Abstract
The effect of prolonged aflatoxin B1 (AFB1) administration on blood serum oestradiol-17β and progesterone concentrations in goats during the luteal phase and the synchronized oestrus was investigated. Thirty-six Greek indigenous primiparous goats were used, during the oestrus period; 12 goats received, per os, 50 μg (treated group T50) and 12 goats received 100 μg (treated group T100) AFB1/day/head, respectively, for approximately 1.5 month, while 12 goats served as controls (C). On day 36 of the experiment, each goat was injected, i.m, 0.5 ml prostaglandin F2α (PGF2α). Blood samples were collected from each goat twice a week, before PGF2α injection, as well as every 4 hours from the onset to the end of the synchronized oestrus. Oestradiol-17β and progesterone concentrations in blood serum were determined using radioimmunoassay. During the whole luteal(s) phase(s), linear regression analysis revealed a significant negative dependence (P < 0.05) of oestradiol-17β and a significant positive dependence (P < 0.05) of progesterone over group (C = 0, T50 = 50, T100 = 100), in a dose dependent manner. During the synchronized oestrus, multiple linear regression analysis revealed a significant negative dependence (P < 0.05) of oestradiol-17β, as well as a significant positive dependence (P < 0.05) of progesterone over group (C = 0, T50 = 50, T100 = 100) and over time (hours, from the onset to the end of the synchronized oestrus). No significant differences were noticed among the three groups, regarding the body weight of the goats from the onset to the end of AFB1 administration, the occurrence or the duration of the synchronized oestrus presented by the goats (P > 0.05). In conclusion, prolonged AFB1 administration at doses of 100 or even of 50 μg/day/head changes the hormonal pattern in blood during the luteal phase and the synchronized oestrus of goats, being in oestrus period.
Keywords: mycotoxins, AFB1, reproduction, ruminants, hormones
Introduction
Aflatoxins (B1, B2, G1 and G2) are toxic metabolites produced by the fungi Aspergillus flavus and Aspergillus parasiticus and considered carcinogenic for animals and humans ( Klich, 2007 ). AFB1 is very regularly detected in feedstuffs and sometimes in feed from households ( Klarić et al., 2009 ) causing various detrimental effects after it is consumed by animals or humans. Furthermore, aflatoxin Μ1 (AFM1), the hydroxylized metabolite of AFB 1, is excreted into the milk of most animal species and of human, as well ( Galvano et al., 1996 ; Abdulrazzaq et al., 2003 ) and considered carcinogenic.
The mechanisms, through which AFB1 affects the reproductive system, remain unexplained, since the effects of the toxin have not been studied extensively ( Shuaib et al., 2010 ). Aflatoxins are easily and rapidly absorbed from both the gastrointestinal tract and through the peritoneum as Bastaki et al. (2010) observed after administrating 20 mg AFB1/kg body weight, orally or intraperitoneally on gestation day 13 in pregnant mice. After AFB1 administration (7.5 mg/kg body weight/day for 14 days) in female rats, inhibition of oocytes growing, reduction of the ovary size and weight, reduced oestradiol-17β concentration and increased progesterone concentration in blood ( Ibeh and Saxena, 1997a ) were observed. During rabbits’ gestation, a considerable decrease in fetal weight was recorded, after AFB1 was administered at 0.1 mg/kg body weight per os ( Wangikar et al., 2005 ). Moreover, the high risk of AFB1 toxicity on the foetus, involve aflatoxicol production, an AFB1 metabolite with carcinogenic potency, from the placenta of the women studied ( Partanen et al., 2010 ); with that risk being more serious in high-risk countries, such as Egypt ( Piekkola et al., 2012 ).
Recently, Storvik et al. (2011) indicated that the chronic exposure to AFB1 might cause endocrine disruption in the human foetoplacental unit due to its effect on the expression of aromatase enzymes (P450s or CYPs enzymes), categorizing AFB1 as a potential endocrine disruptor. Endocrine disruptors could affect steroid ovarian hormones concentrations, either directly or indirectly. The alterations in oestradiol-17β and/or progesterone concentrations during the luteal phase and/or the synchronized oestrus may have detrimental effects on subsequent reproductive life of the animals, such as shortened cycles, lower fertility, negative effects on follicle maturation, ovulation or the presence and/or the signs of the oestrus cycle.
