation of SARS-CoV-2 infection, regardless of clinical signs and symptoms, according to the definitions created by the European Centre for Disease Control.10 A COVID-19 diagnosis was considered laboratory confirmed if a nasopharyngeal swab for SARS-CoV-2 RNA detection, serology for anti-SARS-CoV-2 IgG/IgM by chemiluminescent immunoassay, or the enzyme-linked immunosorbent assaymethod yielded positive results. In the chilblain-like lesions (CLLs) setting, serologic studies using IgM/IgG rapid test were also conducted. Cutaneous signs and dermatologic diagnosis were recorded on admission. Epidemiologic, clinical, therapeutic, and outcome data were collected from patient medical records. Blood investigations were performed in most cases, whereas skin biopsies representative of every subset were performed, whenever possible in some patients. A complete dermatologic history was recorded to distinguish between newly occurred COVID-19-related pathologies, and persistence/reactivation of preexisting dermatologic conditions.
Results
We observed 345 patients with confirmed COVID-19. We recorded the histories for 52 patients (15%), 7 to 94 years of age (mean 54.6 years), having new-onset dermatologic conditions, not preexisting. There was no sex prevalence. Data are detailed in Table 1 . In 38% of the cases, skin involvement was apparent at the onset of the infection, simultaneously with the flu-like presentation typical of the disease (we considered onset through the third day). The remaining 62% of the dermatologic cases were observed between 4 and 90 days after the onset of typical COVID-19 findings.
Table 1.
Demographic and clinical data of patients with cutaneous manifestations COVID-19-related.
| Case | Age (y) | Sex (m/f) | Inpatients / outpatients (i/o) | Cutaneous manifestations | Localization | Cutaneous findings | Eruption duration | Onset after COVID-19 symptoms (d) | Systemic manifestations | Swab | Serology |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 86 | m | i | Maculopapular eruption | Trunk | Itching | 8 | 1 | Fever, dyspnea | r | np |
| 2 | 62 | m | i | Maculopapular eruption | Trunk | Itching | 6 | 1 | Fever, dyspnea | r | np |
| 3 | 60 | f | i | Maculopapular eruption | Trunk | None | 8 | 1 | Fever, dyspnea | r | np |
| 4 | 76 | f | i | Maculopapular eruption | Trunk | None | 6 | 4 | Fever, dyspnea | r | np |
| 5 | 68 | m | i | Maculopapular eruption | Trunk | None | 6 | 6 | Fever, dyspnea | r | np |
| 6 | 49 | m | i | Maculopapular eruption | Trunk | None | 7 | 1 | Fever, dyspnea | r | np |
| 7 | 54 | f | i | Maculopapular eruption | Trunk | None | 6 | 3 | Fever, dyspnea | r | np |
| 8 | 68 | f | i | Maculopapular eruption | Trunk | None | 8 | 2 | Fever, dyspnea | r | np |
| 9 | 44 | m | i | Maculopapular eruption | Trunk | None | 7 | 4 | Fever, dyspnea | r | np |
| 10 | 37 | m | i | Maculopapular eruption | Trunk | None | 3 | 1 | Fever, dyspnea | r | np |
| 11 | 53 | m | i | Maculopapular eruption | Trunk | None | 6 | 3 | Fever, dyspnea | r | np |
| 12 | 50 | f | i | Maculopapular eruption | Trunk | None | 6 | 3 | Fever, dyspnea | r | np |
| 13 | 40 | m | i | Maculopapular eruption | Trunk | None | 8 | 3 | Fever, dyspnea | r | np |
| 14 | 77 | f | i | Maculopapular eruption | Trunk | None | 6 | 2 | Fever, dyspnea | r | np |
| 15 | 90 | f | i | Macular eruption, purpura | Trunk | None | 7 | 7 | Fever, dyspnea, cough | r | np |
| 16 | 83 | m | i | Macular eruption | Trunk | None | 12 | 10 | Fever, dyspnea | r | np |
| 17 | 57 | m | i | Maculopapular eruption | Trunk | Itching | 10 | 11 | Fever, dyspnea, cough | r | np |
