ABSTRACT
Heparan sulfate proteoglycans (HSPGs) play important roles in cancer initiation and progression, by interacting with the signaling pathways that affect proliferation, adhesion, invasion and angiogenesis. These roles suggest the possibility of various strategies of regulation of these molecules. In this review, we demonstrated that the anticancer drugs can regulate the heparan sulfate proteoglycans activity in different ways: some act directly in core protein, and can bind to a specific type of HSPG. Others drugs interact with glycosaminoglycans chains, and others can act directly in enzymes that regulate HSPGs levels. We also demonstrated that the HSPGs drug targets can be divided into four groups: monoclonal antibodies, antitumor antibiotic, natural products, and mimetics peptide. Interestingly, many drugs demonstrated in this review are approved by FDA and is used in cancer therapy (Food and Drug Administration) like trastuzumab, panitumumab, bleomycin and bisphosphonate zoledronic acid (ASCO) or are in clinical trials like codrituzumab and genistein. This review should help researchers to understand the mechanism of action of anticancer drugs existing and also may inspire the discovery of new drugs that regulate the heparan sulfate proteoglycans activity.
KEYWORDS: Heparan sulfate proteoglycans, glycosaminoglycans, heparan sulfate, cancer, anticancer drugs
ABSTRACT
Introduction
Cancer is a major public health problem worldwide. According to estimates from the World Health Organization (WHO), in 2015, cancer was the first or second leading cause of death before age 70 years in 91 of 172 countries, and it ranked third or fourth in additional 22 countries1. Cancer is known as abnormal cell division. It refers to the continuous and unregulated proliferation of cells, accompanied by metabolic and behavioral changes.2 Tumors produce several molecules that facilitate their proliferation, invasion, and maintenance, especially proteoglycans (PGs). The heparan sulfate proteoglycans (HSPGs), can act as a co-receptor of growth factors and proteins of the extracellular matrix (ECM) by increasing the affinity of adhesion molecules to their specific receptors. Changes in the expression of HSPGs have been found in tumor cells, indicating its involvement in cancer.3
PGs are composed of a core protein with long chains of glycosaminoglycans (GAGs) covalently attached. GAGs are negatively charged polysaccharides composed of repeating disaccharide units, each of which is composed of an acetamido sugar (N-acetyl-d-glucosamine or N-acetyl-d-galactosamine) and a uronic acid (d-glucuronic or l-iduronic acid) or d-galactose units. Five glycosaminoglycans chains have been identified: chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate, and the non-sulfated hyaluronic acid (HA).4
The heparan sulfate proteoglycans (HSPGs) are divided in three groups according to location: membrane HSPGs, such as syndecans (SDC) and glypicans (GPC), the secreted extracellular matrix HSPGs, such as agrin, perlecan, and type XVIII collagen, and the secretory vesicle proteoglycan, serglycin. Interestingly, some heparan sulfate proteoglycans can charge chondroitin sulfate chains.5
Syndecans are a family of four members of transmembrane proteoglycans (SDC-1 to SDC- 4) in mammals. They have similar structural organization, consisting of an extracellular domain (ectodomain), a transmembrane domain, and a cytoplasmic domain. The syndecans are implicated in the control of cell–cell, cell–pathogen and cell–matrix interactions via the recruitment of the actin cytoskeleton, as well as in cellular proliferation, differentiation, and migration. Syndecans can be found in cell protrusions and focal adhesions, where they colocalize with actin. Importantly, they can act as co-receptors of other cell surface receptors like growth factor receptors and integrins.6
The perlecan is the main HSPG in the blood vessels ECM. The perlecan core protein consists of five distinct domains that share homology with SEA (sea urchin sperm protein, enterokinase, and agrin) neural cell-adhesion molecule (N-CAM), IgG, low-density lipoprotein (LDL) and laminin. Each domain can bind to a number of linkers, including basement membrane components, cell-adhesion molecules, and growth factors.7 It has been reported that perlecan displays dual functions in regulating angiogenesis, by blocking angiogenesis through the endorepellin (domain V) and also promoting angiogenesis through the HS cleavage by heparanase and core protein cleavage by protease.8
Glypicans are a family of heparan sulfate proteoglycans (HSPGs) that are linked to the exocytoplasmic surface of the plasma membrane by a glycosyl-phosphatidylinositol (GPI) anchor. To date, six members of the glypican family (GPC 1 to GPC 6) have been identified in mammals, and two in Drosophila.9 GPC can be shed into the extracellular environment. This shedding is generated, at least in part, by Notum, an extracellular lipase that releases glypicans by cleaving the GPI anchor.10
In addition, several papers have described the structure, protein interaction, and cell signaling of HSPGs. This review focuses on discussing the pharmacological potential of HSPG as a drug target in anti-cancer therapy.
Heparan sulfate proteoglycans and cancer
HS and HSPGs regulate diverse cancer cell functions, including oncogenic signaling, apoptosis, cellular differentiation, angiogenesis, tissue invasion, and migration, also in the regulation of immune evasion and extracellular matrix modification.3
GAGs are involved in multiple signaling cascades required for angiogenesis, cancer invasion, and metastasis. Interestingly, some GAGs have also been shown to play a role in the inhibition of tumor progression. HS has been shown to promote cell-cell and cell-ECM adhesion, inhibiting invasion and metastasis, and a decrease in the levels of HS, as seen in some cancers, resulted in the malignant cells being more invasive. The CS chains also participate in various interactions within the ECM, which is of particular importance in malignancy.11
The syndecans family has important role in cancer, for example, syndecan-1 is a molecular marker for triple negative inflammatory breast cancer12 and enhances proliferation, migration of human fibrosarcoma cells in cooperation with syndecan-2.13 Syndecan-2 enhances tumorigenic actives of melanoma cells,14 pancreatic15 and colon cancer cells16 and is a biomarker for early detection of colorectal neoplasm.17 Syndecan-3 has a role in tumoral angiogenesis.18 The syndecan-4 expression is correlation with metastatic potential of tumor cells.19 Besides that there a statistical relationship between syndecan-1 presence in high-grade tumors and absence of syndecan-4, whereas syndecan-4 presence in cases positive for estrogen and progesterone receptor associated with syndecan-1 absence.20 Taken together, syndecans appear to be important players in oncogenesis therefore potential therapeutic targets in cancer.
