Supplemental Digital Content is available in the text.
Keywords: correction rates, electronic ICU Collaborative Research Database, hypernatremia, Medical Information Mart for Intensive Care III, mortality
Abstract
Objectives:
Describe the relationship between ICU-acquired hypernatremia and in-hospital mortality and investigate the optimal hypernatremia correction rate.
Design, Setting, Participants, and Measurements:
Observational study including two individual ICU cohorts. We used the Medical Information Mart for Intensive Care III v. 1.4 database consists of all ICU patients admitted to the Beth Israel Deaconess Medical Center in Boston from 2001 to 2012 (n = 46,476). The electronic ICU v. 2.0 database consists of all ICU patients admitted to 208 distinct hospitals across the United States from 2014 to 2015 (n = 200,859). We included all adult patients admitted to an ICU with two consecutive sodium samples within normal range (135–145 mmol/L) and without two consecutive hyponatremic samples (< 135 mmol/L) during the ICU stay.
Results:
Of 23,445 patients identified in Medical Information Mart for Intensive Care III, 9% (n = 2,172) developed hypernatremia during their ICU stay. In electronic ICU, 88,160 patients were identified and 7% (n = 5,790) developed hypernatremia. In both cohorts, patients with hypernatremia had a higher mortality (Medical Information Mart for Intensive Care III: 20% vs 42%; p < 0.01 and electronic ICU: 6% vs 22%; p < 0.01), with hypernatremia increasing the risk of in-hospital mortality (Medical Information Mart for Intensive Care III: odds ratio, 1.15; 95% CI, 1.13–1.17 and electronic ICU: odds ratio, 1.11; 95% CI, 1.10–1.12) and over time using a Cox regression. Rapid sodium correction rate (> 0.5 mmol/L/hr) was associated with an increased in-hospital mortality in both cohorts (Medical Information Mart for Intensive Care III: odds ratio, 1.08; 95% CI, 1.03–1.13 and electronic ICU: odds ratio, 1.10; 95% CI, 1.06–1.13). In the electronic ICU cohort, rapid correction rates were associated with a significant difference in in-hospital mortality, but there was no statistically significant association in the Medical Information Mart for Intensive Care III cohort.
Conclusions:
ICU-acquired hypernatremia is associated with increased in-hospital mortality. Furthermore, a rapid sodium correction rates may be harmful. This suggests it is important to both prevent ICU-acquired hypernatremia and to avoid rapid correction rates if a patient becomes hypernatremic.
Hypernatremia develops in 4–26% of patients during their stay at the ICU (1). ICU-acquired hypernatremia can develop due to infusion of large amounts of isotonic fluids, IV medication administration, high insensible losses, impaired thirst response, and/or an inability to respond to thirst appropriately (1–3). Studies of ICU-acquired hypernatremia suggest frequent under treatment and an increased occurence rate of this condition (1, 4, 5).
Hypernatremia results in hyperosmolality, which can lead to coma, rhabdomyolysis, decreased heart contractility, and impaired glucose metabolism (1, 2). Previous studies have found an association between hypernatremia and increased length of stay (LOS) as well as mortality (6, 7). These studies have also suggested hypernatremia is an independent risk factor for adverse outcomes and is not only an epiphenomenon of illness severity.
Current guidelines suggest that correction of chronic hypernatremia (defined as serum sodium > 145 mmol/L for more than 48 hr) should be slower than the acute hypernatremia (defined as serum sodium > 145 mmol/L for < 48 hr) due to increased risk for cerebral edema at more rapid correction rates (8). However, this strategy is based on findings from animal studies and children (1, 9, 10). A recent study by Chauhan et al (11) using the Medical Information Mart for Intensive Care III (MIMIC-III) dataset provides evidence that the guidelines may be misguided in adults, as there was no evidence of increased mortality with rapid correction of severe hypernatremia (> 155 mmol/L).
The aim of this study is to investigate the relationship between in-hospital mortality and the presence and severity of ICU-acquired hypernatremia in adults, as well as the association between in-hospital mortality and correction rate of hypernatremia. These associations are investigated in two independent cohorts. We hypothesized that ICU-acquired hypernatremia and a correction rate above 12 mmol/L/d is associated with increased mortality.