Specifically in goats, the reproductive effects of AFB1 administration are limited. Furthermore, the studies that have already been performed, mainly in laboratory animals, use quite higher concentrations and/or shorter periods of AFB1 administration. In a previous study ( Kourousekos et al., 2015 ) the prolonged administration of 100 or even of 50 μg AFB1/day/head increased blood serum oestradiol-17β and progesterone concentrations in anoestrous goats, in a dose dependent manner. Thus, the present study was conducted in order to investigate the possible effects by AFB1 administration on blood serum oestradiol-17β and progesterone concentrations, during the luteal phase and the synchronized oestrus and consequently on ovarian activity and oestrus cycle of goats being in oestrus period.
Materials and Methods
Animals and experimental protocol
For the aim of the present study 36 Greek indigenous primiparous goats, 2-3 years old, weighting 32.6 ± 2.4 kg, housed in open-fronted covered yard and being at the oestrus period were used. The study was conducted at the Veterinary Research Institute, in northern Greece, from middle of September to middle of November (longitude 22o51´37´´E and latitude 40o41´19´´N; average temperature 17.7 oC and average humidity 62.7%; day-length 11.2 hours with 12.8 hours darkness). All goats performed parturition about seven months ago without their oestrus cycles previously being synchronized. Throughout the experimental period, the goats were not in contact with other animals, were healthy and no pharmaceutical treatment were given to them. The goats were fed 1 kg/day/head of pelleted concentrate plus grass hay ad libitum ; water was available for the animals 24 hours a day. The pelleted concentrate feed consisted of corn, barley, gluten, soybean meal, molasses, yeast ranching, sodium chloride, calcium carbonate, dicalcium phosphate, vegetable fat, vitamins and minerals. The chemical analysis of the pelleted concentrate feed was: dry matter 52.4%, total proteins 17.2%, fat 3.3%, cellulose 3.9%, ashes 7.7%, humidity 13.0%, calcium 1.2%, phosphorus 0.7%, sodium 0.6% and chlorine 0.04%.
To ensure that the goats did not receive any AFB1 concentrations through their diet, feedstuff samples were analyzed, once a week, for AFB1 presence using high performance liquid chromatography (HPLC), as described by Akiyama et al. (2001) with the assistance of specific columns (MycoSep® 226 columns for AFB 1). The limit of detection for AFB1 was 0.5 μg/kg. None of the samples was found positive in AFB1 presence.
Goats were randomly divided into 3 groups of 12 animals each [control group (C), treated group (T50) and treated group (T100)]. The experiment lasted approximately 1.5 month, during which the goats of T50 or T100 groups received 50 or 100 μg AFB1/day/head, respectively [doses by which AFM1 is excreted into the milk, exceeding the maximum permissible level (50 ng/L) set by the European Union ( Kourousekos et al., 2012 )]. Ten mg of pure AFB1 (AFB1 from Aspergillus flavus , A 6636-10 MG, SIGMA, Sigma Chemical Co, St. Louis, MO, USA) were dissolved in 100 or 200 mL methanol and 1 mL of this dilution was received per os by each goat of T50 or T100 treated groups, respectively. The goats of the control group received only the solvent of AFB 1 (1 mL methanol/day) in order to be equally handled; methanol is the best solvent for AFB1 for in vivo treatment according to Battacone et al. (2003) , while the administration of methanol at these concentrations has no risk for the animal’s health ( http://www.epa.gov/chemfact/s_methan.txt ). The administration of the diluted AFB1 in the goats of T50 or T100 treated groups, as well as the administration of 1 mL methanol in the goats of group C was achieved, at the same hour (07:00 a.m.) every morning, using a dosimetric pistollete-like pump, in order to be controlled and easily accepted.
On day 36 of the experiment, 0.5 ml PGF2α (Estrumat®, Schering-Plugh, USA) were injected, i.m., in each goat for the synchronization of their oestrus cycles. In the goats where no oestrus was detected after 96 hours from the first injection, 0.5 ml PGF 2α was injected for a second time after an 11-day interval.
From the beginning of the experiment and before PGF2α injection, blood samples were collected twice a week from each goat (always at the same days and at the same time about 08:00 a.m.). After PGF2α injection blood samples were collected from each goat at 4 hours intervals from the onset to the end of the synchronized oestrus. Oestrus detection was realized, every 6 hours, by three teaser bucks. Blood samples were collected by jugular venipuncture into evacuated blood collecting tubes (Venoject, Terumo, Belgium). After clotting, blood samples were centrifuged (2500 x g; 20 min; 4oC); serum was aspirated and stored at –20oC until assayed. Moreover, the body weight (kg) of all goats was measured once a week.