| 18 | 19 | f | i | Macular eruption | Trunk, extremities | None | 4 | 2 | Fever, myopericarditis | r | np |
| 19 | 35 | f | o | Macular eruption | Trunk | Itching | 2 | 10 | Fever, cough, headache | r | np |
| 20 | 59 | m | i | Macular eruption, purpura | Trunk, extremities | None | 8 | 15 | Fever, dyspnea | r | np |
| 21 | 84 | m | i | Urticarial eruption | Trunk | Itching | 5 | 1 | Fever, dyspnea | r | np |
| 22 | 50 | m | i | Urticarial eruption | Trunk | Itching | 5 | 3 | Fever, dyspnea | r | np |
| 23 | 41 | m | i | Urticarial eruption | Trunk | Itching | 5 | 1 | Fever, dyspnea | r | np |
| 24 | 55 | m | o | Urticarial eruption | Trunk, extremities | Itching | 10 | 9 | Fever, dyspnea, cough | r | np |
| 25 | 36 | f | o | Urticarial eruption | Whole body | Itching | 7 | 7 | Fever, dyspnea, ageusia, anosmia | r | np |
| 26 | 45 | f | o | Urticarial eruption | Extremities | Itching | 15 | 6 | Fever, dyspnea, ageusia, anosmia | r | np |
| 27 | 67 | m | i | Vesicular eruption | Lower extremities | Itching | 5 | 8 | Fever, dyspnea | r | np |
| 28 | 50 | m | o | Vesicular eruption | Lower extremities | None | 7 | 5 | Fever, dyspnea | r | np |
| 29 | 44 | m | o | Vesicular eruption | Trunk | None | 15 | 5 | Myalgia, asthenia | r | np |
| 30 | 35 | f | o | Vesicular eruption | Extremities | Itching, burning | 10 | 0 | Fever, dyspnea, cough | r | np |
| 31 | 57 | m | i | Vesicular eruption | Trunk | Itching | 7 | 0 | None | r | np |
| 32 | 53 | m | i | Erythema multiforme | Trunk | Itching | 6 | 7 | Fever, dyspnea, ageusia, anosmia | r | np |
| 33 | 65 | m | i | Erythema multiforme | Trunk, neck | Itching | 12 | 7 | Fever, dyspnea | r | np |
| 34 | 40 | f | o | Erythema multiforme | Face, extremities | Burning | 10 | 7 | Fever | n | r |
| 35 | 79 | m | i | Erythema multiforme | Extremities | Itching | 15 | 3 | Fever | n | r |
| 36 | 33 | f | o | Chilblain-like lesions | Feet | None | 20 | 14 | Fever | r | np |
| 37 | 7 | f | o | Chilblain-like lesions | Hands, feet | Pain | 50 | 21 | Fever, headache, ageusia, anosmia | r | np |
| 38 | 15 | m | o | Chilblain-like lesions | Hands, feet | None | 25 | 0 | None | n | r |
| 39 | 31 | f | o | Chilblain-like lesions | Hands, feet | None | 21 | 0 | None | n | r |
| 40 | 54 | f | i | Vasculitis | Extremities | None | 16 | 26 | Fever, dyspnea, cough | r | np |
| 41 | 81 | f | i | Vasculitis | Extremities | Burning | 20 | 15 | Dyspnea, asthenia | r | np |
| 42 | 64 | m | i | Vasculitis | Legs | Pain | 20 | 14 | Fever, dyspnea, cough | r | np |
| 43 | 74 | f | i | Thrombosis | Right foot | Burning | 24 | 15 | Fever, dyspnea, cough | r | np |
| 44 | 94 | f | i | Acro-ischemia | Feet | None | 25 | 10 | Fever, dyspnea, cough | r | np |
| 45 | 60 | f | i | Acro-ischemia | Hands | None | 24 | 12 | Fever, dyspnea, cough | r | np |
| 46 | 50 | f | o | Livedo reticularis | Legs, feet | Pain | 5 | 25 | Fever | r | np |
| 47 | 55 | m | i | Telogen effluvium | Scalp | Trichodynia | 25 (on going) | 90 | Fever, dyspnea, cough | r | np |
| 48 | 59 | f | i | Telogen effluvium | Scalp | Trichodynia | 15 (on going) | 70 | Fever, dyspnea, cough | r | np |
| 49 | 55 | m | i | Telogen effluvium | Scalp | Trichodynia | 20 (on going) | 78 | Fever, dyspnea, cough | r | np |
| 50 | 58 | f | i | Telogen effluvium | Scalp | Trichodynia | 18 (on going) | 85 | Fever, dyspnea, cough | r | np |
| 51 | 42 | m | o | Telogen effluvium | Scalp | None | 20 (on going) | 84 | Fever, cough | r | np |
| 52 | 38 | f | o | Lichen planopilaris | Scalp | Pain | 22 (on going) | 15 | Fever, dyspnea, cough | r | np |
r, reactive; n, nonreactive; np, not performed.