METHODS
Study Population
This cohort study was conducted by extracting data from two independent databases: 1) the MIMIC-III database v. 1.4, which includes all patients admitted to an ICU at the Beth Israel Deaconess Medical Center in Boston, MA, from 2001 to 2012 (n = 46,476) (12) and 2) the electronic ICU (eICU) Collaborative Research Database v. 2.0 (13), which consist of patients admitted to 208 distinct hospitals across the United States from 2014 to 2015 ICUs (n = 200,859) (14). This study includes data from two publicly available, anonymized databases with preexisting institutional review board approval; thus, no further approval was required (12, 15).
Data Extraction
Both databases were queried using pgAdmin (pgAdmin Development Team, 2020), a free and open-source graphical user interface administration tool for PostgreSQL (PostgreSQL Global Development Group, 2020), using data acquired from PhysioNet (16). We extracted patient age, gender, LOS, in-hospital mortality, illness severity scores (Acute Physiology Score III from MIMIC-III and Acute Physiology and Chronic Health Evaluation IV from eICU), admission ward, and all sodium measurements for every patient after admission to the ICU. Full data extraction methodology is available in Supplement Material (http://links.lww.com/CCX/A462). To explore the influence of induced hypernatremia by hyperosmolar therapy, we also extracted the administration of hypertonic saline and sodium bicarbonate from MIMIC-III. We could not extract this from eICU since the database structure did not permit a valid method for extraction.
ICU-Acquired Hypernatremia
We included ICU patients between the age of 18 and 89 years with two consecutive plasma sodium samples within normal range (135–145 mmol/L) upon admission. We excluded patients with hyponatremia, defined as at least two consecutive plasma sodium below 135 mmol/L. To reduce the influence of erroneous laboratory value entries, only patients with two consecutive sodium levels above the normal range (≥ 145 mmol/L) during the ICU stay were defined as having ICU-acquired hypernatremia. For secondary analyses, patients with two consecutive samples above 155 mmol/L were categorized as having severe ICU-acquired hypernatremia, while hypernatremic patients without two consecutive sodium values greater than 155 mmol/L were classified as having moderate hypernatremia. ”Peak sodium” was defined as the maximum sodium value (mmol/L) measured during a patient’s ICU stay. If patients had more than one ICU admission, only the first admission were used; thus, each patient was only included once. If patients never had a hypernatremic period in any of their ICU admissions, their first ICU admission was included for analysis.
For hypernatremic patients, the “hypernatremic duration” was defined as the accumulated length of the hypernatremic periods. The “hypernatremic burden” was defined as area under the curve when the sodium level were above 145 mmol/L. Because sodium measures were taken sporadically, and at clinicians’ discretion, a linear interpolation was performed to estimate a plasma sodium value every hour of the ICU stay. Every measurement drawn in the hypernatremic periods were compared with the interpolated minimum sodium concentration up to 24 hours after the measurement. Dividing the time between these two points resulted in an hourly “sodium correction rate” (Fig. 1B). The highest sodium correction rate for every patient was included in the analysis. The patients’ sodium correction rates were separated into three groups using thresholds previously used in the literature (1, 9, 17): slow (< 0.25 mmol/L/hr), moderate (0.25–0.50 mmol/L/hr), and fast (> 0.50 mmol/L/hr) correction of serum sodium.
Figure 1.
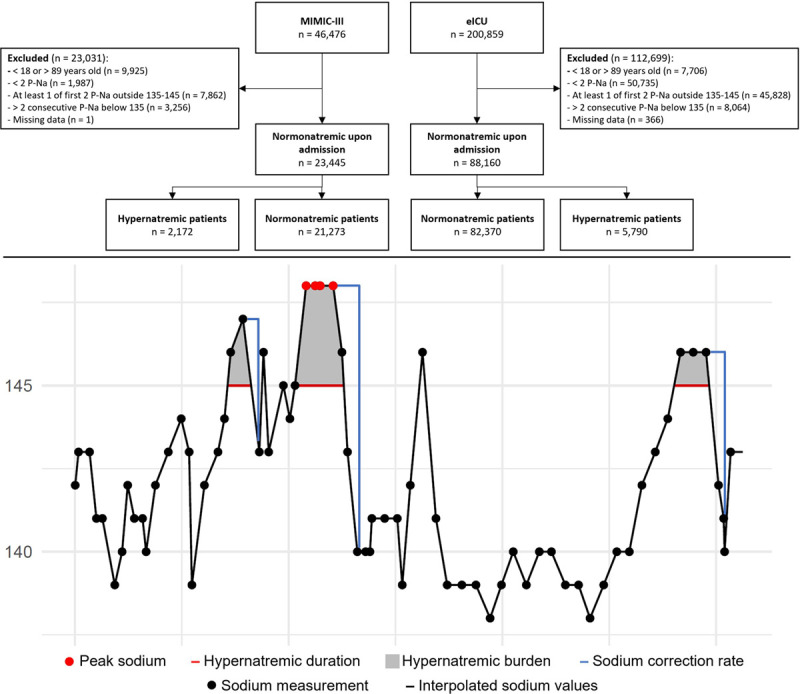
A, Consolidated Standards of Reporting Trial flow diagram. B, An example of variable extraction. The graph shows a patient with three hypernatremic periods (filled gray area) and correction rates (blue lines), where the periods are added together to calculate hypernatremic duration (red lines) and hypernatremic burden. The highest peak sodium and sodium correction rate are extracted. eICU = electronic ICU, MIMIC-III = Medical Information Mart for Intensive Care III, P-Na = plasma sodium.