Perlecan by itself can stimulate angiogenesis in a rabbit ear model of in vivo angiogenesis. In human papillary and invasive ductal breast carcinomas, large deposits of perlecan core protein are found in the tumor stroma and blood vessel walls. Similarly, abundant perlecan core protein is detected in the vessel walls and endothelial cells of human primary liver tumors.8
Glypicans play a significant role in cancer progression by regulating the activity of a wide gamut of soluble and insoluble ligands. Moreover, have the ability to act as both tumor suppressors and promoters, which is dependent on the type and the stage of cancer progression.9 Glypican-1 mediates interstitial flow mechano-transduction to enhance cell migration and metastasis. Beside that is overexpressed in various malignant solid cancers.21 Glypican‐3 is expressed in HCC, OCCC, melanomas, lung squamous cell carcinomas, hepatoblastomas, nephroblastoma (Wilms’ tumors) and yolk sac tumors, as well as, in certain stomach cancers, for example, gastric cancers that produce α‐fetoprotein. The function of secreted and membrane‐anchored GPC3 in these cancers is unknown, but it is almost certainly involved in neoplastic transformation in HCC.22 GPC-5 and GPC-6 has also been implicated in many cancers. Both were associated with an increased risk of invasive epithelial ovarian carcinoma.23
Heparan sulfate proteoglycans as anticancer drug targets
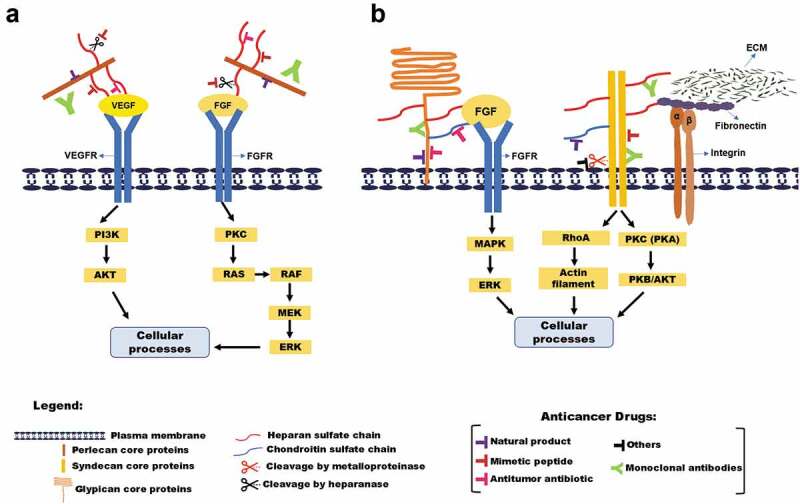
As previously mentioned, HSPGs play a central role in virtually all major stages of tumor progression: the invasiveness and migration of tumor cells, escape from apoptosis and immune surveillance, metastasis, and angiogenesis. These functions make HSPGs an attractive pharmacological target for anti-cancer therapies. According to literature data, the HSPG drug targeted can be divided into four main groups: natural products, monoclonal antibodies, antitumor antibiotic, and mimetics peptides (Table 1).
Table 1.
Effects of anticancer drugs on the heparan sulfate proteoglycans in different cancer cell models
| ANTICANCER DRUG | TARGETS | CANCER TYPE | BIOLOGICAL EFFECT | REFERENCE | |
|---|---|---|---|---|---|
| Natural Products | Calcarea Carbonica | Perlecan | Melanoma | Perlecan expression antagonist | 37 |
| Genistein | Heparan Sulfate (HS) Chondroitin Sulfate (CS) Dermatan Sulfate (DS) |
Colon adenocarcinoma Breast cancer |
Regulate the synthesis and distribution of glycosaminoglycans |
40 | |
| Monoclonal Antibodies | Nbto62 | Syndecan-1 | Multiple myeloma | Blocking syndecan-1 activity | 28 |
| B-B4 | Multiple melanoma | 27 | |||
| OC-46F2 | Melanoma And ovarian carcinoma |
25,26 | |||
| Codrituzumab | Glypican 3 | Hepatocellular Carcinoma | Blocking glypican-3 activity | 30,31 | |
| GPC1-AD | Glypican 1 | Cervical Cancer | Inhibited the growth of glypican-positive cervical cancer cells | 31 | |
| Trastuzumab | Heparan sulfate | Breast cancer | Mediates trastuzumab effect | 33,34 | |
| Syndecan-1 | Decreases syndecan-1 expression | ||||
| Syndecan −4 and Perlecan | Anoikis resistant endothelial cells | Decreases syndecan-4 and perlecan expression | 34 | ||
| Panitumumab | Syndecan-4 | Colon cancer | Decreases the expression of syndecan-4 | 35 | |
| Antitumor antibiotic | Mitoxantrone | Glypican 3 | Gastric carcinoma | GPC3 contributes to resistance of cancer cells to mitoxantrone | 45 |
| Bleomycin | Heparan Sulfate (HS) Chondroitin Sulfate (CS) Dermatan Sulfate (DS) |
Colon Cancer Lung cancer |
Decreases sulfation and change the quantity and disaccharide composition of GAGs | 48 | |
| Mimetic Peptide | NT4 | HSPGs Sulfated Glycosaminoglycans |
Pancreas adenocarcinoma | Binds to HSPGs and GAGs and inhibited adhesion, reduces angiogenesis and invasiveness |