Classification of clinical patterns and associated characteristics
The consensus after direct observation and image review led to the description of seven major clinical patterns:
-
•
Exanthem group representing the first four patterns observed.
-
•
Vascular lesions group with a preponderance of clinicopathologic involvement; that is, vasculitic and CLLs.
-
•
Miscellaneous cutaneous manifestations.
Exanthems (67.3%)
Maculopapular (38.5%)
Erythematous maculopapular lesions were the most common manifestations in SARS-CoV-2 infection (Figure 1 A-C), frequently impacting middle-aged adults (mean 58.3 years). Purpura was usually associated. While the eruption was often asymptomatic, 2% of the patients had pruritus. Lesions frequently appeared early on, and lasted for a mean of 6.7 days.
Fig. 1.
Clinical characteristics of COVID-19 exanthems. A-C, Maculopapular dermatitis. D,E, Urticarial dermatitis in case 25. F, Vesicular dermatitis.
Histopathologic examination found a superficial and/or deep perivascular dermatitis with cuffs of lymphocytes surrounding blood vessels, dermal ducts, and eccrine glands. There were extravasated erythrocytes from damaged vessels in the upper portion of the dermis. Tissue from a few patients revealed a superficial band-like perivascular dermatitis with many necrotic keratinocytes (Figure 2 B-F).
Fig. 2.
Histopathological findings. A, Urticarial dermatitis: cuffs of lymphocytes surrounding blood vessels (red arrow) and dermal ducts (black arrow). B, Maculopapular dermatitis: superficial and deep perivascular dermatitis with lichenoid band-like pattern. C, Maculopapular dermatitis: exocytosis of lymphocytes and necrotic keratinocytes in the epidermis (black arrow); perivascular cuffs of lymphocytes in the dermis are found (red arrow). D, Maculopapular dermatitis: lymphocytic infiltrate surrounding dermal ducts and blood vessels (red arrow). E, Maculopapular dermatitis: dense patchy band-like infiltrate surrounding acrosyringeal ducts. F, Maculopapular dermatitis: lymphocytes surrounding an acrosyringeal duct (red arrow). G, Vesicular dermatitis: superficial spongiotic and perivascular dermatitis with intraepidermal vesicles (red arrow); heavy lymphocytic cuff around dermal vessels (black arrows). H, Vesicular dermatitis: superficial perivascular dermatitis with large intraepidermal nests of Langerhans cells (red arrow).
In our experience, this setting was associated with a more severe course of COVID-19; these patients were often hospitalized, and many were placed on intensive care units.
Urticarial (11.5%)
Urticarial lesions appeared in adults (mean 51.8 years), typically presenting at the beginning of a SARS-CoV-2 infection (Figure 1D,E). These hives mostly affected the trunk and lasted a mean of 7.8 days, with itchiness in many places.
Histopathologic findings revealed a markedly edematous dermis with highly dilated capillaries, and collections of perivascular lymphocytes. Throughout the dermis, there were dilated and vertically arranged vessels with mild interstitial infiltration of eosinophils, together with a periglandular lymphoid infiltrate surrounding the dermal ducts and eccrine glands (Figure 2A).
Urticarial lesions were usually associated with a good prognosis, and these patients did not require hospitalization. Treatment included antihistamines, topical corticosteroids, and rarely systemic corticosteroids.
Vesicular (9.6%)
Small monomorphic vesicles appeared in middle-aged patients (mean 50.6 years), often early in the course of the disease (mean 3.6 days). They commonly affected the trunk (Figure 1F), lasting for a mean of 8.8 days. About half of the patients complained of pruritus.
Histologic study found a band-like, patchy superficial perivascular dermatitis with cuffs of lymphocytes surrounding dermal blood vessels. In addition, there were spongiosis with vesicles and large intraepidermal nests of Langerhans cells, marked exocytosis, and necrotic keratinocytes (Figure 2G,H). The appearance of vesicular eruptions occurred with variable severity, and often required hospitalization. One such patient (case 27) died from respiratory failure.
