Abstract
Background
Streptomycin is used as an epidemiological marker in monitoring programs for antimicrobial resistance in Salmonella serovars and indicates the presence of pentaresistance. However, comprehensive data on streptomycin resistant Salmonella among human, animal, and animal products is lacking in Ethiopia. In this review, we aimed to assess heterogeneity and pooled proportion of Salmonella serovars to streptomycin resistance among human, animal and animal products in Ethiopia.
Methods
We conducted a systematic review and meta-analysis of published literature from Ethiopia. We used the MEDLINE/ PubMed, Embase, Cochrane Library, and Google Scholar databases to identify genetic and phenotypic data on Salmonella isolates. To determine the heterogeneity and pooled proportion, we used metaprop commands and the random-effects model. Relative and cumulative frequencies were calculated to describe the overall preponderance of streptomycin resistance isolates after arcsine-transformed data. Metan funnel and meta-bias using a begg test were performed to check for publication bias.
Results
Overall, we included 1475 Salmonella serovars in this meta-analysis. The pooled proportion of streptomycin resistance was 47% (95% CI: 35–60%). Sub-group analysis by target population showed that the proportion of streptomycin resistance in Salmonella serovars was 54% (95% CI: 35–73%) in animal, 44% (95% Cl: 33–59%) in humans and 39% (95% CI: 24–55%) in animals products. The streptomycin resistant Salmonella serovars were statistically increasing from 0.35(95% CI: 0.12–0.58) in 2003 to 0.77(95% CI: 0.64–0.89) in 2018. The level of multidrug-resistant (MDR) Salmonella serovars was 50.1% in the meta-analysis.
Conclusion
We found a high level of streptomycin resistance, including multidrug, Salmonella serovars among human, animals, and animal products. This resistance was significantly increasing in the last three decades (1985–2018). The resistance to streptomycin among Salmonella serovars isolated from animals was higher than humans. This mandates the continuous monitoring of streptomycin use and practicing one health approach to preventing further development of resistance in Ethiopia.
Registration
We conducted a systematic review and meta-analysis after registration of the protocol in PROSPERO (CRD42019135116) following the MOOSE (Meta-Analysis of Observational Studies in Epidemiology).
Background
Salmonella is a major public health problem affecting both humans and animals. Most mammals and birds can be reservoirs for Salmonella as well as many other vertebrates. Some plants may also be able to act as reservoirs [1, 2]. This bacterial species cause gastroenteritis, typhoid fever, non-typhoidal Salmonella, miscarriage, and bacteremia, depending on the types of serovars and the hosts [3, 4]. The infection is transmitted through unhygienic living conditions, sharing of houses between animals and humans, and consumption of raw or undercooked animal-origin food items [5]. The problem is much worse in developing countries where the aforementioned means of transmission are common [5].
The emergence and spread of drug-resistant Salmonella serovars are one of a current global concern [6]. The widespread use of antimicrobials at suboptimal doses and their prophylactic use in livestock, companion animals, and humans led to the emergence of drug-resistant Salmonella serovars to different groups of antimicrobial agents [7]. The interaction of humans and animals though the food chain is crucial in the transmission of these resistant bacteria [8]. This is multidimensional nature of the problems is currently recognized, and a comprehensive one health research agenda is proposed to address antimicrobial resistance(AMR), mainly among foodborne pathogens such as Salmonella for aminoglycosides, quinolones, cephalosporins and other related antimicrobials [9].
Streptomycin is an aminoglycoside antimicrobial and was one of the first antimicrobial agents to be discovered in the early 1940s [10]. The drug has been used for the treatment of animal and human Salmonella infections since its discovery [10]. The resistance level of Salmonella serovars is increasing globally for this antibiotic. The most common enzymes associated with this antibiotic resistance are acetyltransferases, phosphotransferases, and nucleotidyltransferases, which modify and inactivate streptomycin and other aminoglycosides [9]. The Salmonella serovars (Salmonella enterica) resistant to Ampicillin, Chloramphenicol, and other related antibiotics [11] are also use these enzymes. Therefore, detection of streptomycin resistance is potentially used as an epidemiological marker in monitoring AMR programs among Salmonella serovars.
Ethiopia is particularly at risk of Salmonella infection, including the drug resistance serovars, as 80% of households have direct contact with domestic animals, creating a chance for infection and spread of disease [12]. For example, a study in the country revealed that there is a transmission of drug-resistant Salmonella among humans and animals [13]. The study also showed resistant to first and second-line drugs used in the therapeutic management of salmonellosis, including streptomycin resistance [13]. Despite the medical and veterinary significance of the disease, surveillance and monitoring systems are not in place and the pharmaco-epidemiology of streptomycin resistant Salmonella serovars is not adequately described in Ethiopia. Therefore, this review aimed to answer the following questions: 1) what is the pooled proportion of streptomycin resistant Salmonella serovars among the studies included in this review from Ethiopia? 2) Is the pooled proportion of streptomycin resistant Salmonella serovars different among animals, humans’, and animal products in Ethiopia?
Methods
Study design and data sources
We performed a systematic review and meta-analysis of published studies in Ethiopia. Before the data extraction, the protocol was registered in PROSPERO (International prospective register of systematic reviews) (CRD42019135116), following the MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guideline. For this, we searched original studies from MEDLINE/PubMed, Google Scholar, Cochrane Library, and African Journals Online (AJOL) databases. We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14, 15] to report the study (S1 Checklist). The electronic search was performed to avoid duplication using the following keywords: streptomycin-resistant Salmonella AND Ethiopia AND (‘systematic review’ OR ‘meta-analysis'). Multiple search strings (medical subject headings [MeSH], title/abstract [TIAB]] and text words [TW]) were tried to identify primary studies. The search string that enabled the location of most of the studies was the following: streptomycin AND Salmonella AND Human OR animal AND Ethiopia. In addition, we made a search for cross-referencing of the identified original articles. We did the last search on April 11, 2019.
The study selection
In the first step, all eligible articles which address the study objectives were retrieved by reviewing their title and abstract. In the second step, we deeply assessed every article against the inclusion criteria. The inclusion criteria were:—(i) laboratory-based study reporting streptomycin resistance of Salmonella either from humans, animals, or animal products (ii) published in English, (iii) studies conducted in Ethiopia. We excluded letters, communications, and reports with unclear data and those studies which did not include streptomycin antibiotic susceptibility result. We also did not consider studies reiterated findings from the already included studies.
Data extraction
With the help of a standardized data abstraction format prepared in Microsoft Excel, we (GM and GD) extracted the following characteristics of the study:-first author, year of publication, year of study, region, target population (humans, domestic animals, and animal products), and sampling population (health-care/nosocomial, community, food handler’s, animal species/products, animal handlers, and health status of the sample population), sample size, sampling method, sample type, drug susceptibility test (DST),numbers of isolates, numbers of isolates subjected to DST, numbers of MDR strains, number of streptomycin resistant serovars, and minimum inhibitory concentrations (MICs)/zone diameters (ZD).