Outcome and Covariates
The primary outcome was in-hospital mortality. Consistent with previous studies investigating hypernatremia, we chose age, sex, and an illness severity score as covariates when doing multivariable analyses (15, 18). We included the number of samples in the multivariable models, to account for the fact that multiple measurements would increase the risk of measuring a high sodium. As exploratory analyses, we tested the models after addition of administration of either hypertonic saline or sodium bicarbonate (for MIMIC-III) or admission ward (for eICU).
Data Analysis
The association between hypernatremia and in-hospital mortality was assessed using multivariate logistic regression and Cox proportional hazard regression with the covariates described above. We used univariable logistic regression to characterize the influence of peak sodium, hypernatremic duration, and hypernatremic burden on in-hospital mortality. The analyses were carried out only after ensuring the model assumptions were met, including the proportionality of hazards. The sodium correction rates were analyzed using similar univariable and multivariable analyses. Subgroup analyses for hypernatremic groups and sodium correction rates were carried out using Wilcoxon signed-rank test, and p values are presented only after Bonferroni correction. Analyses were initially carried out in the MIMIC-III cohort and subsequently repeated in the eICU cohort. Data were analyzed using R 4.0.0 (R Core Team, Vienna, Austria).
RESULTS
Patient Characteristics
We identified 23,445 patients in the MIMIC-III cohort who were normonatremic upon admission and 88,160 patients in the eICU cohort (Fig. 1A). Nine percent (n = 2,172) of MIMIC-III patients and 7% (n = 5,790) of eICU patients developed hypernatremia during their ICU admission. Baseline characteristics were notable for higher mortality and longer LOS among both normonatremic and hypernatremic patients in the MIMIC-III database compared with the eICU database (p < 0.001). In both the MIMIC-III and the ICU database, the hypernatremic patients were generally older, had higher illness severity scores, longer LOS, and increased mortality compared with patients with normonatremia. For all characteristics, see Table 1. Approximately the same percentage of patients with normonatremia and hypernatremia had one or more admissions after the analyzed admission (normonatremic: 36%; hypernatremic: 31%).
TABLE 1.
Normonatremic Patients Compared With Patients With ICU-Acquired Hypernatremia
| Variables | Medical Information Mart for Intensive Care III (n = 23,445) | Electronic ICU (n = 88,160) | ||
|---|---|---|---|---|
| Normonatremia (n = 21,273) | Hypernatremia (n = 2,172) | Normonatremic (n = 82,370) | Hypernatremic (n = 5,790) | |
| Age, median (IQR) | 63 (51–75) | 69 (56–78) | 65 (53–75) | 67 (56–77) |
| Male, n (%) | 12,260 (58) | 1,302 (60) | 44,526 (54) | 3,282 (57) |
| Acute Physiology Score III, median (IQR) | 35 (26–45) | 48 (36–63) | — | — |
| Acute Physiology and Chronic Health Evaluation IV, median (IQR) | — | — | 50 (37–65) | 71 (55–91) |
| Number of samples, median (IQR) | 6 (4–9) | 21 (13–32) | 4 (3–7) | 14 (9–22) |
| Sodium (mmol/L), mean ± sd | 139 ± 3.1 | 143 ± 4.7 | 139 ± 3.1 | 144 ± 3.1 |
| Glucose (mg/dL), mean ± sd | 130 ± 57 | 135 ± 61 | 133 ± 57 | 147 ± 57 |
| Length of stay (d), median (IQR) | 6.0 (3.9–9.4) | 15.1 (9.7–24.0) | 3.3 (1.8–5.5) | 9.8 (6.1–15.4) |
| Ward, n (%) | ||||
| Cardiothoracic ICUa | 7,753 (36) | 433 (20) | 18,832 (23) | 969 (17) |
| General ICUb | 13,520 (64) | 1,739 (80) | 57,715 (70) | 4,254 (73) |
| Neurologic ICUc | — | — | 5,823 (7) | 567 (10) |
| Administration of hypertonic saline or sodium bicarbonated, n (%) | 1,201 (6) | 395 (18) | — | — |
| In-hospital mortality, n (%) | 4,170 (20) | 912 (42) | 4,647 (6) | 1,296 (22) |
IQR = interquartile range.