4,38 |
| Synstatin | Syndecan 1 | Breast cancer Hepatocellular carcinoma |
Inhibits the syndecan-1-coupled IGF1R-αvβ3 integrin complex |
51,52 | |
| PG545 | Heparan Sulfate | Breast, prostate, liver, lung, colon, head and neck cancers and melanoma | Inhibits heparanase activity, angiogenesis and induce anti-metastatic effects |
56 | |
| M402 | Pancreatic cancer Melanoma |
Binds to multiples growth factors, adhesion molecules, and cytokines to inhibit tumor angiogenesis, progression, and metastasis | 55 | ||
| Phosphomannopentaose Sulfate (PI-88) | Adenocarcinoma mammary Myeloid leukemia |
Inhibits heparanase activity, angiogenesis and tumor metastasis | 43 | ||
| Others | Bisphosphonate Zoledronic acid (ASCO) | Syndecan-1 Syndecan-2 Syndecan- 4 Glypican |
Breast cancer | Syndecan-1 and glypican 1 downregulation and syndecan-4 upregulation | 44 |
| all-trans retinoic acid (ATRA) | Syndecan −1 | Lung cancer | Modulate the syndecan-1 expression and shedding | 45 |
Monoclonal antibodies
Monoclonal antibody (mAbs)-based treatment of cancer has been established as one of the most successful therapeutic strategies for hematologic malignancies and solid tumors. Moreover, different types of mAbs are used in malignancy treatment.46
OC-46F2 is a recombinant human antibody generated by selecting of a human antibody phage display library on human melanoma cells. OC-46F2 is specific for the extracellular domain of syndecan-1, besides that, inhibits vascular maturation and tumor growth in melanoma.28 In addition, a recent study demonstrated that melanoma cells lose their ability to form tubule-like structures in vitro after blocking syndecan-1 activity by the OC-46F2. The efficacy of OC-46F2 was also demonstrated in pre-clinical in vivo models of human melanoma and ovarian carcinoma.29
B-B4 is a monoclonal antibody, which specifically identifies human plasma cells. It strongly reacts with all multiple myeloma cell lines and with malignant plasma cells of all tumor samples of the multiple myeloma patients tested. Cloning of the B-B4 antigen reveals that the monoclonal antibody recognizes syndecan-1. Wijdenes and collaborators (1996) report the development and characterization of a mAbs that recognizes only myeloma cells in the bone marrow from multiple myeloma patients. The cDNA of the recognized antigen was cloned and found to encode syndecan-1.27
Another mAb specificity for syndecan-1 (CD138) is called nBT062, that is a murine/human chimeric form of B-B4. Studies showed that nBT062 significantly inhibited growth of MM (MM- multiple myeloma) cell lines by inducing G2-M cell-cycle arrest followed by apoptosis associated with cleavage of caspase-3, caspase-8, caspase-9, and poly (ADP-ribose) polymerases. Moreover, nBT062-maytansinoid conjugates blocked adhesion of MM cells to bone marrow stromal cells.26,28
Codrituzumab, also called of GC33 is a humanized monoclonal antibody generated in MRL/LPR mice that recognizes an epitope in the C-terminal portion of glypican-3 (GPC3). GC33 is being evaluated in a phase II clinical trial ability to inhibit HCC cell proliferation or induce apoptosis.30 The antitumor activity of GC33 was shown in preclinical models of hepatocellular carcinoma (HCC).31
As cancer cells present high glypican expression (GPC-1), some researchers developed and isolated a GPC1-targeted antibody-drug conjugate (ADC). The antibody was conjugated with the cytotoxic agent monomethyl auristatin F (MMAF) and was potently cytotoxic to cancer cells highly expressing GPC1, by inhibit tumor growth and mediated G2/M phase cell cycle arrest. Furthermore, the toxicity of GPC-ADC was tolerable within the therapeutic dose range in mice3231.