Quality of studies and risk of bias
We use Joanna Briggs Institute (JBI) critical appraisal tool to assess the methodological quality of each study. This tool was used to detect the occurrence of any real evidence of bias based on (i) target population (humans vs. animals vs. animal products), (ii) sampling population (humans–health-care/nosocomial, community, food handler’s; animals/animal products, abattoir, dairy farms, poultry farm), (iii) sample size (n≤384 vs. higher; n = Z2PQ/d2; where Pexp = 0.5), (iv) sampling method (probability vs. non-probability). In each sampling population, studies with the following characteristics were considered as low-risk studies: sample size (>384) and prospective/consecutive sampling [16]. The Begg and Mazmudar rank correlation test was used to assess bias across studies (small study effects) [17].
Data analysis
We use Microsoft office excel 2013, Stata (Version 15.1) and EpiInfoTM (version 7.2) for data extraction, entry, and data analysis. Then, we presented a detailed description of the original studies in a table and forest plot. The level of streptomycin resistance was classified as: rare (<0.1%), very low (0.1% -1%), low (>1–10%), moderate (10% -20%), high (20% - 50%); very high (50% - 70%) and extremely high, (>70%) [18].
We determined the pooled estimate of the proportion of streptomycin resistant Salmonella using the DerSimonian-Laird for random-effects meta-analysis (random-effects model). We summarized the drug resistance level of Salmonella for streptomycin in each study by calculating the proportion in a 95% confidence interval. To normalize data distribution, Arcsine transformation was used and the result was presented using the original probability scale after corresponding back transformation [19].
We tested the possible cause of publication bias and heterogeneity across studies by means of the Cochrane Q test (presence of heterogeneity) and I2 statistics (amount of heterogeneity) [20]. The existence of heterogeneity was verified using the Cochrane Q test (P < 0.10 indicates statistically significant heterogeneity). The I2 test to measure the level of heterogeneity between studies with the values of 25% (low heterogeneity), 50% (medium heterogeneity), and 75% (high heterogeneity) was used. The Begg’s rank correlation test and Egger weighted regression test was used to statistically assess publication bias. A p-value of< 0.05 was considered indicative of statistically significant publication bias [17].
We performed a sensitivity test to identify which article is the main determinant of the pooled result, and the primary cause of heterogeneity. The test excludes each study one by one in the analysis to show the change in pooled effect size and its heterogeneity. If the point estimate of pooled effect after excluding a study lies within the 95% CI of the overall pooled effect for all studies as a whole, we assumed the study has a non-important impact on the overall pooled effect [17].
We planned subgroup analysis during the study design to examine the source of heterogeneity based on regions, target population and the change in streptomycin resistant Salmonella serovars over time.
Result
Search and selection of studies
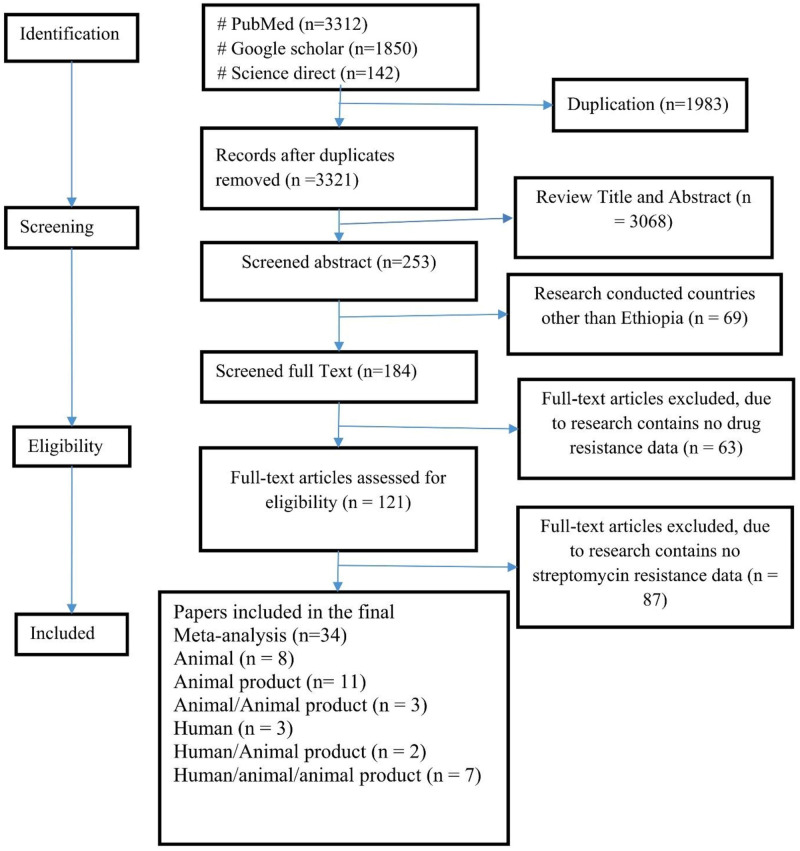
Overall, we identified a total of 3312 articles using the keywords. Out of these, 3278 were excluded either due to duplication, research conducted outside Ethiopia, or not including the research questions. Others were excluded because of either only reporting Salmonella prevalence or resistance for antimicrobial agents other than streptomycin. Lastly, we included only 34 articles in this review and meta-analysis [21–54](Fig 1) (S1 Supplement).
Fig 1. Flowchart of literature search and inclusion/exclusion process.
Characteristics of included studies
Except one retrospective study [21], all others are laboratory-based prospective cross-sectional studies. Based on the source of the specimen for the studies, eleven were conducted among animal products, eight among animal specimens and three used human specimens only. Moreover, seven studies included human, animal, and animal product specimens, three studies included both animal and animal products and the rest three included both human and animal products Table 1.
Table 1. Characteristics of included studies by target population and Salmonella isolate.
| Target population* | Number of Isolates | No of MDR isolates | % of MDR |
|---|---|---|---|
| Animal | 331 | 186 | 56.19 |
| Animal Product | 430 | 275 | 63.95 |
| Animal/Animal product | 84 | 58 | 69.05 |
| Human | 196 | 121 | 61.73 |
| Human/Animal product | 185 | 25 | 13.51 |
| Human/Animal/Animal product | 249 | 74 | 29.72 |
| Total | 1475 | 739 | 50.1 |
* Some article did not differentiate the bacterial isolates by the target population.