Erythema multiforme (7.7%)
Erythema multiforme eruptions were observed in 4 patients (two cases positive to polymerase chain reaction (PCR), two cases positive to serology), occurring on a mean of 6 days after COVID-19 infection onset, and lasted for about 10 days. Clinically, there were infiltrated papules with targetoid lesions on the extremities, sometimes with central vesiculation (Figure 3 ). Many patients had significant pruritus.
Fig. 3.
Clinical characteristics of COVID-19 exanthems: erythema multiforme. A,B, Targetoid lesions on the hands in case 32. C,D, Lesions on the upper extremities in case 35.
Histopathologic examination revealed a diffuse interface dermatitis, cytotoxic CD8+ lymphocytes diffusely infiltrating the epithelium, and scattered necrotic keratinocytes. Lymphocytes accumulated particularly around the acrosyringeal and dermal eccrine gland ducts (Figure 4 A). The patients were considered to have a mild or moderate level of disease severity.
Fig. 4.
Histopathological findings. A, Erythema multiforme: superficial band-like perivascular dermatitis with necrotic keratinocytes (black arrow). In the mid-dermis, dilated blood vessels surrounded by lymphocytic cuff (red arrow). B, Livedo reticularis: large thrombus in the mid-dermis (red arrow). C, Livedo: numerous thrombosed vessels with marked endothelial hyperplasia in the superficial and deep dermis (red arrows). D, Chilblain-like lesions: dense coat-sleeve-like lymphoid infiltrate around medium- and small-caliber dermal vessels, as well as around dermal glands. E, Lichen planopilaris of the scalp: ulcerated epidermis with dense lymphocytic infiltration around hair follicles (red arrow); the dermis is diffusely fibrotic. F, Lichen planopilaris: diffuse thrombosis of the upper dermal vessels (red arrows).
Vascular lesions (21.2%)
Vasculitic lesions (13.5%)
Vasculitis-like skin signs; such as petechiae, purpura, cyanosis, livedo, and necrosis; usually occurred with a delayed onset, being observed on a mean of 16.7 days after the onset of COVID-19 systemic signs. Manifestations included livedo reticularis (n = 1), thrombosis (n = 1), acro-ischemia (n = 2), and vasculitis (n = 3) (Figure 5 ). Livedo reticularis was transitory, whereas acro-ischemia lasted longer. Vasculitis, thrombosis, and acro-ischemia more often affected elderly patients and portended a worse prognosis, often requiring intensive care management with oxygen supplementation.
Fig. 5.
Clinical characteristics of COVID-19 vascular lesions. A, Vasculitis. B, Thrombosis. C, Acro-ischemia. D,E, Chilblain-like lesions in 2 cases. F, Livedo reticularis. b c d e should be rotated 180 degrees
Histology reported varying degrees of vascular occlusion, involving both small and larger vessels. We often observed diffuse angiogenesis with hyperplasia of endothelial cells surrounding the thrombi throughout the dermis (Figure 4B,C). Such patients often required low molecular weight heparin dosage for the thromboses, whereas patients with livedo reticularis underwent complete resolution without systemic therapy.
Chilblain-like lesions (7.7%)
Due to clinical and histologic findings, we decided to include these manifestations in the vascular lesions group. During the study, we observed 32 patients with CLL unrelated to cold exposure or comorbidities. We ruled out other viral infections, including Epstein-Barr virus, cytomegalovirus, Coxsackie, and Parvovirus B19 infection with appropriate serologic studies. PCR for SARS-CoV-2 from nasopharyngeal swab was positive only in 2 cases (in these 2 patients, the swab was taken 3 weeks before the patients developed CLL, due to a suspicion based on respiratory symptoms and fever). Serologic studies, using SARS-CoV-2 IgM/IgG rapid test, were negative in eight tested cases. Antibody testing, utilizing the enzyme-linked immunosorbent assay method, allowed us to establish a previous SARS-CoV-2 infection in two other patients. The serologies were nonreactive in the remaining 20 tested patients. For this reason, and respecting our inclusion criteria, we included only four out of the 32 confirmed positive cases in this study; nevertheless, the overall epidemiologic and clinical characteristics of CLL still strongly point to a COVID-19-related condition. CLL appeared late over the disease course, and typically affected children and young adults. The cutaneous manifestations of this setting ranged from an acral eruption of erythematous-violaceous papules and macules, to possible bullous evolution or digital swelling (Figure 5-D,E). Three of them also exhibited targetoid lesions. Lesions were localized predominantly on the feet, but hands were often affected. Lesions were usually asymptomatic, but occasionally painful. In most cases, CLL were the only manifestation of the disease; rarely, mild systemic symptoms preceded the onset of the lesions from 1 to 8 weeks.