Trastuzumab (Herceptin®) is a humanized recombinant monoclonal antibody (mAb) of the immunoglobulin G1 type, approved for FDA in 1998. Trastuzumab (Tmab) targeting the extracellular domain IV of HER2 and it is used to treat patients of breast and gastric cancer with overexpressing of ErbB2 (HER2). Although this is the Tmab mechanism of action more accurately described, the precise molecular pathways through which trastuzumab exerts its antitumor effects on breast and gastric cancer cells are diverse and still not fully understood. It is known that many patients do not respond to Tmab therapy, even if their tumors overexpress HER2.47
Using breast cancer cell line, as MCF7 and SKBR3, Suarez and collaborators demonstrated the importance of cell surface HS to trastuzumab action. Breast cancer cell lines responsive to trastuzumab present higher amounts of HER2, Syn-1, and HS on the cell surface, but lower levels of secreted HS.33 Interestingly, our group observed that trastuzumab also reduces heparan sulfate, syndecan-4 and perlecan levels and control angiogenesis, adhesion and cell cycle in anoikis resistant endothelial cells.34
Panitumumab is a human monoclonal antibody (pMAb) approved by FDA in 2007 which inhibits the epidermal growth factor receptor (EGFR). Panitumumab is used for the treatment of patients with metastatic colorectal cancer. A study report for the first time that pmAb is a potent inhibitor of certain matrix effectors, such as membrane-type1 metalloproteinase (MT1-MMP), extracellular metalloproteinases inducer (EMMPRIN), uroquinase plasminogen activator (uPA) and syndecan-4. Beside that they show that this inhibition results in a synergistic inhibitory effect on cell functional properties, among them migration and invasion, both of crucial importance for colon cancer progression.35
Natural products
Natural products (NPs) from vegetables, teas and fruits have been extensively studied as anticancer drugs by interfering with the initiation, development, and progression of cancer through the modulation of various mechanisms including: cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis. Curiously, in the area of cancer, half of the FDA approved New chemical entities (NCES) are natural products.48
Calcarea carbonica is derived from the soft white middle layer of the oyster shell that is composed of fine crystalline calcium carbonate (CaCO3) with traces of other minerals, such as magnesium carbonate. It has been reported to have in vitro and in vivo anti-cancer properties in a murine melanoma model,24 besides that, was used for treating polycystic ovarian syndrome (PCOS) patients.49 Guimarães and coworkers, demonstrated that Calcarea carbonica derivate complex(M8) decreases perlecan expression and inhibit the invasion of murine melanoma cells (B16F10) and human colon-rectal cancer cells (HT29). In addition, they showed that perlecan expression also was significantly downregulated in tumor nodules after Calcarea carbonica derivate complex(M8) treatment.24
Genistein (4′, 5, 7-trihydroxyisoflavone or 5, 7-dihydroxy-3-(4-hydroxyphenyl)-4 H-1- benzopyran-4-one) is a natural isoflavone enriched in soybeans. Genistein induces apoptosis in a variety of tumor cell lines. Several possible mechanisms for the anticancer effects of genistein have been proposed. These include topo II inhibition, induction of differentiation, inhibition of protein tyrosine kinase activity and inhibition of angiogenesis. Previous studies have demonstrated that genistein affects the biosynthesis of glycosaminoglycans and proteoglycans in various human cancer cell lines.25,50 Using two human colon cancer cell lines SW-116 and HT-29, Chatzinikolaou and collaborators have suggested that genistein affects the synthesis of HA, GalAG, and HS by the two-estrogen receptor-positive colon cancer cell lines. In the SW-1116 cell line, genistein had a dose-dependent inhibitory effect on the synthesis of both secreted and cell-associated GAG/PG as well as on cell growth, mediated probably through a PTK pathway. The synthesis of GAGs/PGs by HT-29 cells in the presence of genistein was demonstrated to be dependent on their type and localization.25
Antitumor antibiotic
Antibiotics as anticancer drugs clearly form an important part of chemotherapeutics with curative properties. The antibiotics which are used in cancer chemotherapy have various modes of actions. For most of them DNA is one of the primary molecular targets. Some these anticancer antibiotics acts as DNA intercalators or prevent DNA repair among other mechanisms of actions. Anthracyclins, bleomycins, actinomycin D, mitomycins are a few anticancer antibiotics used in therapy51 .
Mitoxantrone – MTX (1,4-dihydroxy-5,8-bis[2-(2-hydroxyethylamino)ethylamino]anthracene-9,10-dione) is a derivative anthracenedione anticancer drug developed in the 1980s as a doxorubicin analogue in a program to find drugs with improved antitumor activity and decreased cardiotoxicity compared with the anthracyclines. The antitumor activity of mitoxantrone is related to its ability to interact with topoisomerase-2, bind to DNA and to inhibit DNA replication and DNA-dependent RNA synthesis, prevents the ligation of DNA strands, and consequently cause G2 phase arrest, delays the cell-cycle progression.52 Mitoxantrone is used primarily in therapy for breast cancer, acute leukemia, lymphoma, and prostate cancer53 . MTX also has been routinely used for its palliative benefit and enhanced clinical remission in combination with other drugs.54 Gastric cells that became resistant to mitoxantrone expressed an increased level of GPC3 and suppression of GPC3 expression by an anti-GPC3 ribozyme not only restored sensitivity to mitoxantrone but also attenuated cross-resistance to etoposide. Furthermore, the degree of drug resistance correlated with the level of GPC3 mRNA.36
Bleomycin (BLM) is a cytotoxic antibiotic produced by the bacterium streptomyces that have ability to make single-and double-strand DNA breaks in mammalian cells. Since approved by FDA in 1973 is used as an anticancer agent the therapy of testicular, cervical, ovarian, esophageal, head-neck, thyroid carcinomas, melanoma, sarcoma, and Hodgkin’s and non-Hodgkin’s lymphomas.55 DNA cleavage by bleomycin anticipates the presence of oxygen and ferrous ions involving the following three actions: (i) recognition of a particular base or base sequence on a DNA double strand, (ii) formation of radical species that propagates free radical-based mechanism of action and (iii) oxidation reactions leading to DNA strand scission.56 Li and collaborators observed that heparan sulfate GAG was significantly undersulfated and the quantity and disaccharide compositions of GAGs were changed in bleomycin-treated cells in a concentration- and time-dependent manner. They revealed that bleomycin-induced cytotoxicity was directly related to cell surface GAGs.37
Mimetic peptide
Mimetic peptides are synthetic compounds that are identical to amino acid sequence synthesized by an organism and can interact with growth factor receptors and provide antiaging clinical effects.57
The ectodomains interaction of Sdc1 with αvβ3 provides a docking site that captures and activates the insulin-like growth factor-1 receptor (IGF1R) leading to autophosphorylation of IGF1R and activation of the integrin. The clustering of syndecans-1 with IGF1R and integrin αvβ3 is crucial for mediating cell functions such as proliferation, adhesion, and angiogenesis as well as tumor survival and metastasis. Moreover, this complex is highly expressed on tumor cells and is activated in endothelial cells during angiogenesis.58 A selective peptide, called synstatin (SSTN92-119) inhibits the complex formation between syndecan-1, IGF1R, and integrin αvβ3 and reduce the activation of the angiogenic growth factors, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF/FGF-2), inhibits angiogenic and decreasing tumor growth in mammary carcinoma in mouse and hepatocellular carcinoma in rats.39,40
NT4 is a tetra-branched peptide specifically binds to sulfated glycosaminoglycans on cancer cell membranes, such as colorectal cancer, pancreas adenocarcinoma, and urinary bladder cancer and can efficiently and selectively deliver drugs or liposomes for cancer cell imaging or therapy. NT4 inhibits cancer cell adhesion and migration on different proteins,4,38 however, the exact mechanism of action is still unclear.