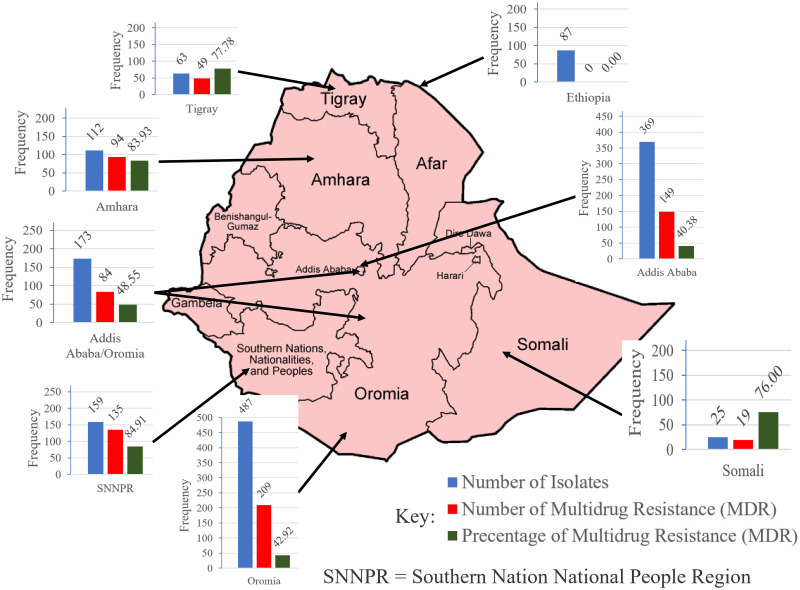
A total of 1475 Salmonella serovars were isolated from 34 included studies. Of these, 430 were only from animal products [22–24, 26, 32, 36, 37, 40, 47, 48, 53], 331 were only from animal specimens [25, 27, 30, 43, 45, 46, 51, 52], 249 were from human/animal/animal product [28, 29, 34, 35, 41, 49, 54] and the rest were from others samples [21, 31, 33, 38, 39, 42, 44, 50] as presented on Table 1. Based on the region, the majority of the isolates (n = 487) were from Oromia region followed by Addis Ababa (n = 369), SNNRP (N = 159) and Amhara (n = 173) [26, 50, 52] (Fig 2).
Fig 2. Characteristics of included studies by place of the studies and Salmonella isolate.
Antibiotic susceptibility tests were performed for streptomycin in 34 studies and 97.0% (33/34) of them reported that there is at least one resistant serovar among the isolates. Overall, about 50.1% (739/1475) of the Salmonella isolates in the included studies were MDR. The majority of the Salmonella serovars isolated from animal and animal product specimens were, 69.05% (58/84) MDR, followed by animal products, 63.95(275/430), humans 61.73% (121/196) and animals 56.19% (186/331) Table 1. The proportion of MDR isolates were different across the regions with higher in SNNPR (84.91%, n = 159) followed by Amhara (83.93%, n = 112) and Somali region (77.78%, n = 63).
Quality assessment and risk appraisal
The methodological qualities and risks of bias of included studies are given in (S2 Supplement). Fig 3 presents the proportion of studies by methods. Target population, sampling population, prospective/consecutive sampling, and specimen specified; correct interpretation and isolates subjected to DST were each reported in 50% or more of the studies (Fig 3A).
Fig 3. Radar plots of proportions of studies by methods.
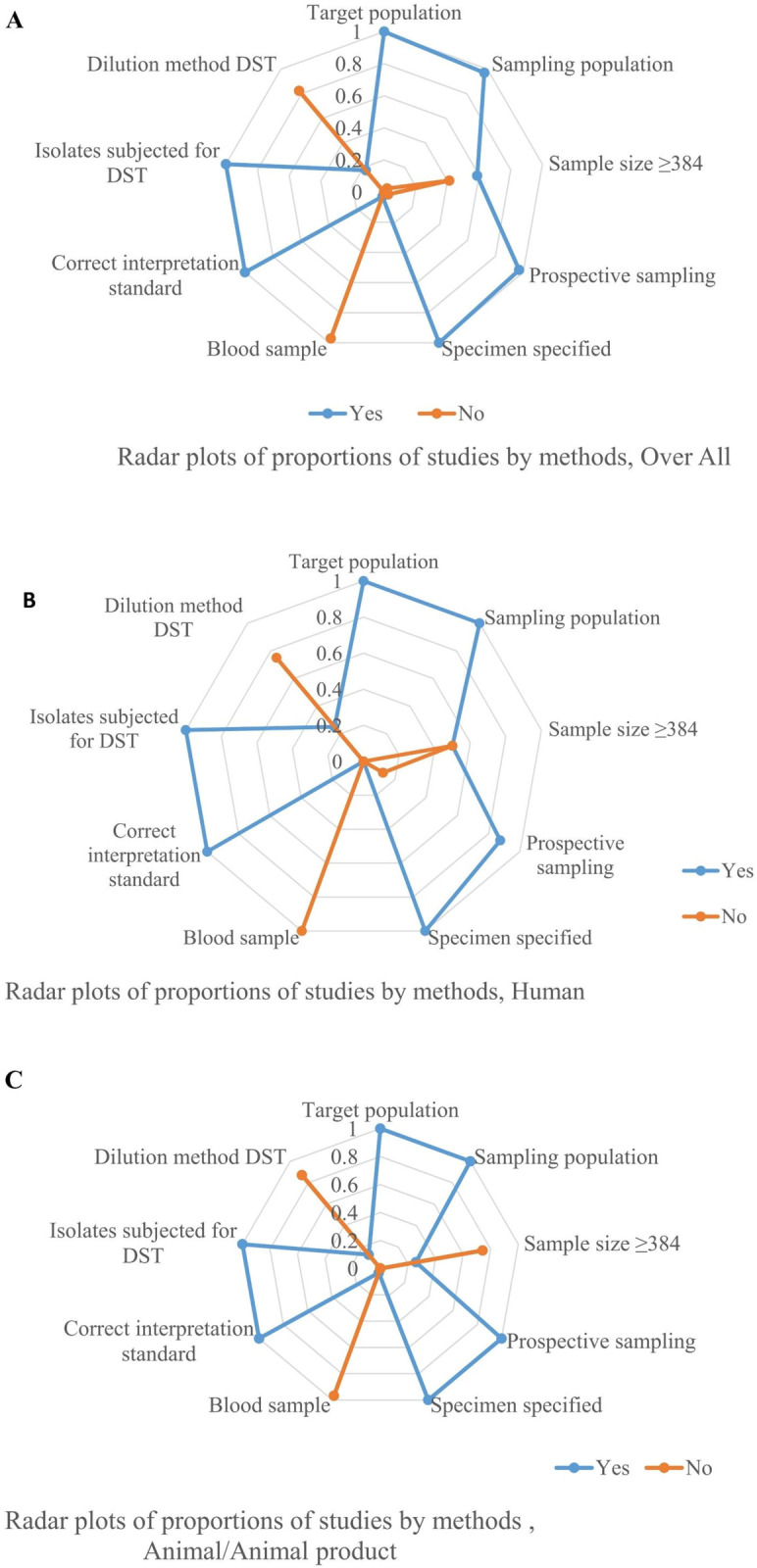
All studies (A), All human studies (B), All Animal and animal product studies (C).