Oestradiol-17β and progesterone assays
Oestradiol-17β and progesterone concentrations in blood serum were determined, in duplicate, using radioimmunoassay (RIA), after extraction, as described by Martin et al. (1987) , following minor modification. The radiolabelled solutions of oestradiol-17β and progesterone were provided by Amersham Biotech, (Buckinghamshire, UK), while oestradiol-17β and progesterone antiserums, were developed by the Institute of Molecular Biology, Iraklion, Crete, Greece ( Theodosiadou et al., 2004 ). The sensitivity (lower limit of detection) for oestradiol-17β was 3.90 pg/mL, while for progesterone was 19 pg/mL (0.019 ng/mL). The intra-assay variability was 3.4-6.0% (n = 8) and 2.8-4.8% (n = 8), while the inter assay variability was 9.5% (n = 72) and 8.5% (n = 72) for oestradiol-17β and progesterone, respectively. The recovery rate was estimated to be 88.3% ± 3.4% (Mean ± SD; n = 72) and 90.5% ± 2.4% (Mean ± SD; n = 72) for oestradiol-17β and progesterone, respectively.
Statistical analysis
One-way analysis of variance (one-way ANOVA) was used to compare the body weight, the occurrence and the duration of the synchronized oestrus, as well as blood serum oestradiol-17β or progesterone concentrations among the three groups studied. Levene’s test was used for the control of homogeneity of variances and statistical differences were estimated using Tukey’s HSD test. Linear regression analysis was used in order to trace the variability of oestradiol-17β or progesterone concentration over group (C = 0, T50 = 50, T100 = 100) and over time (in days for the luteal phase, or in hours for the synchronized oestrus) (multiple), or over group, or over time. Statistical analysis was performed using SPSS® software (Version 15.0, 2006, SPSS Inc., Athens, Greece) for MS Windows; in all cases, a probability of P < 0.05 was the minimum level of significance.
Results
Body weight (kg)
No significant differences (P > 0.05) were observed among the three groups studied (C: 33.6 ± 1.9; T50: 32.8 ± 2.5; T100: 31.5 ± 2.9; Mean ± SD) during the whole experimental period.
Luteal phase
During the whole luteal(s) phase(s), oestradiol-17β concentration of the treated groups T50 or T100 presented significantly lower (P < 0.05), while progesterone concentration presented significantly higher (P < 0.05) than those of the goats of group C ( Figs. 1 - 2 ). Analytically, at the first 3 days after AFB1 administration, progesterone concentration showed no significant differences (P > 0.05) among the three groups studied. From 7 to 35 days after AFB1 administration progesterone concentration of the treated groups T50 or T100 presented significantly higher (P < 0.05) compared to that of group C, while no significant difference (P > 0.05) was observed between T50 and T100 groups ( Fig. 2 ).
Figure 1. Oestradiol-17β concentration in blood serum (pg/mL) during the luteal(s) phase(s) of the goats [control group (C); treated group T50 (50 μg AFB1/goat/day); treated group T100 (100 μg AFB1/goat/day); (Mean ± SD)].
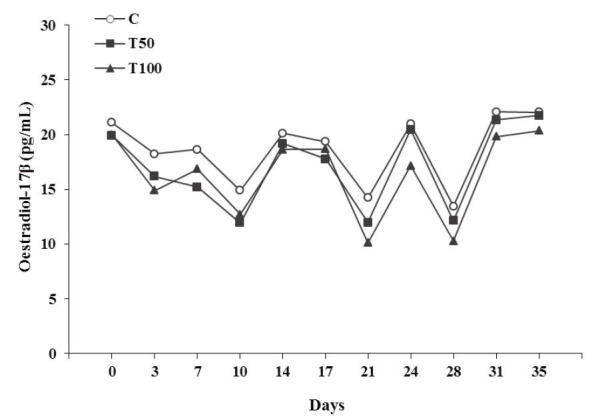
Figure 2. Progesterone concentration in blood serum (ng/mL) during the luteal(s) phase(s) of the goats [control group (C), treated group T50 (50 μg AFB1/goat/day); treated group T100 (100 μg AFB1/goat/day); (Mean ± SD)]. a,b,c Significant differences among the three groups at each time point studied (P < 0.05).