M402 is a rationally engineered, non-cytotoxic heparan sulfate (HS) mimetic, designed to inhibit multiple factors implicated in tumor-host cell interactions, including VEGF, FGF2, SDF-1a, P-selectin, and heparinase. Immunohistological analyses of the primary tumor revealed a decrease in microvessel density in M402 treated animals, suggesting anti-angiogenesis effect to be one of the mechanisms involved in-vivo. M402 treatment also normalized circulating levels of myeloid-derived suppressor cells in tumor-bearing mice59. M402 was shown in nonclinical studies to modulate tumor-stroma interactions involved in the metastatic, invasive and desmoplastic pathways by simultaneously inhibiting two distinct pathways: Shh signaling and the activity of heparanase. M402 regulates a variety of polysaccharide-based binding proteins, which provides a rationale for the clinical investigation of M402 in a range of cancers.42
PG545 is a heparan sulfate (HS) mimetic that inhibits tumor angiogenesis by sequestering angiogenic growth factors in the extracellular matrix (ECM), thus limiting subsequent binding to receptors.41 PG545 also acts as a heparanase inhibitor which may differentiate its mechanisms of action from approved angiogenesis inhibitors.60 PI-88 is another heparan sulfate mimetic that have antiangiogenic, antitumor, and anti-metastatic activity. The drug is derived from the extracellular phosphomannan produced by the yeast Pichia holstii and is presently under evaluation in phase II clinical trials for anticancer efficacy.43
Other types of anticancer drugs
Bisphosphonates
Bisphosphonates are stable analogues of naturally occurring inorganic pyrophosphate in which the oxygen atom linked to two phosphate groups (P-O-P) is replaced by a geminal central carbon atom (P-C-P) to prevent hydrolysis. Side chains R1 and R2 are attached to the carbon atom and influence the bisphosphonates’ affinity for bone and antiresorptive ability. Bisphosphonates containing a primary nitrogen atom in the R2 sidechain (eg, pamidronate) are more potent than nonnitrogen bisphosphonates (such as clodronate). Modifying the primary amine to form a tertiary amine (e.g., ibandronate) results in even more potent molecules. The most potent bisphosphonates are those containing a tertiary amine within a ring structure, such as zoledronic acid.61
Bisphosphonate zoledronic acid (ASCO) has been approved for treatment of patients with advanced lung cancer, renal cancer, and others solid tumors with bone metastases or multiple myeloma and for the management of tumor-induced hypercalcemia.62,63 ASCO have ability to prevent cancer treatment-induced bone loss (CTIBL) and reduce the risk of disease recurrence and metastasis, also been shown to prevent bone loss associated with ovarian failure due to chemotherapy.64
A study demonstrated that syndecan-1, −2, and glypican-1 expression are downregulated, whereas syndecan-4 expression is upregulated upon treatment with ASCO. The authors concluded that zoledronate acid is a critical modulator of ECM gene expression and powerful anticancer agent inhibiting growth, migration, and the matrix-associated invasion of breast cancer cells.44
Retinoid
All-trans retinoic acid (ATRA) is an active metabolite of a nutrient vitamin A that the body needs in small amounts to function and helps cells to grow and develop, especially in the embryo.65,66 The synthetic form of ATRA made in the laboratory is used to treat the skin, to treat conditions such as acne and can be used as chemotherapeutic and chemo preventive agents in a variety of malignancies, such as leukemias, uterine leiomyomas, as well as colon, gastric, ovarian, lung and breast cancers.67 The ATRA have been investigated extensively for the prevention and treatment of cancer, predominantly because of their ability to induce cellular differentiation and arrest proliferation.65,68
ATRA upregulates the expression of perlecan in murine smooth muscle cell (SMCs). Through the use of SMCs that express HS-deficient perlecan, they confirmed that much of the ATRA dependent inhibition of SMC proliferation is mediated by perlecan and dependent on its HS chains, which are important for the activation of PTEN and inactivation of Akt.6970 Also, a study indicated a chemoprotective effect of ATRA against changes in lung epithelial cell membrane syndecan-1 expression in B(α)P-induced lung cancer model. Therefore, ATRA could serve as effective chemotherapeutic agent against cancer invasion/metastasis, at least in the lungs.45
Concluding remarks
Cancer is a multifactorial disease and its genesis and progression are extremely complex. The biggest problem in the anticancer drug development is acquiring of multidrug resistance and relapse.70 Besides that, few anticancer drugs have defined targets and elucidated mechanisms of action. Most anticancer drugs reported in this work alters heparan sulfate proteoglycans expression, have an antitumor effect and decrease or inhibit the activity of HSPGs or enzymes responsible for their regulation. Interestingly, some anticancer drugs reported here, such as ascorbic acid (ASCO), trastuzumab, bleomycin and all-trans retinoid acid (ATRA), are on the world health organization’s list of essential medicines, the most important medications needed in a basic health system.