aCardiothoracic ICU includes cardiac surgery recovery unit, coronary care unit, cardiac ICU, cardiac surgical ICU, cardiothoracic ICU, and coronary-cardiothoracic ICU.
bGeneral ICU includes medical ICU, surgical ICU, trauma/surgical ICU, and medical-surgical ICU.
cThere is not a neurologic ICU in Medical Information Mart for Intensive Care III.
dThis information was not available in electronic ICU.
Dash indicates when data is not available in the specific database.
ICU-Acquired Hypernatremia and Mortality
In both cohorts, ICU-acquired hypernatremia was associated with increased risk of in-hospital mortality compared with patients with normonatremia (Table 2). Higher in-hospital mortality was associated with both a higher peak sodium (MIMIC-III: odds ratio [OR], 1.03; 95% CI, 1.02–1.03; p < 0.01 and eICU: OR, 1.02; 95% CI, 1.02–1.02; p < 0.01) and a higher hypernatremic burden (MIMIC-III: OR, 1.02; 95% CI, 1.01–1.02; p < 0.01 and eICU: OR, 1.00; 95% CI, 1.00–1.00; p < 0.01). Longer duration of hypernatremia was associated with lower mortality (MIMIC-III: OR, 1.00; 95% CI, 0.99–1.00; p = 0.12 and eICU: OR, 0.99; 95% CI, 0.99–1.00; p < 0.01) (Fig. 2).
TABLE 2.
Multivariable Analyses of ICU-Acquired Hypernatremia
| Multivariable Logistic Regression | Medical Information Mart for Intensive Care III (n = 23,445) | Electronic ICU (n = 88,160) | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (numeric, per 10 yr) | 1.04 (1.04–1.04) | < 0.001 | 1.00 (1.00–1.00) | 0.001 |
| Male (binary) | 1.00 (0.99–1.01) | 0.907 | 1.04 (0.87–1.24) | 0.683 |
| Hypernatremia (binary) | 1.15 (1.13–1.17) | < 0.001 | 1.11 (1.10–1.12) | < 0.001 |
| APS III (numeric, per 10 points) | 1.06 (1.05–1.06) | < 0.001 | — | — |
| APACHE IV (numeric, per 10 points) | — | — | 1.04 (1.04–1.04) | < 0.001 |
| Number of samples (numeric, per 10 samples) | 1.00 (1.00–1.00) | 0.375 | 0.98 (0.98–0.99) | < 0.001 |
| Multivariable Cox Regression | HR (95% CI) | p | HR (95% CI) | p |
| Age (numeric, per 10 yr) | 1.29 (1.27–1.32) | < 0.001 | 1.11 (1.09–1.14) | < 0.001 |
| Male (binary) | 1.01 (0.95–1.06) | 0.889 | 0.99 (0.94–1.05) | 0.862 |
| Hypernatremia (binary) | 1.60 (1.47–1.75) | < 0.001 | 1.86 (1.72–2.00) | < 0.001 |
| APS III (numeric, per 10 points) | 1.28 (1.27–1.32) | < 0.001 | — | — |
| APACHE IV (numeric, per 10 points) | — | — | 1.46 (1.45–1.47) | < 0.001 |
| Number of samples (numeric, per 10 samples) | 0.92 (0.89–0.95) | < 0.001 | 0.82 (0.79–0.86) | < 0.001 |
APACHE IV = Acute Physiology and Chronic Health Evaluation IV, APS III = Acute Physiology Score III, HR = hazard ratio, OR = odds ratio.
Dash indicates when data is not available in the specific database. Hypernatremia-related variables is marked as bold.
Figure 2.