Of the studies on isolates of human origins, 90% were relatively homogeneous concerning the target population, sampling population and methods (prospective sampling, specifying the specimen for isolation of the bacteria, correct interpretation of test method, and subjecting all isolates for drug susceptibility testing) Fig 3B. Similarly, the studies on animals and animal products were more than 90% homogeneous with respect to the target population, sampling population, perspective sampling, specimen specified for identification, correct interpretation, and subjecting all isolates to DST Fig 3C. Accordingly, meta- and frequency analyses were performed depending on the number of studies by category, the number of the target population, and risks of bias/heterogeneity.
Meta analyses
Heterogeneity and publication bias
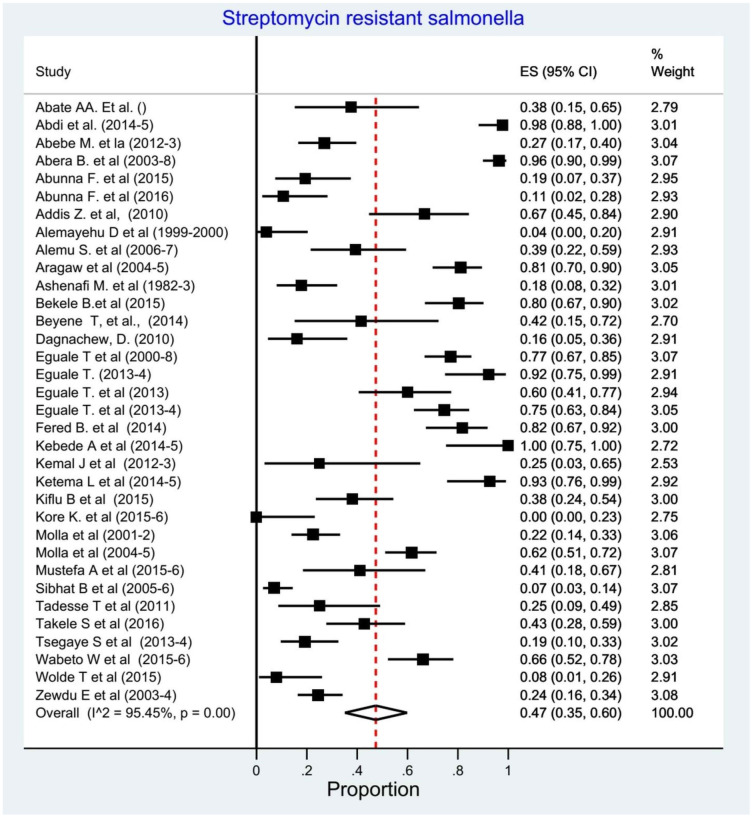
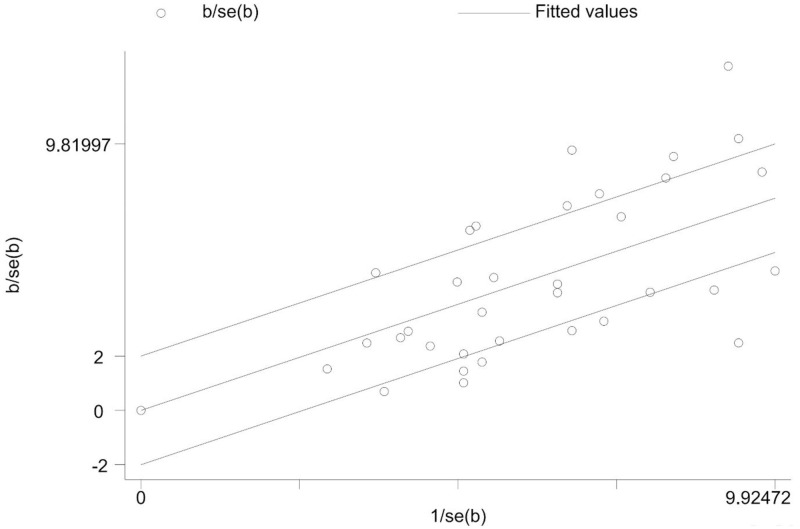
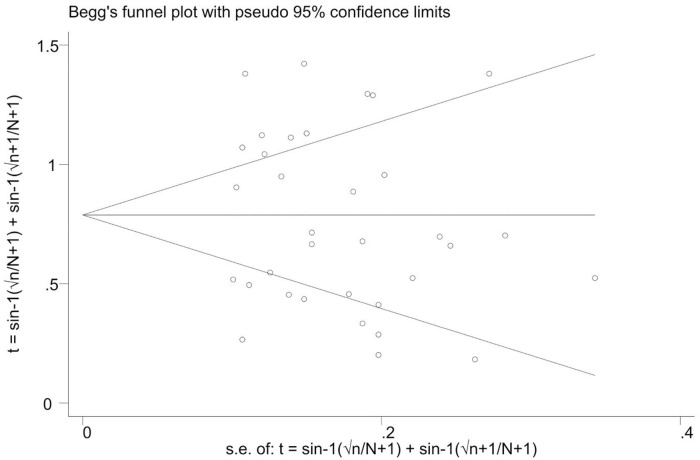
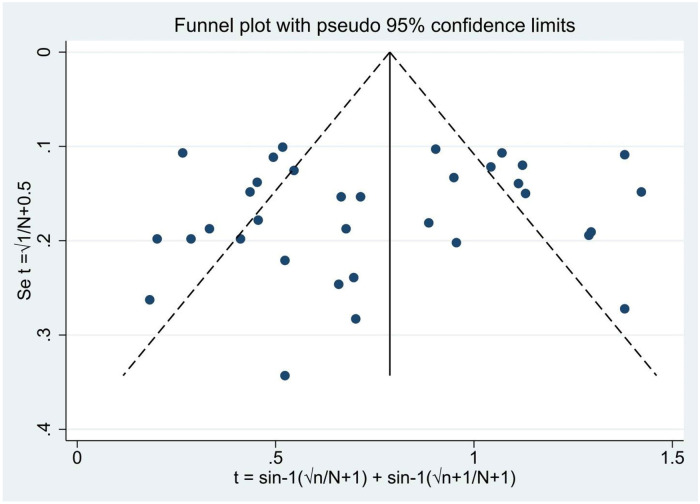
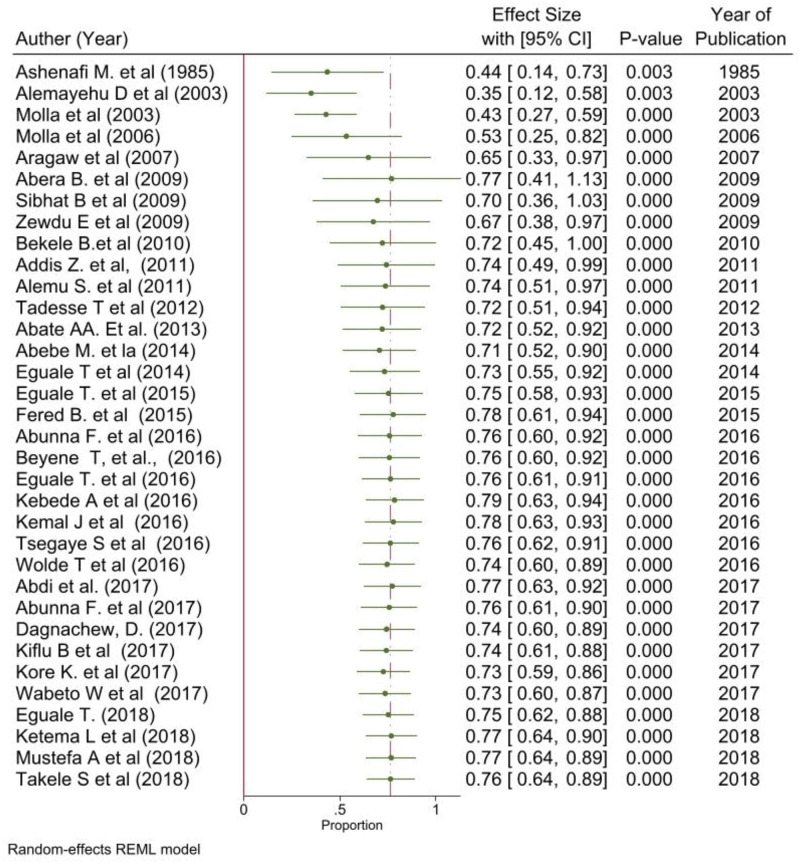
We assessed for heterogeneity and publication bias of studies. The analysis showed substantial heterogeneity among studies (Cochran’s Q test = 725.94, p<0.001; I2 = 95.45.0%, p<0.001) (Fig 4). This considerable level of heterogeneity among studies was also visualized by using Galbraith plot (Fig 5) test. The Begg and Mazmudar rank correlation test did not suggest study bias/low study effects (Kendall’s score = -28; P = 0.689 (Fig 6). Meta-Funnel plot (Fig 7) test also showed that there is no publication bias. So, trim and fill analysis is not necessary because there is no publication bias.