The data reviewed in this article support that the HSPGs as anticancer drugs targets may be a promising strategy for the treatment of different types of cancers. We also believe that HSPGs may contribute to development of others potential anticancer drugs, such as inhibitors, mimetic peptide and monoclonal antibodies.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico [XX]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [XX-Xx]; Financiadora de Estudos e Projetos [XX]; Fundação de Amparo à Pesquisa do Estado de São Paulo [2019/19739-2].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author’s contributions
Onyeisi, J.O.S. and Lopes, C.C., participated in study design, acquisition of data, analysis and interpretation of data. Ferreira, B.Z contributed to acquisition of data. Nader, H.B and Lopes, C.C Were responsible for overall supervision. Onyeisi, J.O.S drafted the manuscript, which was revised by Lopes, C.C. All authors read and approved the final manuscript.
Reference
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clini. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011. Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013.PMID:21376230 [DOI] [PubMed] [Google Scholar]
- 3.Blackhall FH, Merry CL, Davies E, Jayson GC. Heparan sulfate proteoglycans and cancer. Br J Cancer. 2001;85(8):1094. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunetti J, Depau L, Falciani C, Gentile M, Mandarini E, Riolo G, Lupetti P, Pini A, Bracci L. Insights into the role of sulfated glycans in cancer cell adhesion and migration through use of branched peptide probe. Scientific reports 2016; 6:27174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7):a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopes CC, Dietrich CP, Nader HB. Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling. Braz J Med Biol Res. 2006;39(2):157–167. doi: 10.1590/S0100-879X2006000200001. [DOI] [PubMed] [Google Scholar]
- 7.Noonan DM, Hassell JR. Perlecan, the large low-density proteoglycan of basement membranes: structure and variant forms. Kidney Int. 1993;43(1):53–60. doi: 10.1038/ki.1993.10. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Couchman JR. Perlecan and tumor angiogenesis. J Histochemistry & Cytochemistry. 2003;51(11):1393–1410. doi: 10.1177/002215540305101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur SP, Cummings BS. Role of glypicans in regulation of the tumor microenvironment and cancer progression. Biochem Pharmacol. 2019;168:108–118. doi: 10.1016/j.bcp.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9(5):224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morla S. Glycosaminoglycans and glycosaminoglycan mimetics in cancer and inflammation. Int J Mol Sci. 2019;20(8):1963. doi: 10.3390/ijms20081963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, Greve B, El-Shinawi M, Mohamed MM, Götte M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Molecular cancer 2017; 16: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Péterfia B, Füle T, Baghy K, Szabadkai K, Fullár A, Dobos K, Zong F, Dobra K, Hollósi P, Jeney A. Syndecan-. 1 enhances proliferation, migration and metastasis of HT-1080 cells in cooperation with syndecan-2. PloS one 2012; 7:e39474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J-H, Park H, Chung H, Choi S, Kim Y, Yoo H, Kim T-Y, Hann H-J, Seong I, Kim J. Syndecan-2 regulates the migratory potential of melanoma cells. J Biol Chem. 2009;284(40):27167–27175. doi: 10.1074/jbc.M109.034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Oliveira T, Abiatari I, Raulefs S, Sauliunaite D, Erkan M, Kong B, Friess H, Michalski CW, Kleeff J. Syndecan-2 promotes perineural invasion and cooperates with K-ras to induce an invasive pancreatic cancer cell phenotype. Molecular cancer 2012; 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Choi Y, Jun E, Kim I-S, Kim S-E, Jung S-A, Oh E-S. Shed syndecan-2 enhances tumorigenic activities of colon cancer cells. Oncotarget 2015; 6: 3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Park SC. Syndecan-2 methylation as a new biomarker for early detection of colorectal neoplasm. Gut Liver. 2018;12(5):479. doi: 10.5009/gnl18286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roskams T, De Vos R, David G, Van Damme B, Desmet V. Heparan sulphate proteoglycan expression in human primary liver tumours. The J Pathol: A J Pathol Soc G B Irel. 1998;185(3):290–297. [DOI] [PubMed] [Google Scholar]
- 19.Carneiro BR, Pernambuco Filho PC, Mesquita AP, da Silva DS, Pinhal MA, Nader HB, Lopes CC. Acquisition of anoikis resistance up-regulates syndecan-4 expression in endothelial cells. PloS one 2014; 9:e116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA, Couchman JR. Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J Histochemistry & Cytochemistry. 2011;59(6):615–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada E, Serada S, Fujimoto M, Takahashi Y, Takahashi T, Hara H, Nakatsuka R, Sugase T, Nishigaki T, Saito Y. Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma. Oncotarget. 2017;8(15):24741. doi: 10.18632/oncotarget.15799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T. Cancer immunotherapy‐targeted glypican‐3 or neoantigens. Cancer science 2018; 109:531–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amankwah EK, Lin HY, Tyrer JP, Lawrenson K, Dennis J, Chornokur G, Aben KK, Anton‐Culver H, Antonenkova N, Bruinsma F. Epithelial‐mesenchymal transition (EMT) gene variants and epithelial ovarian cancer (EOC) risk. Genetic epidemiology 2015; 39:689–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immunity Archive 2012; 12:14. [PMC free article] [PubMed] [Google Scholar]