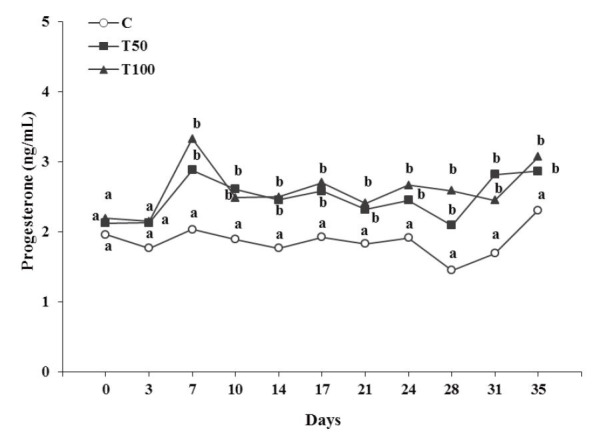
More specifically, linear regression analysis revealed a significant decrease of oestradiol-17β concentration (F = 9.98, df = 306, P = 0.002; Constant = 19.67 ± 0.81, t = 24.38, P = 6.94E-021; Group = -1.16 ± 0.37, t = -3.16, P = 0.002), as well as, a significant increase of progesterone concentration (F = 66.62, df = 306, P = 8.29E-015; Constant = 1.59 ± 0.09, t = 16.39, P = 6.94E-021; Group = 0.36 ± 0.04, t = 8.16, P = 8.29E-015) over group (C = 0, T50 = 50, T100 = 100), in a dose dependent manner.
The luteal phase was defined by progesterone concentrations >1 ng/mL and oestradiol-17β concentrations <27.2 pg/mL ( Chemineau et al., 1982 ; Bauernfeind and Holtz, 1991 ). All other values during the period of the 35 days treatment were excluded, since those values represented the natural oestrus of the goats.
Synchronized oestrus
After the first PGF2α injection, oestrus was detected in 26 goats (C: n = 9; T50: n = 8; T100: n = 9). After the second PGF2α injection (in the goats that did not respond to the first injection) oestrus was detected in another 8 goats (C: n = 2; T50: n = 3; T100: n = 3). Two goats did not respond at any PGF2α injection and excluded from the study. No significant differences were observed among the three groups studied (P > 0.05), regarding the number of goats that responded to PGF2α injection.
Regarding the duration of the synchronized oestrus that followed either the first or the second PGF2α injection, no significant differences were observed among the three groups studied (P > 0.05). The mean oestrus duration in hours (Mean ± SD) was 38.9 ± 5.0 (n = 11), 36.7 ± 6.6 (n = 11) and 38.3 ± 6.7 (n = 12) for group C, T50 and T100, respectively.
During the synchronized oestrus, oestradiol-17β concentration of the goats of the treated groups T50 or T100 presented significantly lower (P < 0.05), than that of group C ( Fig. 3 ). Analytically, at the first 4 hours from the onset of the synchronized oestrus no significant differences were observed among the three groups studied (P > 0.05). From 8 to 20 hours oestradiol-17β concentration of the goats of T100 group presented significantly lower (P < 0.05) than that of the goats of T50 or C group, while no significant difference was observed between T50 and C groups (P > 0.05). From 24 hours until the end of the synchronized oestrus oestradiol-17β concentration was significantly lower (P < 0.05) at the goats of the treated groups T50 or T100 compared to that of group C; the lowest concentration was observed in T100 group ( Fig. 3 ).
Figure 3. Oestradiol-17β concentration in blood serum (pg/mL) during the synchronized oestrus of the goats [control group (C); treated group T50 (50 μg AFB1/goat/day); treated group T100 (100 μg AFB1/goat/day); (Mean ± SD)]. a,b,c Significant differences among the three groups at each time point studied (P < 0.05).
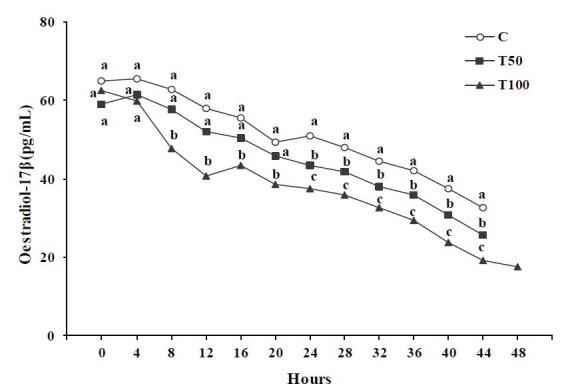
Specifically, multiple linear regression analysis revealed a significant decrease of oestradiol-17β concentration over group (C = 0, T50 = 50, T100 = 100) and over time (hours from the onset to the end of the synchronized oestrus) (F = 285.49, df = 355, P = 0.0; Constant = 74.12 ± 1.43, t = 51.99, P = 3.86E-021; Group = –5.79 ± 0.56, t = –10.28, P = 3.86E-021; Time = –0.79 ± 0.04, t = –21.65, P = 3.86E-021).