The histologic features were similar to classic erythema pernio, with a dense coat-sleeve-like lymphoid infiltrate, a prevalence of cytotoxic CD8 lymphocytes, around medium- and small-caliber dermal vessels, and around dermal glands (Figure 4D). Thickening of the vessel wall and hyperplasic endothelial cells with nuclear enlargement were documented. The severity of the vasculitis may be so intense that it induced detachment at the dermal–epidermal junction and necrosis of the epidermis. Occasional deep dermal vessel microthrombi were observed.
Most lesions resolved in 3 to 4 weeks without treatment, whereas some patients experienced a prolonged course of 2 months. CLL were associated with a good prognosis.
Other cutaneous manifestations
Alopecia (11.5%)
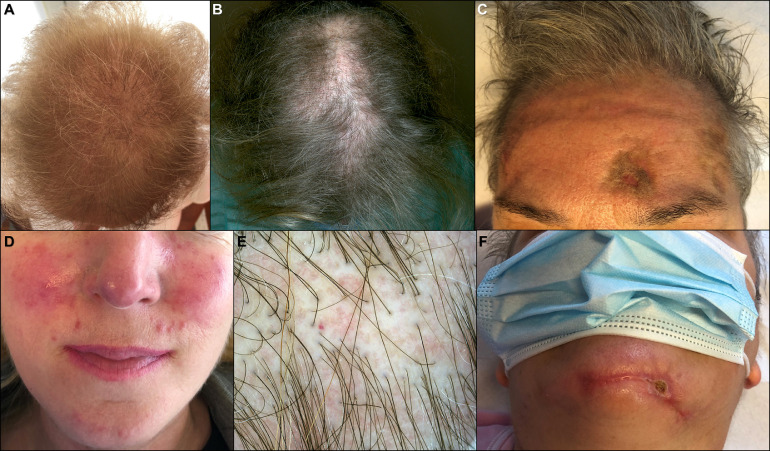
We observed five cases of severe telogen effluvium in patients previously hospitalized for COVID-19 (Figure 6 A-C). Three cases required intensive care. Latency of effluvium was consistent with hair follicle damage manifesting 3 months after the SARS-CoV-2 infection. Patients complained of acute diffuse hair loss accompanied by trichodynia. In addition, a case of lichen planopilaris, resulting in cicatricial alopecia, was observed (Figure 6B-E). In this case, a biopsy documented an ulcerated epidermis with a dense lymphocytic infiltration around hair follicles, and a few thrombosed vessels at the hair bulge. The dermis was diffusely fibrotic (Figure 4E,F).
Fig. 6.
Indirect cutaneous manifestations related to COVID-19. A, Telogen effluvium. B, Lichen planopilaris. C,F, Pressure sores and telogen effluvium. D, Rosacea. E, Trichoscopy of lichen planopilaris.
Indirect cutaneous manifestations
A few patients reported reactivation of other skin diseases such herpes simplex, herpes zoster, and pityriasis rosea. Cutaneous manifestations after therapies or hospitalization were also reported, such as cutaneous adverse drug reactions, and pressure sores due to pronation (Figure 6C-F). Personal protective equipment and hand hygiene practices sometimes aggravated preexisting skin conditions (eg, acne, rosacea, seborrheic dermatitis, and contact dermatitis) (Figure 6D). The group of “indirect cutaneous manifestations” has not contributed to prevalence calculation of cutaneous manifestations related to COVID-19.
Discussion
SARS-CoV-2 infection has been found to affect the skin; however, data about prevalence of cutaneous involvement are lacking. Two previous contributions reported a prevalence of 20.4% and 7.8%, respectively.3 , 4 In the present study, we observed a prevalence of 15%. This study included both patients that were hospitalized and outpatients. We believe that many other patients with a COVID-19-related cutaneous dermatitis did not seek medical advice, due to the absence of systemic symptoms, and therefore, escaped dermatologic diagnosis. Skin lesions may have also been neglected, as their duration can be very short and symptoms mild; hence, cutaneous lesions are likely to have been underestimated. Similarly, the number of COVID-19 positive patients in the general population is likely underestimated, due to the low number of virologic tests performed. Thus, as far as prevalence is concerned, both numerator and denominator are underestimated. We believe that our prevalence estimate could be reliable, because in our study we included both inpatients and outpatients. This cohort of patients could reflect the prevalence of SARS-CoV-2-related skin involvement in the general population accurately. Sex prevalence was uniform and middle-aged adults were the most involved group, even if the disease can affect age transversely.