Fig 4. Forest plots of streptomycin resistant Salmonella serovars proportions.
Fig 5. Galbraith plot of streptomycin resistant Salmonella serovars proportions among studies.
Fig 6. Publication bias (meta-bias) for the proportion of streptomycin resistant Salmonella serovars.
Fig 7. Meta-funnel for the proportion of streptomycin resistant Salmonella serovars.
Prevalence of streptomycin resistant Salmonella serovars
We determined the pooled proportion of streptomycin resistant Salmonella serovars among 1475 isolates of the 34 studies. The overall pooled proportion of any streptomycin resistant Salmonella detection using the random effect model was 0.47% (95% CI: 0.35–0.60, I2 = 95.45%, p<0.001) (Fig 4).
Subgroup analysis
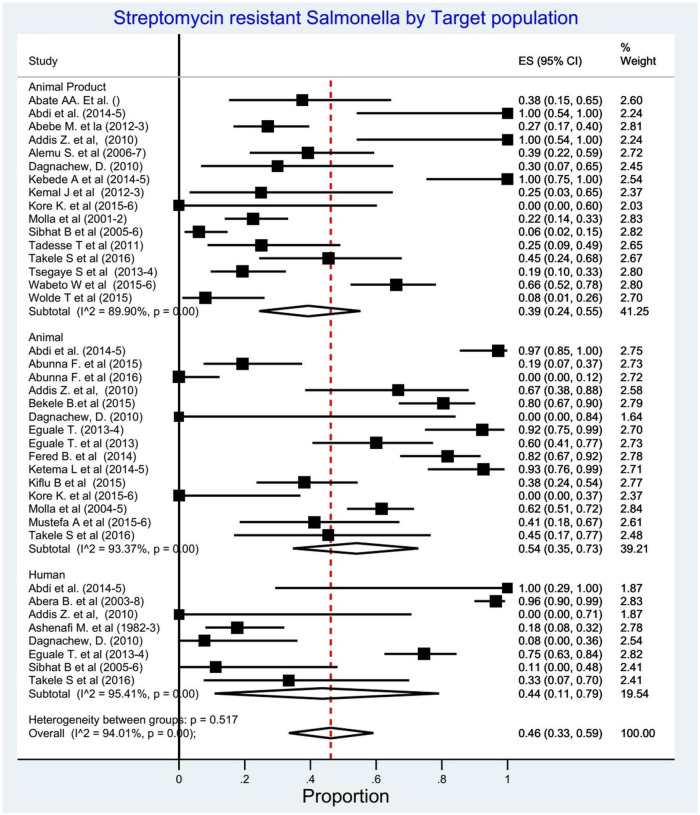
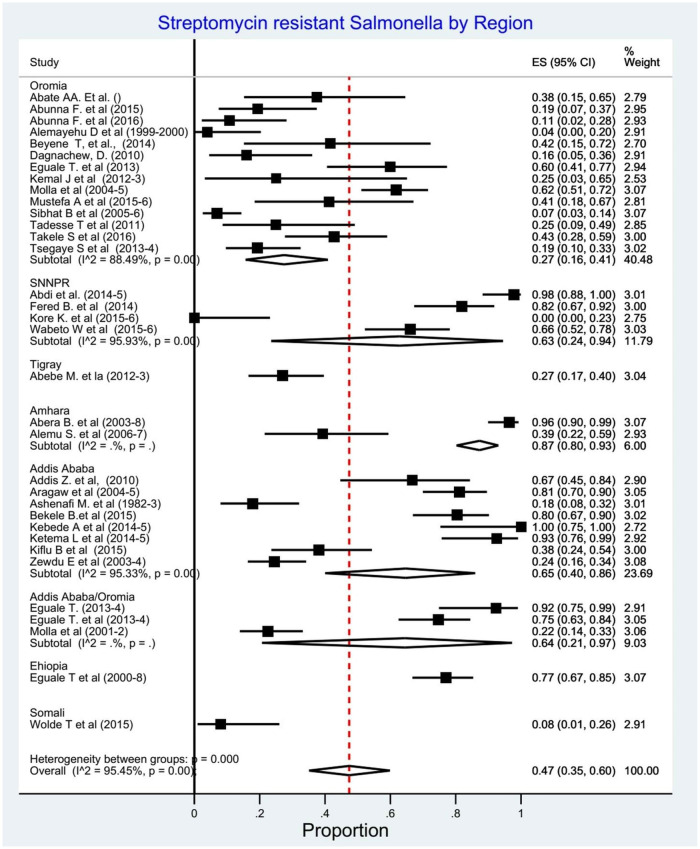
In the subgroup analysis based on target population (n = 38), the pooled proportion of streptomycin resistant Salmonella serovars was highest in animals 54% (95% CI; 35–73%), followed by humans 44% (95% CI; 33–59%) and animal products 39% (95% CI;24–55%) (Fig 8). Five studies were not included in this subgroup analysis as the target population was not clearly stated [49] and the streptomycin resistance by the target population is not available [29, 33, 38, 53]. Another subgroup analysis by region, the pooled proportion of the streptomycin resistance Salmonella serovars was 87% (95% CI; 80–93%) in Amhara,65% (95% CI; 40–86%) in Addis Ababa and 63% (95% CI; 24–94) in SNNPR (Fig 9).