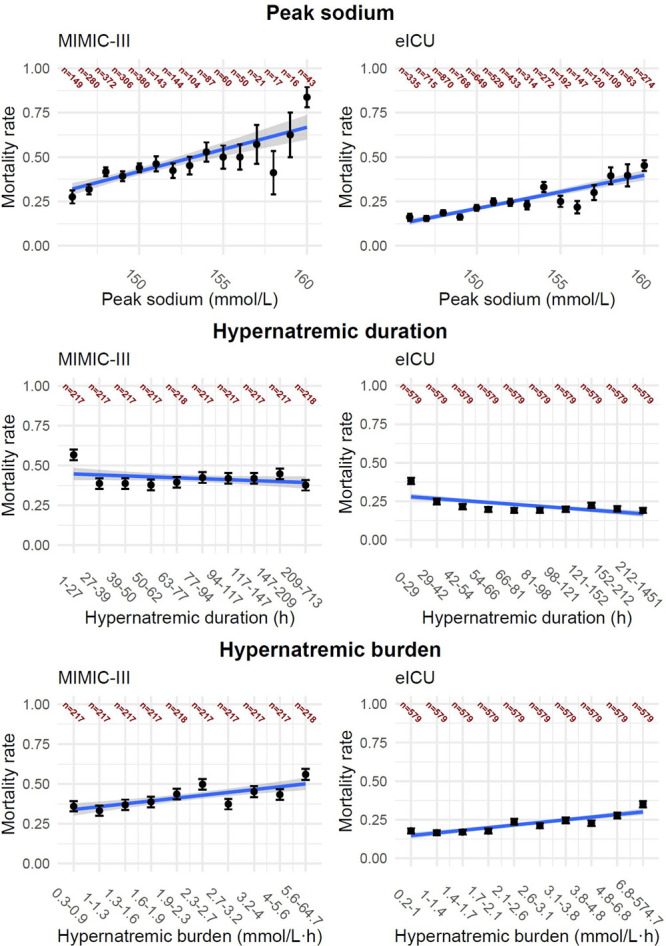
Variables from the Medical Information Mart for Intensive Care III (MIMIC-III) (left column) and electronic ICU (eICU) (right column) database associated with in-hospital mortality. Top row presents the mortality rate based on maximum sodium value (peak sodium). Patients with peak sodium within normal range but below 140 mmol/L were combined in one group, and patients with maximum values above 156 mmol/L were combined another group. Middle row presents the accumulated hypernatremic duration and the association with in-hospital mortality, and the bottom row shows the association between hypernatremic burden and mortality. The line (blue) shows the univariable regression line. The groups for duration and burden are stratified based on group size, which are presented in dark red.
Subgroup analysis of in-hospital mortality based on the three sodium categories (normonatremia, moderate hypernatremia, and severe hypernatremia) demonstrated increased mortality in each of the hypernatremia groups compared with the normonatremia group, as well as increased mortality in the severe hypernatremia group compared with the moderate hypernatremia group in both cohorts (Supplemental Table 1, http://links.lww.com/CCX/A469).
Including administration of hypertonic saline or sodium bicarbonate (for MIMIC-III) and admission to a neurologic ICU (for eICU) into exploratory analyses did not change the relationship between ICU-acquired hypernatremia and in-hospital mortality, but the administration of hypertonic saline or sodium bicarbonate (OR, 1.06; 95% CI, 1.04–1.09; p < 0.001) and neurologic ICU admission (OR, 1.02; 95% CI, 1.01–1.03; p < 0.001) in itself were associated with in-hospital mortality (Supplemental Table 2, http://links.lww.com/CCX/A463).
Sodium Correction Rates and Mortality
A more rapid sodium correction rate was associated with an increased in-hospital mortality in both cohorts (MIMIC-III: OR, 1.08; 95% CI, 1.03–1.13; p < 0.01 and eICU: OR, 1.10; 95% CI, 1.06–1.13; p < 0.01) (Table 3). When grouping correction rates into slow, moderate, and fast, there was significantly higher mortality in the fast correctors compared to the slow (p < 0.001) and moderate (p < 0.001) correctors in eICU patients, but no difference among groups in MIMIC-III patients (Fig. 3).
TABLE 3.