An uncommon aspect of SARS-CoV-2 compared to other viruses is its ability to determine a wide spectrum of cutaneous manifestations. We are used to linking each exanthem to a particular virus or bacteria, but in this new pandemic, usual schemes have to be reviewed.
We described seven major cutaneous clinical patterns associated with COVID-19, under three main groups.
Exanthems were the most commonly reported skin manifestations. They are characteristic of an early viremic phase, similar to eruptions occurring in the course of other common viral infections. Purpura, observed in a few cases, could represent a secondary phenomenon during the natural evolution of the exanthem, perhaps enhanced by the altered coagulated state linked to SARS-CoV-2. In the group of vascular lesions, likely due to a delayed vasculopathic mechanism, a distinction was made between vasculitic lesions and CLL subset, because the latter was related to a well-defined setting of young patients, with reproducible lesions and an always good prognosis.
Cutaneous manifestations (excluding the telogen effluvium subset) began on a mean of 6.9 days after the onset of COVID-19 symptoms. In 38% of the cases, skin involvement was present from the beginning. The duration of dermatologic manifestations was short (mean 11.3 days). In the vascular lesions setting, skin signs raised later and lasted longer (mean 22.7 days). The sites of involvement in the exanthem group were mainly the trunk, whereas in the vascular lesions group, they were mainly the extremities. Mucosal lesions were not recorded.
Histopathology of COVID-19-related skin lesions found different patterns, comparable to those already described in known dermatoses. These patterns have been linked to an immune dysregulation, either cell-mediated, such as in erythema multiforme and lichen planus, or mediated by circulating immune complexes, such as in urticaria. Both viral and bacterial infections are common triggers of these patterns.
Treatment of skin involvement was based mostly on administering antihistamines, and sometimes topical or systemic corticosteroids. Patients developing thromboses required proper low molecular weight heparin therapy, whereas patients with livedo reticularis and CLL underwent complete resolution without systemic therapy.
Erythematous maculopapular eruptions were associated with a worse prognosis for COVID-19 infection, whereas urticarial and vesicular eruptions usually had a portended good outcome. In the vascular lesions group, a diagnosis of CLL was related to a good prognosis, whereas the other vascular manifestations (livedo reticularis, thrombosis, acro-ischemia, and vasculitis), which typically involved the elderly, were linked to a worse outcome.
An “epidemic” of CLL has been reported in several countries during the COVID-19 pandemic. The epidemic and clinical characteristics in these cases (ie, the sudden outbreak of CLL parallel with COVID-19, the unusual seasonal and exposure conditions, plus the frequent occurrence in siblings and in relatives of COVID-19 patients) strongly suggest a link with SARS-CoV-2 infection. Unfortunately, most cases in the reported series to date lack confirmation of SARS-CoV-2 infection by PCR or serologic testing.5 , 11, 12, 13, 14, 15, 16, 17, 18 The prototype patient was asymptomatic or with minimal findings in a young adult. CLL should be considered a different phenotype than acro-ischemic lesions presenting in critical patients.19 In a previous contribution, we hypothesized that the lesions might result from a delayed immunologic reaction against viral particles.5 An increased type I interferon reaction has also been implicated.20 Serologic non-reactivity could be due to the limitation of available commercial serologic tests. Alternatively, a vigorous innate immune response against the virus, able to control the infection without effectively generating antibodies through the adaptive response, may be implicated. If we consider in the CLL subset even the non-confirmed cases, data suggest that CLL develops in subjects with mild infection and low viral shedding who are unable to generate neutralizing antibodies. As a result, affected patients might not be protected from COVID-19 reinfection.
Vascular lesions are probably due to an unbalanced coagulation state, and might represent a COVID-19-specific complication. SARS-CoV-2 may induce a prothrombotic state, leading to the formation of microthrombi in the dermal vessels due to inflammation, platelet activation, and endothelial dysfunction.8 , 21 For these reasons, thromboprophylaxis is recommended for all hospitalized COVID-19 patients.22 It is unknown if the cutaneous vascular lesions might signal vessel damage to other organs. With this assumption that CLL could be a marker of systemic vessel damage, such patients might benefit from antiplatelet or antiinflammatory intervention.