- 25.Orecchia P, Conte R, Balza E, Petretto A, Mauri P, Mingari MC, Carnemolla B. A novel human anti-syndecan-1 antibody inhibits vascular maturation and tumour growth in melanoma. European journal of cancer 2013; 49:2022–33 [DOI] [PubMed] [Google Scholar]
- 26.Orecchia P, Conte R, Balza E, Pietra G, Mingari MC, Carnemolla B. 2015. Targeting Syndecan-1, a molecule implicated in the process of vasculogenic mimicry, enhances the therapeutic efficacy of the L19-IL2 immunocytokine in human melanoma xenografts. Oncotarget. 6(35):37426–37442. doi: 10.18632/oncotarget.605510.18632/oncotarget.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijdenes J, Vooijs WC, Clement C, Post J, Morard F, Vita N, Laurent P, Sun R-X, Klein B, Dore J-M. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94(2):318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research 2009; 15:4028–37 [DOI] [PubMed] [Google Scholar]
- 29.Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588(2):377–382. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu AX, Gold PJ, El-khoueiry A, Abrams TA, Morikawa H, Ohishi N, Ohtomo T, Philip PA. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clinical Cancer Research 2013:clincanres. 2616.012. [DOI] [PubMed]
- 31.Matsuzaki S, Serada S, Hiramatsu K, Nojima S, Matsuzaki S, Ueda Y, Ohkawara T, Mabuchi S, Fujimoto M, Morii E. Anti‐glypican‐1 antibody‐drug conjugate exhibits potent preclinical antitumor activity against glypican‐1 positive uterine cervical cancer. International journal of cancer 2018; 142:1056–66 [DOI] [PubMed] [Google Scholar]
- 32.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clinical Cancer Research. 2009;15(24):7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez ER, Paredes-Gamero EJ, Del Giglio A, Dos Santos Tersariol IL, Nader HB, Pinhal MAS. Heparan sulfate mediates trastuzumab effect in breast cancer cells. BMC Cancer. 2013;13(1):444. doi: 10.1186/1471-2407-13-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onyeisi JOS, Castanho de Almeida Pernambuco Filho P, de Araujo Lopes S, Nader HB, Lopes CC. Heparan sulfate proteoglycans as trastuzumab targets in anoikis-resistant endothelial cells. J Cell Biochem 2019; 120:13826–40 [DOI] [PubMed] [Google Scholar]
- 35.Gialeli C, Theocharis A, Kletsas D, Tzanakakis G, Karamanos N. Expression of matrix macromolecules and functional properties of EGF-responsive colon cancer cells are inhibited by panitumumab. Invest New Drugs. 2013;31(3):516–524. doi: 10.1007/s10637-012-9875-x. [DOI] [PubMed] [Google Scholar]
- 36.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 37.Guimarães FS, Andrade LF, Martins ST, Abud AP, Sene RV, Wanderer C, Tiscornia I, Bollati-Fogolín M, Buchi DF, Trindade ES. In vitro and in vivo anticancer properties of a Calcarea carbonica derivative complex (M8) treatment in a murine melanoma model. BMC cancer 2010; 10: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das D, Das I, Das J, Koyal SK, Khuda-Bukhsh AR. Efficacy of two commonly used potentized homeopathic drugs, Calcarea carbonica and Lycopodium clavatum, used for treating polycystic ovarian syndrome (PCOS) patients: II Modulating effects on certain associated hormonal levels. TANG. 2016;6(1):41–47. [Google Scholar]
- 39.Lan Y, Li X, Liu X, Hao C, Song N, Ren S, Wang W, Feng N, Zhang L. Genistein Enhances or Reduces Glycosaminoglycan Quantity in a Cell Type-Specific Manner. Cellular Physiology and Biochemistry 2018; 47:1667–81 [DOI] [PubMed] [Google Scholar]
- 40.Chatzinikolaou G, Nikitovic D, Stathopoulos E, Velegrakis G, Karamanos N, Tzanakakis G. Protein tyrosine kinase and estrogen receptor-dependent pathways regulate the synthesis and distribution of glycosaminoglycans/proteoglycans produced by two human colon cancer cell lines. Anticancer Res. 2007;27(6B):4101–4106. [PubMed] [Google Scholar]
- 41.Bhattacharya B, Mukherjee S. Cancer therapy using antibiotics. J Cancer Ther. 2015;6(10):849. doi: 10.4236/jct.2015.610093. [DOI] [Google Scholar]
- 42.Li J-M, Yang Y, Zhu P, Zheng F, Gong F-L, Mei Y-W. Mitoxantrone exerts both cytotoxic and immunoregulatory effects on activated microglial cells. Immunopharmacol Immunotoxicol. 2012;34(1):36–41. doi: 10.3109/08923973.2011.572890. [DOI] [PubMed] [Google Scholar]
- 43.Enache M, Toader A, Enache M. Mitoxantrone-surfactant interactions: a physicochemical overview. Molecules. 2016;21(10):1356. doi: 10.3390/molecules21101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004;63(12 suppl 6):S15–S18. doi: 10.1212/WNL.63.12_suppl_6.S15. [DOI] [PubMed] [Google Scholar]
- 45.Wichert A, Stege A, Midorikawa Y, Holm PS, Lage H. Glypican-3 is involved in cellular protection against mitoxantrone in gastric carcinoma cells. Oncogene. 2004;23(4):945. doi: 10.1038/sj.onc.1207237. [DOI] [PubMed] [Google Scholar]
- 46.Rahaman ST. Bleomycin: an overview on anti cancer drug. Int J Recent Advances in Multi Res. 2018;5(2):3618–3622. [Google Scholar]
- 47.Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29(5):371–387. doi: 10.1016/S0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Lan Y, He Y, Liu Y, Luo H, Yu H, Song N, Ren S, Liu T, Hao C. Heparan Sulfate and Chondroitin Sulfate Glycosaminoglycans Are Targeted by Bleomycin in Cancer Cells. Cellular physiology and biochemistry 2017; 43:1220–34 [DOI] [PubMed] [Google Scholar]