Multivariable Analyses of Sodium Correction Rate and Association With In-Hospital Mortality
| Multivariable Logistic Regression | Medical Information Mart for Intensive Care III (n = 2,982) | Electronic ICU (n = 8,594) | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (numeric, per 10 yr) | 1.04 (1.03–1.05) | < 0.001 | 1.00 (1.00–1.01) | 0.296 |
| Male (binary) | 1.02 (0.98–1.07) | 0.308 | 1.02 (0.99–1.04) | 0.196 |
| Sodium correction rate (numeric, per 1 mmol/L/hr) | 1.08 (1.03–1.13) | 0.003 | 1.10 (1.06–1.13) | < 0.001 |
| APS III (numeric, per 10 points) | 1.04 (1.03–1.05) | < 0.001 | — | — |
| APACHE IV (numeric, per 10 points) | — | — | 1.03 (1.03–1.03) | < 0.001 |
| Number of samples (numeric, per 10 samples) | 0.98 (0.97–0.99) | < 0.001 | 0.97 (0.97–0.98) | < 0.001 |
| Multivariable Cox Regression | HR (95% CI) | p | HR (95% CI) | p |
| Age (numeric, per 10 yr) | 1.12 (1.07–1.18) | < 0.001 | 1.00 (0.96–1.02) | 0.975 |
| Male (binary) | 1.07 (0.94–1.22) | 0.313 | 1.08 (0.96–1.21) | 0.225 |
| Sodium correction rate (numeric, per 1 mmol/L/hr) | 1.32 (1.16–1.51) | < 0.001 | 1.48 (1.33–1.64) | < 0.001 |
| APS III (numeric, per 10 points) | 1.13 (1.09–1.16) | < 0.001 | — | — |
| APACHE IV (numeric, per 10 points) | — | — | 1.18 (1.16–1.20) | < 0.001 |
| Number of samples (numeric, per 10 samples) | 0.85 (0.81–0.89) | < 0.001 | 0.80 (0.76–0.85) | < 0.001 |
APACHE IV = Acute Physiology and Chronic Health Evaluation IV, APS III = Acute Physiology Score III, HR = hazard ratio, OR = odds ratio.
Dash indicates when data is not available in the specific database. Hypernatremia-related variables is marked as bold.
Figure 3.
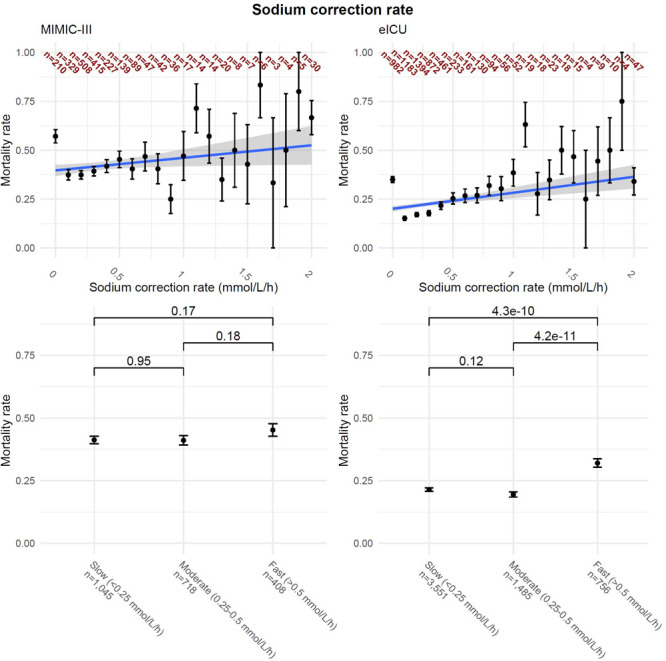
Correction rates of hypernatremic patients. Left column is Medical Information Mart for Intensive Care III (MIMIC-III), while the right column is electronic ICU (eICU). Upper row presents mortality rate based on maximum correction rate with aggregated values greater than 15 to 15 mmol/L, with numbers in each group presented with dark red. Lower row presents the mortality rate based on three groups. p value shown is the Fisher exact test, with Bonferroni correction.
Including administration of hypertonic saline or sodium bicarbonate (for MIMIC-III) and admission to a neurologic ICU (for eICU) into exploratory analyses did not change the relationship between and in-hospital mortality, but only admission to a neurologic ICU was in this model associated with in-hospital mortality (OR, 1.07; 95% CI, 1.03–1.11; p < 0.001) (Supplemental Table 3, http://links.lww.com/CCX/A464).