Conclusions
Cutaneous involvement during COVID-19 infection has been well documented. Prevalence in affected patients is likely around 15%. The power of this study is that all our cases had a laboratory confirmed diagnosis of COVID-19.
The spectrum of skin manifestations is heterogeneous and can be grouped in three main settings: an exanthem, usually simultaneous with the onset of the extracutaneous COVID-19 findings; a second subset, consisting of vascular lesions arising later and lasting longer; and a miscellaneous group of various cutaneous manifestations, not directly linked to the viral action on the skin.
These results may help physicians to promptly identify COVID-19-related skin signs, and enhance our understanding of the pathogenetic mechanisms of the underlying disease. The recognition of cutaneous manifestations in asymptomatic patients could be helpful for epidemiologic control, especially in areas where diagnostic tests are scarce. Lastly, these findings may prove useful for providing better patient management and prognosis.
SARS-CoV-2 has completely changed our era and has modified our way of thinking about an infective disease. We are reminded of the time of HIV discovery a generation ago, and raises new questions waiting to be answered. How many other new clinical manifestations do we expect? How many other diseases can it promote or reactivate? How long will the virus persist in affected patients? Studies on COVID-19 pathogenesis must continue, and we believe that unexpected findings might might emerge in the future.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The following collaborators aassisted in data collection and patient management: Tania Barbagallo, Francesca Prestinari, Lucrezia Adina Frasin, Silvia Tonolo, Francesco Luzzaro, Cristina Riva, and Emanuele Dainese.
References
- 1.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Du Z, Zhu F. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16387. e212-e213. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgi V, Recalcati S, Jia Z. Cutaneous manifestations related to coronavirus disease 2019 (COVID-19): a prospective study from China and Italy. J Am Acad Dermatol. 2020;83:674–675. doi: 10.1016/j.jaad.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recalcati S, Barbagallo T, Frasin LA. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16533. e346-e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recalcati S, Piconi S, Franzetti M. Colchicin treatment of covid-19 presenting with cutaneous rash and myopericarditis. Dermatol Ther. 2020;33:e13891. doi: 10.1111/dth.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recalcati S, Fantini F. Chilblain-like lesions during the COVID-19 pandemic: early or late sign? Int J Dermatol. 2020;59 doi: 10.1111/ijd.14975. e268-e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianotti R, Recalcati S, Fantini F. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID-19 in the northern part of Italy: analysis of the many faces of the viral-induced skin diseases in previous and new reported cases. Am J Dermatopathol. 2020;42:564–570. doi: 10.1097/DAD.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianotti R., Veraldi S., Recalcati S. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100(8):adv00124. doi: 10.2340/00015555-3490. Date of last accessed 14/01/2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control . 2020. Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. Available at: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition, Date of last access: 14/01/2021. [Google Scholar]
- 11.Freeman EE, McMahon DE, Lipoff JB. American academy of dermatology ad hoc task force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landa N, Mendieta-Eckert M, Fonda-Pascual P. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59:739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccolo V, Neri I, Filippeschi C. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16526. e291-e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolivras A, Dehavay F, Delplace D. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galván Casas C, Català A, Carretero Hernández G. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83 doi: 10.1016/j.jaad.2020.04.093. e61-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saenz Aguirre A, De la Torre Gomar FJ, Rosés-Gibert P. Novel outbreak of acral lesions in times of COVID-19: a description of 74 cases from a tertiary university hospital in Spain. Clin Exp Dermatol. 2020;45:1065–1067. doi: 10.1111/ced.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Docampo-Simón A, Sánchez-Pujol MJ, Juan-Carpena G. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review. J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16665. e445-e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccolo V, Bassi A. Acral findings during the COVID-19 outbreak: chilblain-like lesions should be preferred to acro-ischemic lesions. J Am Acad Dermatol. 2020;83:e231. doi: 10.1016/j.jaad.2020.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouaziz JD, Duong T, Jachiet M. Vascular skin symptoms in COVID-19: a French observational study. J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16544. e451-e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikdeli B, Madhavan MV, Jimenez D. Global COVID-19 thrombosis collaborative group, endorsed by the ISTH, NATF, ESVM, and the IUA, supported by the ESC working group on pulmonary circulation and right ventricular function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollias A, Kyriakoulis KG, Dimakakos E. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]