- 49.Gazitaeva ZI, Drobintseva AO, Chung Y, Polyakova VO, Kvetnoy IM. Cosmeceutical product consisting of biomimetic peptides: antiaging effects in vivo and in vitro. Clin Cosmet Investig Dermatol. 2017;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka Y, Tateishi R, Koike K. Proteoglycans are attractive biomarkers and therapeutic targets in hepatocellular carcinoma. Int J Mol Sci. 2018;19(10):3070. doi: 10.3390/ijms19103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rapraeger AC. Synstatin: a selective inhibitor of the syndecan‐1‐coupled IGF1R–αvβ3 integrin complex in tumorigenesis and angiogenesis. Febs J. 2013;280(10):2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metwaly HA, El-Gayar AM, El-Shishtawy MM. Inhibition of the signaling pathway of syndecan-1 by synstatin: A promising anti-integrin inhibitor of angiogenesis and proliferation in HCC in rats. Archives of biochemistry and biophysics; 2018. [DOI] [PubMed] [Google Scholar]
- 53.Bracci L, Mandarini E, Brunetti J, Depau L, Pini A, Terzuoli L, Scali S, Falciani C. The GAG-specific branched peptide NT4 reduces angiogenesis and invasiveness of tumor cells. PloS one 2018; 13:e0194744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Roy S, Cochran E, Zouaoui R, Chu CL, Duffner J, Zhao G, Smith S, Galcheva-Gargova Z, Karlgren J. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PloS one 2011; 6:e21106. [DOI] [PMC free article] [PubMed]
- 55.Galcheva-Gargova Z, Chu CL, Long A, Duffner J, Holte K, Schultes BC. Role of M402, a novel heparan sulfate mimetic, in pancreatic cancer cell invasion and metastasis: Inhibition of the Sonic Hedgehog pathway and heparanase activity. Journal of Clinical Oncology; 2012; 30: 25– [Google Scholar]
- 56.Dredge K, Hammond E, Handley P, Gonda T, Smith M, Vincent C, Brandt R, Ferro V, Bytheway I. PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. British journal of cancer 2011; 104: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hammond E, Brandt R, Dredge K. PG545, a heparan sulfate mimetic, reduces heparanase expression in vivo, blocks spontaneous metastases and enhances overall survival in the 4T1 breast carcinoma model. PloS One. 2012;7(12):e52175. doi: 10.1371/journal.pone.0052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao B-Y, Wang Z, Hu J, Liu W-F, Shen Z-Z, Zhang X, Yu L, Fan J, Zhou J. PI-88 inhibits postoperative recurrence of hepatocellular carcinoma via disrupting the surge of heparanase after liver resection. Tumor Biology 2016; 37:2987–98 [DOI] [PubMed] [Google Scholar]
- 59.Coleman R. Potential use of bisphosphonates in the prevention of metastases in early-stage breast cancer. Clin Breast Cancer. 2007;7:S29–S35. [DOI] [PubMed] [Google Scholar]
- 60.Hortobagyi GN, Van Poznak C, Harker WG, Gradishar WJ, Chew H, Dakhil SR, Haley BB, Sauter N, Mohanlal R, Zheng M. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA oncology 2017; 3:906–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman R, Body -J-J, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25(suppl_3):iii124–iii137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 62.Hadji P, Coleman RE, Wilson C, Powles T, Clézardin P, Aapro M, Costa L, Body J-J, Markopoulos C, Santini D. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Annals of Oncology 2015; 27:379–90 [DOI] [PubMed] [Google Scholar]
- 63.Dedes P, Gialeli C, Tsonis A, Kanakis I, Theocharis A, Kletsas D, Tzanakakis G, Karamanos N. Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochimica et Biophysica Acta (BBA)-General Subjects [DOI] [PubMed]
- 64.Siddikuzzaman GC, Berlin Grace V. All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol. 2011;33(2):241–249. doi: 10.3109/08923973.2010.521507. [DOI] [PubMed] [Google Scholar]
- 65.Lokman NA, Ho R, Gunasegaran K, Bonner WM, Oehler MK, Ricciardelli C. Anti-tumour effects of all-trans retinoid acid on serous ovarian cancer. J Exp & Clin Cancer Res. 2019;38(1):10. doi: 10.1186/s13046-018-1017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhao J, Sun J, Huang L, Li Q. Targeting lung cancer initiating cells by all‑trans retinoic acid‑loaded lipid‑PLGA nanoparticles with CD133 aptamers. Exp Ther Med. 2018;16(6):4639–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connolly RM, Nguyen NK, Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res. 2013;19(7):1651–1659. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran-Lundmark K, Tannenberg P, Rauch BH, Ekstrand J, Tran PK, Hedin U, Kinsella MG. Perlecan Heparan Sulfate Is Required for the Inhibition of Smooth Muscle Cell Proliferation by All-trans-Retinoic Acid. J Cell Physiol. 2015. Feb;230(2):482–7. doi: 10.1002/jcp.24731.PMID:25078760 [DOI] [PubMed] [Google Scholar]
- 69.Ramya D, Siddikuzzaman, Grace VB. Effect of all-trans retinoic acid (ATRA) on syndecan-1 expression and its chemoprotective effect in benzo (α) pyrene-induced lung cancer mice model. Immunopharmacol Immunotoxicol. 2012;34(6):1020–1027. doi: 10.3109/08923973.2012.693086. [DOI] [PubMed] [Google Scholar]
- 70.Kumar B, Singh S, Skvortsova I, Kumar V. Promising targets in anti-cancer drug development: recent updates. Curr Med Chem. 2017;24(42):4729–4752. [DOI] [PubMed] [Google Scholar]