DISCUSSION
We report an occurence rate of ICU-acquired hypernatremia of 7–9% among all ICU patients, which is lower than previously described (1). This is likely due to our more restrictive definition of hypernatremia, with two consecutive hypernatremic samples required. After controlling for multiple potential confounders, in-hospital mortality was higher in patients with ICU-acquired hypernatremia. Subgroup analysis based on severity of hypernatremia demonstrated that even mild cases (145–155 mmol/L) were associated with increased in-hospital mortality, corroborating findings from other studies (1, 19). Higher peak sodium values were significantly associated with increased in-hospital mortality, which aligns with a previous study analyzing data from the eICU dataset (15).
Our work generally corroborates studies suggesting the safe daily correction rate of chronic hypernatremia is between 10 and 12 mmol/L (1, 9). However, our findings differ from prior studies in notable ways as well.
We did not observe any increase in mortality with slower rates of correction, as has previously been described in patients with chronic hyponatremia corrected at less than 0.25 mmol/L/hr (9). This could be explained by the difference in cohort sizes and inclusion criteria, as other studies included hospitalized patients with severe hypernatremia (> 155 mmol/L) both at admission and acquired during the hospitalization; patients admitted with hypernatremia may have different etiologies of sodium derangement.
Fast correction of hypernatremia (> 0.5 mmol/L/hr) was associated increased in-hospital mortality in eICU database but not the MIMIC database. A recent study using the MIMIC-III dataset during the same time period also found no evidence of higher mortality or any significant neurologic events at patients corrected at rates great than 0.5 mmol/L/hr (11), which supports the validity of our analytic technique. Nevertheless, we did find an increase in mortality with faster correction in the eICU cohort using the same techniques. One possible explanation for the discrepancy is that in the MIMIC-III cohort patients were at baseline a sicker population with a higher overall mortality and LOS, which may have been more heavily determined by factors independent of sodium correction rate, whereas eICU patients had proportionally less sodium-independent factors contributing to mortality. In addition, MIMIC-III is from a single center, which limits the external validity of the results from this dataset, as there may be practice patterns or patient characteristics at that hospital which uniquely impact outcomes. eICU is a multicenter database, and therefore, the results carry more external validity and are more likely to apply to a general patient population.
Given the higher mortality seen with even mild cases of hypernatremia, our results suggest it would be beneficial to avert the occurence rate of hypernatremia (> 145 mmol/L) in ICU patients (1, 19),excluding those with elevated intracranial pressure or other intracranial pathologies for which therapeutic hypernatremia may be indicated (20–22). Rapid correction should be avoided regardless of the chronicity of the hypernatremia; in contrast to other studies (23), longer duration of hypernatremia was not associated with increased mortality in our analysis, suggesting quickly correcting back to normal is not imperative once an individual becomes hypernatremic.
The major strength is the use of the same methodology in two separate, large databases, added both power and external validity. This study is limited by the inherent retrospective nature of the two databases. The data from the databases were collected prospectively but not systematically to ascertain the associations examined in this study (24). Blood samples were taken sporadically, and to estimate the hourly sodium values, we interpolated the values, which is susceptible to bias. We did not differentiate between acute and chronic hypernatremia (persistent for more than 48 hr) in our analysis of sodium correction rates, an important distinction that should be addressed in future studies. While we corrected for illness severity score, there were likely unmeasured confounders contributing to mortality.
In conclusion, ICU-acquired hypernatremia is associated with increased in-hospital mortality even at relatively low levels, with increased peak sodium and increased hypernatremic burden both associated with increased risk of death. Rapid correction of hypernatremia was associated with increased in-hospital mortality, contradicting recent evidence that it may be benign, and there was no evidence that longer duration of hypernatremia increased mortality. Overall, our study suggests that preventing even mild hypernatremia, while avoiding aggressive correction if hypernatremia develops, may be the best strategy.
ACKNOWLEDGMENTS
We would like to acknowledge the team behind PhysioNet, Medical Information Mart for Intensive Care III, and electronic ICU Collaborative Research Database for the availability of the data and multiple data extraction guidelines. Furthermore, we would especially like to acknowledge the organizers of Aarhus Critical Care Datathon 2019, where the coauthors were introduced, and the concept of the study was outlined.
Supplementary Material
Footnotes
For this type of study, formal consent is not required.
The anonymized databases did not require ethical approval before use, but a short course regarding how data must be handled and good clinical practice.