Progesterone concentration of the goats of the treated groups T50 or T100 presented significantly higher (P < 0.05) than that of group C ( Fig. 4 ). Analytically, from the onset to 20 hours of the synchronized oestrus progesterone concentration of the goats of the treated groups T50 or T100 presented significantly higher (P < 0.05) than that of the goats of group C, while no significant difference was observed between T50 and T100 groups (P > 0.05). From 24 hours until the end of the synchronized oestrus progesterone concentration remained significantly higher (P < 0.05) at the goats of the treated groups T50 or T100 compared to that of group C; the highest concentration was observed in T100 group ( Fig. 4 ).
Figure 4. Progesterone concentration in blood serum (ng/mL) during the synchronized oestrus of the goats [control group (C); treated group T50 (50 μg AFB1/goat/day); treated group T100 (100 μg AFB1/goat/day); (Mean ± SD)]. a,b,c Significant differences among the three groups at each time point studied (P < 0.05).
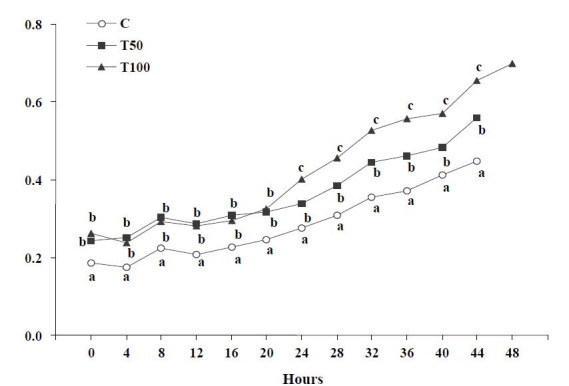
Specifically, multiple linear regression analysis revealed a significant increase of progesterone concentration over group (C = 0, T50 = 50, T100 = 100) and over time (hours from the onset to the end of the synchronized oestrus) (F = 315.09, df = 355, P = 0.0; Constant = 0.08 ± 0.01, t = 6.26, P = 1.15E-09; Group = 0.06 ± 0.01, t = 11.13, P = 3.86E-021; Time = 0.01 ± 0,0003, t = 22.58, P = 3,86E-021). The decrease of oestradiol-17β concentration and the increase of progesterone concentration, over time (hours from the onset to the end of the synchronized oestrus), in each group, are presented in Table 1 .
Table 1. Linear regression analysis results showing the negative or the positive dependence of blood serum oestradiol-17β or progesterone concentration over time (hours from the onset to the end of the synchronized oestrus) of the goats, in each group studied [control group (C); treated group T50 (50 μg AFB1/goat/day); treated group T100 (100 μg AFB1/goat/day)].
| Oestradiol-17β (pg/mL) | Progesterone (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | Sign. F | Time | Constant | df | F | Sign. F | Time | Constant | |||||
| C | 117 | 122.52 | 4.78E-019 | -0.72 | 67.01 | C | 117 | 126.58 | 4.78E-019 | 0.006 | 0.15 | |||
| Standard error | 0.07 | 1.55 | Standard error | 0.001 | 0.01 | |||||||||
| t | -11.07 | 43.32 | t | 11.25 | 12.39 | |||||||||
| Significance of t | 4.78E-019 | 4.78E-019 | Significance of t | 4.78E-019 | 4.78E-019 | |||||||||
| T50 | 110 | 149.56 | 6.39E-019 | -0.77 | 62.14 | T50 | 110 | 149.47 | 6.38E-019 | 0.006 | 0.22 | |||
| Standard error | 0.06 | 1.42 | Standard error | 0.001 | 0.01 | |||||||||
| t | -12.23 | 43.82 | t | 12.26 | 19.02 | |||||||||
| Significance of t | 6.39E-019 | 6.39E-019 | Significance of t | 6.38E-019 | 6.38E-019 | |||||||||
| T100 | 125 | 200.59 | 3.52E-019 | -0.87 | 58.30 | T100 | 125 | 284.26 | 3.52E-019 | 0.009 | 0.20 | |||
| Standard error | 0.06 | 1.44 | Standard error | 0.001 | 0.01 | |||||||||
| t | -14.16 | 40.37 | t | 16.86 | 15.06 | |||||||||
| Significance of t | 3.52E-019 | 3.52E-019 | Significance of t | 3.52E-019 | 3.52E-019 | |||||||||
Discussion
The results of the present study are confirmed by those of other researchers, but in different species. Ibeh and Saxena (1997a ; b) administered in female rats 7.5 mg AFB1/kg body weight/day for 14 days or 15 mg AFB1/kg body weight/day for 21 days, respectively, and observed that blood oestradiol-17β and progesterone appeared significantly lower and higher, respectively, in rats receiving aflatoxin. The authors proposed either a direct effect of AFB1 on ovarian secreting cells or on the hypothalamus-hypophysis-ovaries axis. In the present study, AFB1 was administered in quite lower doses, but for a longer period, and that could have influenced the ovarian activity or the above-mentioned axis. In the study of Hasanzadeh et al. (2011) the hormonal pattern was investigated in male rats after the administration of 0.8, 1.6 and 3.2 ppm AFB1 orally for 48 days. The concentrations of blood serum LH, testosterone and oestradiol-17β were significantly lower in the group of rats receiving the highest dose of AFB1. The authors proposed either a direct effect of AFB1 on testes secreting cells or on the hypothalamus-hypophysis-testes axis. Furthermore during the anoestrus period of the goats the prolonged per os administration of 100 or even of 50 μg AFB1/day/head increased blood serum oestradiol-17β and progesterone concentrations, in a dose dependent manner ( Kourousekos et al., 2015 ).