Fig 8. Forest plots: Proportions of streptomycin resistant Salmonella serovars by target population.
Fig 9. Forest plots: Proportions of streptomycin resistant Salmonella serovars by region.
The time base analysis revealed that, despite not linear, the proportion of streptomycin resistant Salmonella serovars increased from 0.35 (95% CI; 0.12–0.58) in 2003 to0.77 (95% CI; 0.64–0.89) in 2018 among the study population (Fig 10).
Fig 10. Forest plots: Cumulative analysis of proportion of streptomycin resistant Salmonella serovars.
Sensitivity analysis
Sensitivity analysis is a vital part of meta-analysis as its goal is to determine the robustness of the observed outcomes to the assumptions considered in executing the analysis. Therefore, we did a sensitivity analysis to investigate the influence of one study on the overall meta-analysis estimate. The result presented that there is no sensitive article that influences either negatively or positivity the proportion of streptomycin resistant Salmonella serovars in Ethiopia.
Discussion
Antimicrobial resistant bacteria are a worldwide problem that affects all countries globally. In the case of zoonotic disease-causing microorganisms, the prevention of emerging antibiotic resistant strains is multifaced. This means; to prevent and control AMR in such organisms, compressive data from humans, animals and animal products should be available. To the best of our knowledge, this is the first meta-analyses assessing streptomycin resistant Salmonella serovars among humans, animals, and foods of animal origin in Ethiopia. In the review process, we found huge number of very heterogeneous literature conducted using different study designs, varied sample sizes, and different microbiological diagnostic methods; and most of them were excluded. However, we still found an adequate number of studies that enable us to produce data to answer our research questions.
In this meta-analysis, the pooled proportion of streptomycin resistant Salmonella serovars among animals and humans was 54% and 44%, respectively. This is higher than a meta-analysis among poultry (22.5%) and humans (12.4%) in Brazil [55]; and Broiler Production (2.8%) in a Nigerian national study [56]. The difference might be related to the difference in Salmonella serovars isolated, the AMR prevention and control practice across the countries, the microbiological diagnostic methods used, or the nature of the study population. In this case, the study conducted in Nigeria was the national surveillance against the meta analysis in our cases.
A data from time series analysis of the AMR pattern is much more important than the data taken in a snap shot approach (in a single year) to evaluate the program implemented and to design alternative AMR preventive and control strategies in a country. In this study, we observed a significant increase (from 0.35 to 0.77) of the streptomycin resistant Salmonella serovars from 2003 to 2018. The significant level of self-antibiotic prescription, self-medication, wrong indication, wrong duration, improper route of administration, immature discontinuation of antibiotics, and use of leftover antibiotics might be the possible reasons for this change as described in the recent meta-analysis conducted in the country [57].
The emergent MDR bacteria are the worst face of the ongoing overall increase of the resistance bacterial globally. The effect is particularly worrisome in developing countries where alternative antimicrobial drugs are lacking, routine testing of the isolates for antimicrobial resistance is limited and empirical treatment is used most of the time. In our review, we found that more than half of the Salmonella isolates (50.1%) were MDR. This is lower than a study conducted in India (80%) [58] but higher than that seen in a continent meta-analysis conducted in Africa (34.6%) [59]. The variation could also be explained with the different drug usage policies of the countries. In addition, our finding is lower than the other study done in Ethiopia (71.4%) [60]. In their study, they determined the MDR Salmonella serovars for other antimicrobials in addition to streptomycin that may vary the result with this study.
This is the first systematic review and meta-analysis addressed streptomycin resistance for Salmonella serovars in Ethiopia. It has analyzed articles from most parts of the country where eligible studies have been retrieved from. It has included those articles that were conducted to isolate streptomycin resistant Salmonella serovars in Ethiopia up to the time of data extraction.
Nevertheless, this study has several limitations. The studies used for analysis were heterogeneous as some were done in healthy participants and others in patients, a different group of animals and animal products, though this was dealt with a random-effects model. It was difficult to analyze the streptomycin resistance pattern of each bacterial serovars. The studies from some regions such as Tigray and Somali are limited in number, so the study result may not be representative of the remaining part of the country. We detected the high level of heterogeneity across all analyses, so the readers should interpret the pooled analysis and subgroup with caution.
Conclusion
In this review, about half of Salmonella serovars were streptomycin and multidrug resistance. This resistance was high when analyzed separately among human, animal and animal products. Moreover, a significantly increased level of streptomycin resistance was found in the last three decades. This shows a need for regular epidemiological surveillance to monitoring the occurrence and transmission of streptomycin resistance Salmonella serovars among human, animal and animal products. Judicial use of streptomycin in both humans and animals could prevent further development of resistance and prevention of resistant serovar transmission across the susceptible population is not left to tomorrow. Lastly, this review call to practice the one health approach to preventing further development of resistance in Ethiopia.