Dr. Olsen involved in concept and design of the study, analysis of data, writing of the article, important intellectual content, and approval of final article. Drs. Møller, Andersson, Sherak, and Jeppesen involved in concept and design of the study, important intellectual content, and approval of final article. Dr. Romano involved in concept and design of the study, data acquisition, important intellectual content, and approval of final article. Drs. Mlodzinski and Raines involved in important intellectual content, approval of final article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Dr. Sherak received funding from the Albert Einstein Office of Medical Student Research to travel to the Datathon. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lindner G, Funk G-C. Hypernatremia in critically ill patients. J Crit Care. 2013; 28:216.e11–e20 [DOI] [PubMed] [Google Scholar]
- 2.Kim SW. Hypernatemia: Successful treatment. Electrolyte Blood Press. 2006; 4:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarahian S, Pouria MM, Ing TS, et al. Hypervolemic hypernatremia is the most common type of hypernatremia in the intensive care unit. Int Urol Nephrol. 2015; 47:1817–1821 [DOI] [PubMed] [Google Scholar]
- 4.Sterns RH. Hypernatremia in the intensive care unit: Instant quality–just add water. Crit Care Med. 1999; 27:1041–1042 [DOI] [PubMed] [Google Scholar]
- 5.Oude Lansink-Hartgring A, Hessels L, Weigel J, et al. Long-term changes in dysnatremia incidence in the ICU: A shift from hyponatremia to hypernatremia. Ann Intensive Care. 2016; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darmon M, Timsit JF, Francais A, et al. Association between hypernatraemia acquired in the ICU and mortality: A cohort study. Nephrol Dial Transplant. 2010; 25:2510–2515 [DOI] [PubMed] [Google Scholar]
- 7.Lindner G, Funk GC, Lassnigg A, et al. Intensive care-acquired hypernatremia after major cardiothoracic surgery is associated with increased mortality. Intensive Care Med. 2010; 36:1718–1723 [DOI] [PubMed] [Google Scholar]
- 8.Sterns RH. Disorders of plasma sodium–causes, consequences, and correction. N Engl J Med. 2015; 372:55–65 [DOI] [PubMed] [Google Scholar]
- 9.Alshayeb HM, Showkat A, Babar F, et al. Severe hypernatremia correction rate and mortality in hospitalized patients. Am J Med Sci. 2011; 341:356–360 [DOI] [PubMed] [Google Scholar]
- 10.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000; 342:1581–1589 [DOI] [PubMed] [Google Scholar]
- 11.Chauhan K, Pattharanitima P, Patel N, et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin J Am Soc Nephrol. 2019; 14:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016; 3:160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard TJ, Johnson AEW, Raffa JD, et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018; 5:180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollard T, Johnson A, Raffa J, et al. eICU Collaborative Research Database v2.0. PhysioNet. 2019 doi: 10.13026/C2WM1R. Available at: . Accessed December 2, 2020. [DOI]
- 15.Waite MD, Fuhrman SA, Badawi O, et al. Intensive care unit-acquired hypernatremia is an independent predictor of increased mortality and length of stay. J Crit Care. 2013; 28:405–412 [DOI] [PubMed] [Google Scholar]
- 16.Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation. 2000; 101:E215–E220 [DOI] [PubMed] [Google Scholar]
- 17.Hoorn EJ, Betjes MG, Weigel J, et al. Hypernatraemia in critically ill patients: Too little water and too much salt. Nephrol Dial Transplant. 2008; 23:1562–1568 [DOI] [PubMed] [Google Scholar]
- 18.Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007; 50:952–957 [DOI] [PubMed] [Google Scholar]
- 19.Stelfox HT, Ahmed SB, Khandwala F, et al. The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care. 2008; 12:R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng MY, Al-Rawi PG, Czosnyka M, et al. Enhancement of cerebral blood flow using systemic hypertonic saline therapy improves outcome in patients with poor-grade spontaneous subarachnoid hemorrhage. J Neurosurg. 2007; 107:274–282 [DOI] [PubMed] [Google Scholar]
- 21.Tyagi R, Donaldson K, Loftus CM, et al. Hypertonic saline: A clinical review. Neurosurg Rev. 2007; 30:277–289; discussion 289 [DOI] [PubMed] [Google Scholar]
- 22.Vedantam A, Robertson CS, Gopinath SP. Morbidity and mortality associated with hypernatremia in patients with severe traumatic brain injury. Neurosurg Focus. 2017; 43:E2. [DOI] [PubMed] [Google Scholar]
- 23.Nur S, Khan Y, Nur S, et al. Hypernatremia: Correction rate and hemodialysis. Case Rep Med. 2014; 2014:736073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann CJ. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg Med J. 2003; 20:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