The similar chemical structure between aflatoxins and oestradiol-17β triggered some researchers to study the possible oestrogenic action of aflatoxins. Kyrein (1974) observed that aflatoxins B1, G1 and G2 did not present any binding affinity to oestrogen receptors derived from healthy uterus of calves. On the contrary, AFM1 presented an extent of receptors binding, although in quite higher concentrations. Furthermore, aflatoxicol, another AFB1 metabolite, showed a small binding ability to oestrogen receptors ( Blankenship et al., 1982 ). In our study, the possible binding of oestrogen receptors by AFB1 metabolites could have disturbed gonadotrophins secretion and consequently reduced oestrogen production.
Recently, Storvik et al. (2011) categorized AFB1 as a potential endocrine disruptor. It is known that AFB 1 is metabolized by cytochrome P450 (CYPs) enzymes ( Buhler and Wang-Buhler, 1998 ). Furthermore, Storvik et al. (2011) and Huuskonen et al. (2013) supported that AFB1 increases the expression of CYP19A1 in human placenta cells. More specifically, Huuskonen et al. (2013) indicated that AFB1 affected the placental steroid hormone synthesizing, metabolizing and conjugating enzymes and that these alterations may lead to anomalies in the foetoplacental hormonal homeostasis, while Storvik et al. (2011) suggested that AFB1, after being metabolized in aflatoxicol, had effects on genes important in endocrine regulation in placental cells. Furthermore, since CYPs have been found to take part in steroid hormones synthesis, the increase of the expression of such enzyme by AFB 1 in the placenta could result in increased progesterone production. In our study the increased blood serum progesterone concentration, in a dose dependent manner, might have been a result of such alterations of CYPs enzymes on the ovaries due to prolonged AFB1 administration.
At this point, the study of the effects of high progesterone or low oestradiol-17β concentrations during the oestrus period would be particularly useful. Menchaca and Rubianes (2001) supported that premature progesterone exposure early in the ovulatory cycle of the goat affected its length inducing short or shortened cycles. In the study of Theodosiadou et al. (2004) the synchronization of oestrus by administration of the standard dose of progesterone resulted in a decrease/increase of oestradiol-17β/progesterone, respectively, in blood plasma and oviductal wall, compared to natural oestrus. Regarding low oestradiol-17β concentrations, Gilad et al. (1993) reported that in cows with low plasma oestradiol-17β, the mean and basal concentrations and amplitudes of gonadotrophins were significantly lower by heat stress compared to cows with high plasma oestradiol-17β concentrations. Furthermore, Theodosiadou et al. (2014) revealed that at artificial insemination time (performed either at fixed-time or after oestrus detection in synchronized ewes), pregnant ewes had lower progesterone and higher oestradiol-17β concentrations in blood serum, and lower electrical resistance values in the cervical mucus (ERCM) than the non-pregnant ones. The increased progesterone concentrations in the periestrual period might have negatively affected spermatozoa, causing pregnancy failure, since a significant positive relation between progesterone concentrations and ERCM values was found. Moreover, Theodosiadou and Tsiligianni (2015) observed that blood serum progesterone concentrations and ERCM values at oestrus were lower in synchronized ewes that conceived after they were mated to fertile rams compared to those that did not conceive, either at oestrous or at anoestrous period. Finally, Carter et al. (2010) mentioned that elevated concentration of progesterone do not affect the ability of the early cow embryo to reach the blastocyst stage in vivo, but do result in subtle changes to the transcriptome of the embryo, due to advanced elongation post-hatching. Taking into account the above mentioned studies, the decreased/increased blood serum oestradiol-17β/progesterone concentrations, observed in our study, may cause detrimental effects on fertilization, conception and/or embryo development.