Supporting information
(DOC)
(XLSX)
(XLSX)
Abbreviations
- AMR
Antimicrobial Resistance
- CLSI
Clinical Laboratory Standard Institute
- DST
Drug Susceptibility Test
- JBI
Joanna Briggs Institute
- MDR
Multi drug resistant
- MIC
Minimum inhibitory concentration
- MOOSE
Meta-Analysis of Observational Studies in Epidemiology
- NTS
Non-typhoidal Salmonella
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SNNPR
Southern Nation Nationality and people region
- USA
United States of America
- ZD
Zone Diameters
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Eguale T, Asrat D, Alemayehu H, Nana I, Gebreyes WA, Gunn JS, et al. Phenotypic and genotypic characterization of temporally related nontyphoidal Salmonella strains isolated from humans and food animals in central Ethiopia. Zoonoses and public health. 2018;65(7):766–76. 10.1111/zph.12490 [DOI] [PubMed] [Google Scholar]
- 2.Djeffal S, Bakour S, Mamache B, Elgroud R, Agabou A, Chabou S, et al. Prevalence and clonal relationship of ESBL-producing Salmonella strains from humans and poultry in northeastern Algeria. BMC veterinary research. 2017;13(1):132 10.1186/s12917-017-1050-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal-Bayard J, Ramos-Morales F. Molecular Mechanisms Used by Salmonella to Evade the Immune System. Current issues in molecular biology. 2018;25:133–68. 10.21775/cimb.025.133 [DOI] [PubMed] [Google Scholar]
- 4.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bulletin of the World Health Organization. 2004;82(5):346–53. [PMC free article] [PubMed] [Google Scholar]
- 5.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet (London, England). 2012;379(9835):2489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejo M, Garedew L, Alebachew Z, Worku W. Prevalence and Antimicrobial Resistance of Salmonella Isolated from Animal-Origin Food Items in Gondar, Ethiopia. BioMed research international. 2016;2016:4290506 10.1155/2016/4290506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huehn S, La Ragione RM, Anjum M, Saunders M, Woodward MJ, Bunge C, et al. Virulotyping and antimicrobial resistance typing of Salmonella enterica serovars relevant to human health in Europe. Foodborne pathogens and disease. 2010;7(5):523–35. 10.1089/fpd.2009.0447 [DOI] [PubMed] [Google Scholar]
- 8.Zou W, Chen HC, Hise KB, Tang H, Foley SL, Meehan J, et al. Meta-analysis of pulsed-field gel electrophoresis fingerprints based on a constructed Salmonella database. PLoS One. 2013;8(3):e59224 10.1371/journal.pone.0059224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye J, Jackson C. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from U.S. food animals. Frontiers in Microbiology. 2013;4(135). 10.3389/fmicb.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schatz A, Bugie E, Waksman SA. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. 1944. Clin Orthop Relat Res. 2005(437):3–6. [DOI] [PubMed] [Google Scholar]
- 11.Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2016;22(12):968–74. [DOI] [PubMed] [Google Scholar]
- 12.Tilahun H, Schmidt E. Spatial analysis of livestock production patterns in Ethiopia. 2012. [Google Scholar]
- 13.Tadesse G. A meta-analysis of the proportion of animal Salmonella isolates resistant to drugs used against human salmonellosis in Ethiopia. BMC infectious diseases. 2015;15:84 10.1186/s12879-015-0835-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4(1):1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Systematic Reviews. 2017;6(1):263 10.1186/s13643-017-0663-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. The Lancet Infectious diseases. 2010;10(6):417–32. 10.1016/S1473-3099(10)70072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kale Janhavi, Nirpharake Anwaya. Comparison of Different Methods of Detecting Publication Bias. PhUSE. 2017. [Google Scholar]
- 18.ECDC E. EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control), 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in. 2014;13:4036.
- 19.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. Journal of Epidemiology and Community Health. 2013;67(11):974–8. 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 21.Abera B, Biadglegne F. Antimicrobial resistance of fecal isolates of Salmonella and shigella spp at bahir dar regional health research laboratory, northwest Ethiopia. Ethiopian Pharmaceutical Journal. 2009;27(1). [Google Scholar]
- 22.Wolde T, Abate M, Sileshi H, Mekonnen Y. Prevalence and antimicrobial susceptibility profile of Salmonella species from ready-to-eat foods from catering establishments in Jigjiga City, Ethiopia. African Journal of Microbiology Research. 2016;10(37):1555–60. [Google Scholar]
- 23.Tsegaye S, Beyene W, Tesfaye B, Tesfaye S, Feleke A. Prevalence and antimicrobial susceptibility pattern of Salmonella species from exotic chicken eggs in Alage, Ziway and Shashemene, Ethiopia. Africa Journal of Basic and Applied Science. 2016;8(3):180–4. [Google Scholar]
- 24.Tadesse T, Dabassa A. Prevalence and antimicrobial resistance of Salmonella isolated from raw milk samples collected from Kersa district, Jimma Zone, Southwest Ethiopia. Journal of Medical Sciences. 2012;12(7):224–8. [Google Scholar]
- 25.Mustefa BA, Gebremedhin EZ. Carriage and antimicrobial resistance of non-typhoidal Salmonella in cattle slaughtered in Ambo municipality abattoir, West Shewa zone, Oromia, Ethiopia-a point prevalence survey. Ethiopian Veterinary Journal. 2018;22(2):94–109. [Google Scholar]
- 26.Molla B, Mesfin A, Alemayehu D. Multiple antimicrobial-resistant Salmonella serotypes isolated from chicken carcass and giblets in Debre Zeit and Addis Ababa, Ethiopia. Ethiopian Journal of Health Development. 2003;17(2):131–9. [Google Scholar]
- 27.Ferede B, Desissa F, Feleke A, Tadesse G, Moje N. Prevalence and antimicrobial susceptibility of Salmonella isolates from apparently healthy slaughtered goats at Dire Dawa municipal abattoir, Eastern Ethiopia. Journal of Microbiology and Antimicrobials. 2015;7(1):1–5. [Google Scholar]
- 28.Dagnachew D. Distribution and antimicrobial resistance of Salmonella serotypes in minced beef, calves and humans in Bishoftu and Addis Ababa, Ethiopia. Journal of Parasitology and Vector Biology. 2017;9(5):64–72. [Google Scholar]
- 29.Beyene T, Yibeltie H, Chebo B, Abunna F, Beyi A, Mammo B, et al. Identification and antimicrobial susceptibility profile of Salmonella isolated from selected dairy farms, abattoir and humans at Asella town, Ethiopia. J Vet Sci Technol. 2016;7(3):320. [Google Scholar]
- 30.Bekele B, Ashenafi M. Distribution of drug resistance among enterococci and Salmonella from poultry and cattle in Ethiopia. Tropical animal health and production. 2010;42(5):857–64. 10.1007/s11250-009-9499-0 [DOI] [PubMed] [Google Scholar]
- 31.Ashenafi M, Gedebou M. Salmonella and Shigella in adult diarrhoea in Addis Ababa—prevalence and antibiograms. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1985;79(5):719–21. 10.1016/0035-9203(85)90201-9 [DOI] [PubMed] [Google Scholar]
- 32.Alemu S, Zewde BM. Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Tropical animal health and production. 2012;44(3):595–600. 10.1007/s11250-011-9941-y [DOI] [PubMed] [Google Scholar]