In conclusion prolonged AFB1 administration in goats, being at oestrus period, at the doses of 100 or even of 50 μg/day/head could lead to a decrease/ increase of blood serum oestradiol-17β/progesterone concentrations during the luteal phase and during the synchronized oestrus, in a dose dependent manner. These disturbances may not have obvious effects on the oestrus presence or duration but they could negatively affect the reproductive system of the goats. Further research regarding the direct or indirect effect of the aflatoxins on the reproductive system of the goats could interestingly be useful. Consequently, since aflatoxins are thought to be carcinogenic and teratogenic, the systematic control of the feedstuffs for the presence of AFB1 is strongly proposed.
Acknowledgement
This work was supported by the General Secretariat of Research and Technology of the Greek Ministry of Development ( PENED 2001-7/4/2003, 01ED 282 ).
References
- Abdulrazzaq YM , Osman N , Yousif ZM , Al-Falahi S. Aflatoxin M1 in breast-milk of U.A.E. women. Ann Trop Paediatr. 2003;23:173–179. doi: 10.1179/027249303322296484. [DOI] [PubMed] [Google Scholar]
- Akiyama H , Goda Y , Tanaka T , Toyoda M. Determination of aflatoxins B1, B2, G1 and G2 in spices using a multifunctional column clean-up . J Chromatogr A. 2001;932:153–157. doi: 10.1016/s0021-9673(01)01211-0. [DOI] [PubMed] [Google Scholar]
- Bastaki SA , Osman N , Kochiyil J , Shafiullah M , Padmanabhan R , Abdulrazzaq YM. Toxicokinetics of aflatoxin in pregnant mice. Int J Toxicol. 2010;29:425–431. doi: 10.1177/1091581810369565. [DOI] [PubMed] [Google Scholar]
- Battacone G , Nudda A , Cannas A , Cappio Borlino A , Bomboi G , Pulina G. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1 . J Dairy Sci. 2003;86:2667–2675. doi: 10.3168/jds.s0022-0302(03)73862-4. [DOI] [PubMed] [Google Scholar]
- Bauernfeind M , Holtz W. Progesterone and estrogen levels in serum of cyclic goats measured by enzyme immunoassay . Small Rum Res. 1991;6:95–102. [Google Scholar]
- Blankenship LT , Dickey JF , Bodine AB. In vitro mycotoxin binding to bovine uterine steroid hormone receptors . Theriogenology. 1982;17:325–329. doi: 10.1016/0093-691x(82)90092-9. [DOI] [PubMed] [Google Scholar]
- Buhler DR , Wang-Buhler JL. Rainbow trout cytochrome P450s: purification, molecular aspects, metabolic activity, induction and role in environmental monitoring . Comp Biochem Physiol. 1998;121:107–137. doi: 10.1016/s0742-8413(98)10033-6. [DOI] [PubMed] [Google Scholar]
- Carter F , Rings F , Mamo S , Holker M , Kuzmany A , Besenfelder U , Havlicek V , Mehta JP , Tesfaye D , Schellander K , Lonergan P. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct . Biol Reprod. 2010;83:707–719. doi: 10.1095/biolreprod.109.082354. [DOI] [PubMed] [Google Scholar]
- Chemineau P , Gauthier D , Poirier JC , Saumande J. Plasma levels of LH, FSH, Prolactin, Oestradiol-17β and Progesterone during natural and induced oestrus in the dairy goat . Theriogenology. 1982;17:313–323. doi: 10.1016/0093-691x(82)90091-7. [DOI] [PubMed] [Google Scholar]
- Galvano F , Galofaro V , Galvano G. Occurrence and stability of aflatoxin M1 in milk and milk products: a worldwide review . J Food Prot. 1996;59:1079–1090. doi: 10.4315/0362-028X-59.10.1079. [DOI] [PubMed] [Google Scholar]
- Gilad E , Meidan R , Berman A , Graber Y , Wolfenson D. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows . J Reprod Fetril. 1993;99:315–321. doi: 10.1530/jrf.0.0990315. [DOI] [PubMed] [Google Scholar]
- Hasanzadeh Sh , Hosseini E , Rezazadeh L. Effects of aflatoxin B1 on profiles of gonadotropic (FSH and LH), steroid (testosterone and 17β-estradiol) and prolactin hormones in adult male rat . Iranian J Vet Res. 2011;12:332–336. [Google Scholar]
- Huuskonen P , Myllynen P , Storvik M , Pasanen M. The effects of aflatoxin B1 on transporters and steroid metabolizing enzymes in JEG-3 cells . Toxicol Lett. 2013;218:200–206. doi: 10.1016/j.toxlet.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Ibeh IN , Saxena DK. AFB1 and reproduction. I. Reproductive performance in female rats . Afr J Reprod Health. 1997;1:79–84. a. [PubMed] [Google Scholar]