- 33.Alemayehu D, Molla B, Muckle A. Prevalence and antimicrobial resistance pattern of Salmonella isolates from apparently healthy slaughtered cattle in Ethiopia. Tropical animal health and production. 2003;35(4):309 10.1023/a:1025189204496 [DOI] [PubMed] [Google Scholar]
- 34.Abunna F, Bedasa M, Beyene T, Ayana D, Mamo B, Duguma R. Salmonella: isolation and antimicrobial susceptibility tests on isolates collected from poultry farms in and around Modjo, Central Oromia, and Ethiopia. JAPSC. 2016;5(2):21–35. [Google Scholar]
- 35.Abunna F, Ashenafi D, Beyene T, Ayana D, Mamo B, Duguma R. Isolation, identification and antimicrobial susceptibility profiles of Salmonella isolates from dairy farms in and around Modjo town, Ethiopia. Ethiopian Veterinary Journal. 2017;21(2):92–108. [Google Scholar]
- 36.Abebe M, Tafese B, Adane H. Antimicrobial resistance of Salmonella serovars isolated from food of bovine origin in selected Woredas of Tigray, Ethiopia. World J Med Sci. 2014;11(3):342–7. [Google Scholar]
- 37.Abate AA, Rakshit SK, Anal AK. Genotypic and phenotypic characterization of antimicrobial resistance patterns of Salmonella strains isolated from raw milk in Sebeta, Ethiopia. Int J Adv Lif Sci. 2013;6:192–9. [Google Scholar]
- 38.Zewdu E, Cornelius P. Antimicrobial resistance pattern of Salmonella serotypes isolated from food items and personnel in Addis Ababa, Ethiopia. Tropical animal health and production. 2009;41(2):241–9. 10.1007/s11250-008-9181-y [DOI] [PubMed] [Google Scholar]
- 39.Abdi RD, Mengstie F, Beyi AF, Beyene T, Waktole H, Mammo B, et al. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC infectious diseases. 2017;17(1):352 10.1186/s12879-017-2437-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. Journal of health, population, and nutrition. 2017;36(1):52 10.1186/s41043-017-0131-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takele S, Woldemichael K, Gashaw M, Tassew H, Yohannes M, Abdissa A. Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at the Jimma municipal abattoir, south-West Ethiopia. Tropical diseases, travel medicine and vaccines. 2018;4:13 10.1186/s40794-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibhat B, Molla Zewde B, Zerihun A, Muckle A, Cole L, Boerlin P, et al. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in ethiopia. Zoonoses and public health. 2011;58(2):102–9. 10.1111/j.1863-2378.2009.01305.x [DOI] [PubMed] [Google Scholar]
- 43.Molla B, Berhanu A, Muckle A, Cole L, Wilkie E, Kleer J, et al. Multidrug resistance and distribution of Salmonella serovars in slaughtered pigs. Journal of veterinary medicine B, Infectious diseases and veterinary public health. 2006;53(1):28–33. 10.1111/j.1439-0450.2006.00900.x [DOI] [PubMed] [Google Scholar]
- 44.Kore K, Asrade B, Demissie K, Aragaw K. Characterization of Salmonella isolated from apparently healthy slaughtered cattle and retail beef in Hawassa, southern Ethiopia. Preventive veterinary medicine. 2017;147:11–6. 10.1016/j.prevetmed.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 45.Kiflu B, Alemayehu H, Abdurahaman M, Negash Y, Eguale T. Salmonella serotypes and their antimicrobial susceptibility in apparently healthy dogs in Addis Ababa, Ethiopia. BMC veterinary research. 2017;13(1):134 10.1186/s12917-017-1055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ketema L, Ketema Z, Kiflu B, Alemayehu H, Terefe Y, Ibrahim M, et al. Prevalence and Antimicrobial Susceptibility Profile of Salmonella Serovars Isolated from Slaughtered Cattle in Addis Ababa, Ethiopia. BioMed research international. 2018;2018:9794869 10.1155/2018/9794869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemal J, Sibhat B, Menkir S, Beyene D. Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. Journal of infection in developing countries. 2016;10(11):1230–5. 10.3855/jidc.7885 [DOI] [PubMed] [Google Scholar]
- 48.Kebede A, Kemal J, Alemayehu H, Habte Mariam S. Isolation, Identification, and Antibiotic Susceptibility Testing of Salmonella from Slaughtered Bovines and Ovines in Addis Ababa Abattoir Enterprise, Ethiopia: A Cross-Sectional Study. International journal of bacteriology. 2016;2016:3714785 10.1155/2016/3714785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eguale T, Marshall J, Molla B, Bhatiya A, Gebreyes WA, Engidawork E, et al. Association of multicellular behaviour and drug resistance in Salmonella enterica serovars isolated from animals and humans in Ethiopia. Journal of applied microbiology. 2014;117(4):961–71. 10.1111/jam.12579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, Engidawork E. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC infectious diseases. 2015;15:497 10.1186/s12879-015-1235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eguale T, Engidawork E, Gebreyes WA, Asrat D, Alemayehu H, Medhin G, et al. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC microbiology. 2016;16:20 10.1186/s12866-016-0638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: prevalence and antimicrobial resistance. BMC veterinary research. 2018;14(1):217 10.1186/s12917-018-1539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aragaw K, Molla B, Muckle A, Cole L, Wilkie E, Poppe C, et al. The characterization of Salmonella serovars isolated from apparently healthy slaughtered pigs at Addis Ababa abattoir, Ethiopia. Preventive veterinary medicine. 2007;82(3–4):252–61. 10.1016/j.prevetmed.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 54.Addis Z, Kebede N, Worku Z, Gezahegn H, Yirsaw A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC infectious diseases. 2011;11:222 10.1186/1471-2334-11-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voss-Rech D, Potter L, Vaz CS, Pereira DI, Sangioni LA, Vargas AC, et al. Antimicrobial Resistance in Nontyphoidal Salmonella Isolated from Human and Poultry-Related Samples in Brazil: 20-Year Meta-Analysis. Foodborne pathogens and disease. 2017;14(2):116–24. 10.1089/fpd.2016.2228 [DOI] [PubMed] [Google Scholar]
- 56.Oloso NO, Adeyemo IA, van Heerden H, Fasanmi OG, Fasina FO. Antimicrobial Drug Administration and Antimicrobial Resistance of Salmonella Isolates Originating from the Broiler Production Value Chain in Nigeria. Antibiotics (Basel). 2019;8(2). 10.3390/antibiotics8020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muhie OA. Antibiotic use and resistance pattern in ethiopia: systematic review and meta-analysis. International journal of microbiology. 2019;2019 10.1155/2019/2489063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh B, Singh P, Agrawal S, Teotia U, Verma A, Sharma S, et al. Prevalence of multidrug resistant Salmonella in coriander, mint, carrot, and radish in Bareilly and Kanpur, northern India. Foodborne pathogens and disease. 2007;4(2):233–40. 10.1089/fpd.2006.0082 [DOI] [PubMed] [Google Scholar]
- 59.Founou LL, Amoako DG, Founou RC, Essack SY. Antibiotic Resistance in Food Animals in Africa: A Systematic Review and Meta-Analysis. Microbial drug resistance (Larchmont, NY). 2018;24(5):648–65. 10.1089/mdr.2017.0383 [DOI] [PubMed] [Google Scholar]
- 60.Tadesse G. A meta-analysis of the proportion of antimicrobial resistant human Salmonella isolates in Ethiopia. BMC pharmacology & toxicology. 2014;15:51 10.1186/2050-6511-15-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.