- Ibeh IN , Saxena DK. AFB1 and reproduction. II. Gametotoxicity in female rats . Afr J Reprod Health. 1997;1:85–89. b. [PubMed] [Google Scholar]
- Klarić MS , Cvetnić Z , Pepeljnjak S , Kosalec I. Co-occurrence of aflatoxins, ochratoxin A, fumonisins, and zearalenone in cereals and feed, determined by competitive direct enzyme-linked immunosorbent assay and thin-layer chromatography . Arch Ind Hyg Toxicol. 2009;60:427–434. doi: 10.2478/10004-1254-60-2009-1975. [DOI] [PubMed] [Google Scholar]
- Klich MA. Aspergillus flavus the major producer of aflatoxin. Mol Plant Pathol. 2007;8:713–722. doi: 10.1111/j.1364-3703.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Kourousekos GD , Theodosiadou E , Belibasaki S , Deligiannis K , Koukoulas Th , Zoulfos K , Lymberopoulos AG. Effects of aflatoxin B1 administration on Greek indigenous goats’ milk . Int Dairy J. 2012;24:123–129. [Google Scholar]
- Kourousekos GD , Theodosiadou EK , Lymberopoulos AG , Belibasaki S , Boscos C. Effect of prolonged aflatoxin B1 administration on blood serum oestradiol-17β and progesterone concentrations of goats during the anoestrus period . Czech J Anim Sci. 2015;60:164–170. [Google Scholar]
- Kyrein HJ. The binding affinity of aflatoxins on the uterine oestrogen receptor . Z Lebensm Unters Forsch. 1974;154:285–287. [Google Scholar]
- Martin GB , Sutherland SRD , Lindsay DR. Effects of nutritional supplements on testicular size and the secretion of LH and testosterone in Merino and Booroola rams . Anim Reprod Sci. 1987;12:267–281. [Google Scholar]
- Menchaca A , Rubianes E. Effect of high progesterone concentrations during the early luteal phase on the length of the ovulatory cycle of goats . Anim Reprod Sci. 2001;68:69–76. doi: 10.1016/s0378-4320(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Partanen HA , El-Nezami HS , Leppanen JM , Myllynen PK , Woodhouse HJ , Vahakangas KH. Aflatoxin B1 transfer and metabolism in human placenta . Toxicol Sci. 2010;113:216–225. doi: 10.1093/toxsci/kfp257. [DOI] [PubMed] [Google Scholar]
- Piekkola S , Turner PC , Abdel-Hamid M , Ezzat S , El-Daly M , El-Kafrawy S , Savchenko E , Poussa T , Woo JCS , Mykkanen H , El-Nezami H. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women . Food Addit Contam. 2012;29:962–971. doi: 10.1080/19440049.2012.658442. [DOI] [PubMed] [Google Scholar]
- Shuaib FM , Ehiri J , Abdullahi A , Williams JH , Jolly PE. Reproductive health effects of aflatoxins: a review of the literature . Reprod Toxicol. 2010;9:262–270. doi: 10.1016/j.reprotox.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Storvik M , Huuskonen P , Kyllonen T , Lehtonen S , El-Nezami H , Auriola S , Pasanen M. Aflatoxin B1 – a potential endocrine disruptor – up-regulates CYP19A1 in JEG-3 cells . Toxicol Lett. 2011;202:161–167. doi: 10.1016/j.toxlet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Theodosiadou E , Goulas P , Kouskoura T , Smokovitis A. Oestrogen and progesterone concentrations in plasma and oviductal tissue of ewes exhibiting a natural or induced oestrus . Anim Reprod Sci. 2004;80:59–67. doi: 10.1016/S0378-4320(02)00223-3. [DOI] [PubMed] [Google Scholar]
- Theodosiadou E , Amiridis SG , Tsiligianni T. Relationship between electrical resistance of cervical mucus and ovarian steroid concentration at the time of artificial insemination in ewes . Reprod Biol. 2014;14:234–237. doi: 10.1016/j.repbio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Theodosiadou E , Tsiligianni T. Determination of the proper time for mating after oestrus synchronization during anoestrus or oestrus by measuring electrical resistance of cervical mucus in ewes . Vet Med-Czech. 2015;60:87–93. [Google Scholar]
- Wangikar PB , Dwivedi P , Sinha N , Sharma AK , Telang AG. Effects of AFB1 on embryo fetal development in rabbits . Food Chem Toxicol. 2005;43:607–615. doi: 10.1016/j.fct.2005.01.004. [DOI] [PubMed] [Google Scholar]


